Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
XIAO Chengbin (肖诚斌), NING Jun (宁君), YAN Hai (闫海), SUN Xudong (孙旭东)and HU Jiye (胡继业)
Biodegradation of Aniline by a Newly Isolatedsp. XYJ6*
XIAO Chengbin (肖诚斌)1, NING Jun (宁君)1, YAN Hai (闫海)2,**, SUN Xudong (孙旭东)2and HU Jiye (胡继业)2
1Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing 100085, China2Department of Biological Science and Technology, School of Applied Science, University of Science and Technology Beijing, Beijing 100083, China
A promising gram-negative bacterial strain for the biodegradation of aniline as the sole carbon, nitrogen and energy sources was successfully isolated and identified assp. XYJ6. The optimal temperature and pH for both the growth ofsp. XYJ6 and the biodegradation of aniline were 30°C and 7.0, respectively. Initial aniline of 2000 mg·L-1could be completely removed by the strain at 22 h, which showed thatsp. XYJ6 had a strong ability in the biodegradation of aniline. It indicated that aniline was firstly converted to catechol catalyzed by aniline dioxygenase as a first product, which was then further biodegraded to,-muconic acid catalyzed by the catechol 1,2-dioxygenase ofsp. XYJ6 as a second product.,-muconic acid could also be further biodegraded to other small compound again. The pathway for the biodegradation of aniline bysp. XYJ6 was not previously reported.
aniline,sp., biodegradation, pathway
1 INTRODUCTION
Aniline is widely used as an intermediate in the production of dye industry and raw material in the manufacturing of synthetic organic chemicals and polymers including polyurethanes, rubber additives, pharmaceuticals, pesticides, and herbicides [1-3], which is increasingly produced and released to the environment at a growing rate, and is frequently detected in both terrestrial and aquatic environments [4]. Because of its potent toxicity to both wildlife and humans, and recalcitrant to be biodegraded in naturally indigenous microorganisms, aniline was classified as a persistent organic pollutant by USA and China [5-7].
Biodegradation is a common method for the removal of organic pollutants, because of its low cost and little collateral destruction of indigenous flora and fauna [8-10]. Some researchers had studied on the biodegradation of aniline and a few species of aniline- biodegrading bacteria including[11],[12],[13],[14],[15],[16] and[17] were successfully isolated from environment. As for the biodegradation pathway of aniline, it showed that aniline could firstly be convertedaniline dioxygenase to catechol in the aerobic environment, and then catechol was further biodegraded to,-muconic acid (ccMA) catalyzed by the catechol 1,2-dioxygenase (the-cleavage pathway) or 2-hydroxymuconic semialdehyde by catechol 2,3-dioxygenase (the- cleavage pathway) [8, 11, 18, 19]. On the other hand, aniline could be metabolized-aminobenzoate as the first intermediate under anaerobic denitrifying conditions[20]. Furthermore, the genes for the biodegradation of aniline was cloned and identified [14, 21].
In this study, a promising bacterial strain for the efficient biodegradation of aniline was successfully isolated and identified assp. XYJ6. Both the kinetics and the pathway of aniline biodegradation by this newly isolatedsp. XYJ6 were further investigated, and a new biodegradation pathway of aniline bywas confirmed. The study was very important in both the basic research and application in environmental sciences.
2 MATERIALS AND METHODS
2.1 Materials
Aniline used in this study was bought from the Institute of Environmental Monitor, Ministry of Agriculture, China. All other chemicals in the experiment were analytical grade. The aniline-degrading bacterium was isolated from the wastewater sediment in a Shandong Pesticide Factory.
2.2 Culture medium and conditions
The basal liquid medium to isolate and culture strain XYJ6 consisted of 0.20 g of MgSO4·7H2O, 0.02 g of CaCl2, 0.008g of FeSO4, 0.20 g of KH2PO4, 1.00 g of Na2HPO4·12H2O and 1.0 ml of trace elements solution in 1000 ml distilled water. Initial pH of medium was adjusted to 7.0 with 1.0 mol·L-1NaOH or HCl. Aniline with different initial concentrations was added in the medium as the sole carbon and nitrogen sources. Both medium and all experiment utensils were sterilized at 121°C for 20 min.
Strain XYJ6 was inoculated in the sterilized culture medium, and grew in a 100 ml flask that contained 20 ml liquid medium. The culture condition was at the temperature of 30°C with the shake rate of 200 r·min-1. The optical density at wavelength of 680nm (OD680) was measured, which represented the growth of Strain XYJ6. Culture broth of 1.0 ml was taken and centrifuged at 12000 r·min-1for 10 min and the supernatant was diluted and directly used to determine the concentration of aniline on high performance liquid chromatography (HPLC). The data presented were the average values derived from three measurements and their relative standard deviations were less than 10%.
2.3 Isolation of bacterial strain
Wastewater sediment samples were inoculated into the liquid culture medium using aniline of 500 mg·L-1as the sole carbon and nitrogen sources, and cultured with a shake rate of 200 r·min-1at 30°C for 3 days. After three consecutive cultures, the mixed bacteria were diluted and spread onto the solid medium containing 1.5% agar in the presence of 500 mg·L-1aniline. Single colonies that grew on the plates were isolated and inculcated into liquid medium containing aniline of 500 mg·L-1to test its biodegradation ability. The process was repeated until a pure bacterial strain that had the ability in the biodegradation of aniline was obtained.
2.4 Identification of bacterial strain
A newly isolated strain XYJ6 was identified by the morphological and physiological traits, which was further identified by the analysis of 16S rDNA sequences. Gram staining was performed as described previously [22] and the genomic DNA of strain was extracted according to the previous report [23]. The 16S rDNA was selectively amplified with polymerase chain reaction (PCR, Eppendorf-5331) for a total of 30 cycles of 94°C for 5 min, then 50°C for 1min and finally 72°C for 3 min. The upstream primer was 5′-AGA GTT TGA TCA TGG CTC AG-3′ and the downstream primer was 5′-CTA CGG TTA CCT TGTTACG AC-3′. The amplified DNA was sent to the Bejing Sunbiotech Co. (China) for sequencing. The nucleotide sequences were compared to the most similar sequences of bacterium on the Gene Bank, which was used to identify this newly isolated bacterial strain.
2.5 Analysis by HPLC and LC/MS
Aniline was measured using an HPLC system (Shimadzu LC-l0ATVP, Shimadzu Co., Japan) with a UV Diode Array Detector at 230 nm connected to an SB-C 18 (4.6 mm×250 mm, 5 μm) column from Agilent.The mobile phase was 50% (by volume) methanol/watersolution with a flow rate of 1.0 ml·min-1. The calibration curve was established between the peak areas and the concentration of aniline, which was used to calculate the unknown concentration of aniline in the experiment.
The mass spectrometer (Agilent 6110, Quadrupole LC/MS) was used to identify the products of aniline biodegradation, which was operated in the negative mode using electrospray ionization (ESI) source with a dried gas flow of 10 L·min-1at temperature of 350°C. The nebulizer pressure was set to 310.26 kPa (45 psi) and the data were acquired in a full-scan mode (/50-600).
3 RESULTS AND DISCUSSION
3.1 Identification of strain
Using aniline as the sole carbon, nitrogen and energy sources, an aniline-biodegrading bacterial strain XYJ6 was successfully isolated, which formed a circle yellow colony on solid medium. The observation under microscope indicated that the cells of strain XYJ6 were gram-negative, no spore and short-rod. Strain XYJ6 could be grown in pH range from 5.0 to 10.0 on solid medium with a maximum NaCl of 2.0%. It also showed that strain XYJ6 had no antibiotic resistance to four antibiotics of streptomycin, erythromycin, ampicillin and kanamycin, except for cefotaxime sodium.
16S rDNA of strain XYJ6 was amplified and sequenced for the identification of its phylogenetic placement. Based on the analysis of 16S rDNA sequences, strain XYJ6 appeared to be closely related tosp. The relationship of strain XYJ6 to other closely-related members was shown in Fig. 1. The complete sequence of the 1373 bp 16S rDNA fragment formsp. XYJ6 had been deposited in the GenBank database under accession number EU707799. Based on morphological and physiological traits as well as the phylogenetic analysis of 16S rDNA sequences, strain XYJ6 was identified assp. XYJ6.
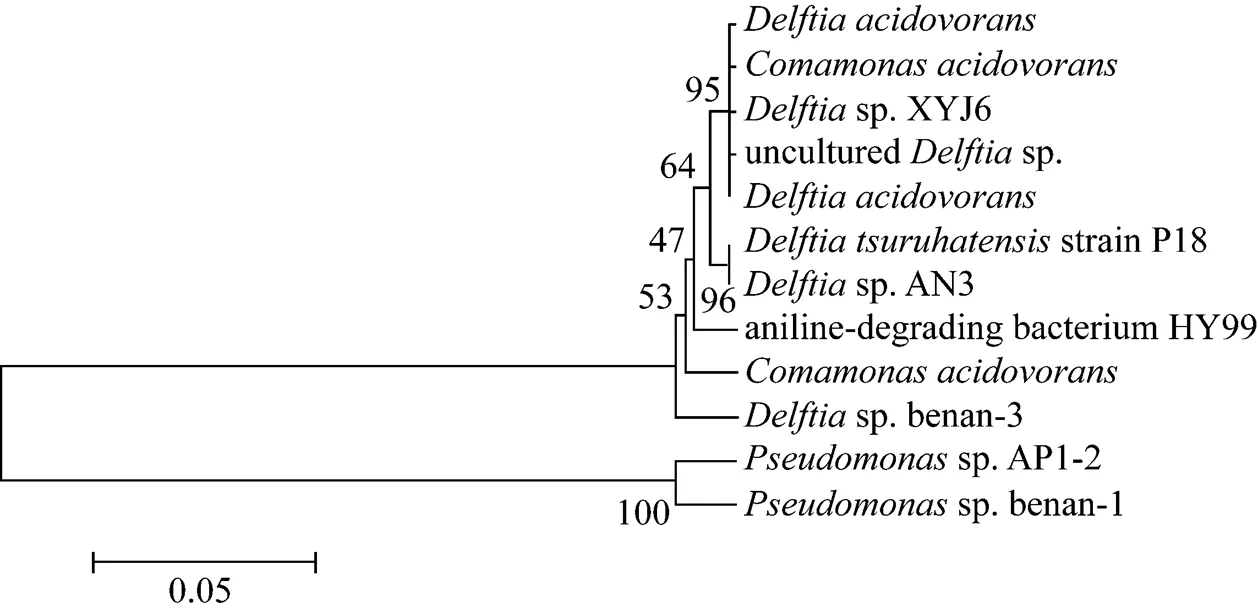
Figure 1 Phylogenetic tree ofsp. XYJ6
3.2 Biodegradation of aniline by Delftia sp. XYJ6
Figure 2 was the biodegradation kinetics of aniline bysp. XYJ6 at different initial concentrations of aniline, which showed that the biodegradation of aniline was apparently inhibited when the initial concentration of aniline increased to 3000 mg·L-1. However, initial aniline of 1000 and 2000 mg·L-1could be completely biodegraded bysp. XYJ6 at 22 h, which indicated that biodegradation rate of aniline bysp. XYJ6 was much higher than that of GXA7 reported by Bi[24].
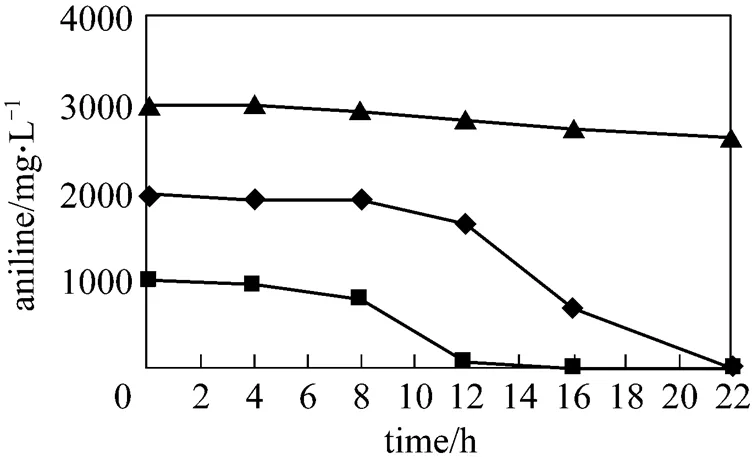
Figure 2 Kinetics of aniline biodegradation by an isolated bacterial strain XYJ6
▲ 3000 mg·L-1;◆ 2000 mg·L-1;■ 1000 mg·L-1
Figure 3 showed the biodegradation kinetics of aniline and the growth ofsp. It indicated that the growth process ofsp. XYJ6 underwent a lag phase before 4 h, exponential phase between 4 h to 12 h and stationary phase from 12 h to 32 h, respectively. With the growth of strain XYJ6, the concentration of aniline slowly decreased from 0 to 4 h, and sharply declined from 4 h to 12 h. The maximum biodegradation rate appeared at the most rapid growth phase ofsp. XYJ6 and initial aniline of 1000 mg·L-1was completely removed at 22 h. Because aniline was almost removed, the biomasses ofsp. XYJ6 gradually decreased after 12 h.

Figure 3 Growth of strain XYJ6 and biodegradation of aniline
3.3 Effects of pH and temperature on the growth of Delftia sp. XYJ6 and the biodegradation of aniline
The growth ofsp. XYJ6 and biodegradation of aniline under the different pH and temperature were shown in Fig. 4. It showed that both the growth ofsp. XYJ6 and the biodegradation of aniline were promoted at pH 7.0 in the pH range from 6.0 to 8.0. It also indicated that the temperature of 30°C in the range from 25°C to 35°C was an optimal temperature for both the growth ofsp. XYJ6 and the biodegradation of aniline.

Figure 4 Effects of pH and temperature on the growth ofsp. XYJ6 and the biodegradation of aniline pH: ◆ 6.0;■ 7.0; △ 8.0 temperature/°C: ▲ 25; ● 30; ◇ 35
3.4 Pathway for the biodegradation of aniline by Delftia sp. XYJ6
During the biodegradation of aniline bysp. XYJ6, the first (1) and second (2) intermediate products at retention time of 4.6 min and 2.6 min were apparently observed at the profile of HPLC chromatography in Fig. 5. It showed that both the retention time and UV scanning profile of product 1 was same to those of the standard catechol. Moreover, the same outcomes were obtained when the flow rate of HPLC was changed to 0.8 ml·min-1.

Figure 5 HPLC chromatograms and scanning profiles of degradation of aniline and standard aniline, catechol and ccMA1—catechol; 2—ccMA
Initial catechol of 300 mg·L-1was added into the media in order to determine product 2, and could also be completely biodegraded at 27 h in the presence ofsp. XYJ6 (Fig. 5). With the decrease of catechol peak, product 2 at the retention time of 2.6 min increased with time from 0 h to 4 h and then decreased from 4 h to 16 h. Both the retention time and UV scanning profile of product 2 was same to those of the standard ccMA, and the same outcomes were obtained when the flow rate of HPLC was changed to 0.8 ml·min-1. The characteristic peak of product 2 decreased after 10 h soon (Fig. 5), which meant that it could also be further biodegraded to other small molecular compounds.
The products of aniline biodegradation were further determined by LC/MS in order to measure their molecular weight (W) and to presume their structures. Total ion current chromatograms (TIC) of products 1 and 2 were shown in Fig. 6. The/of the deprotonated molecular ion, [M—H]-, of products 1 and 2 were 109.1 and 141.1, which were corresponding to the molecular weight of 110.1 and 142.1 for catechol and ccMA, respectively.
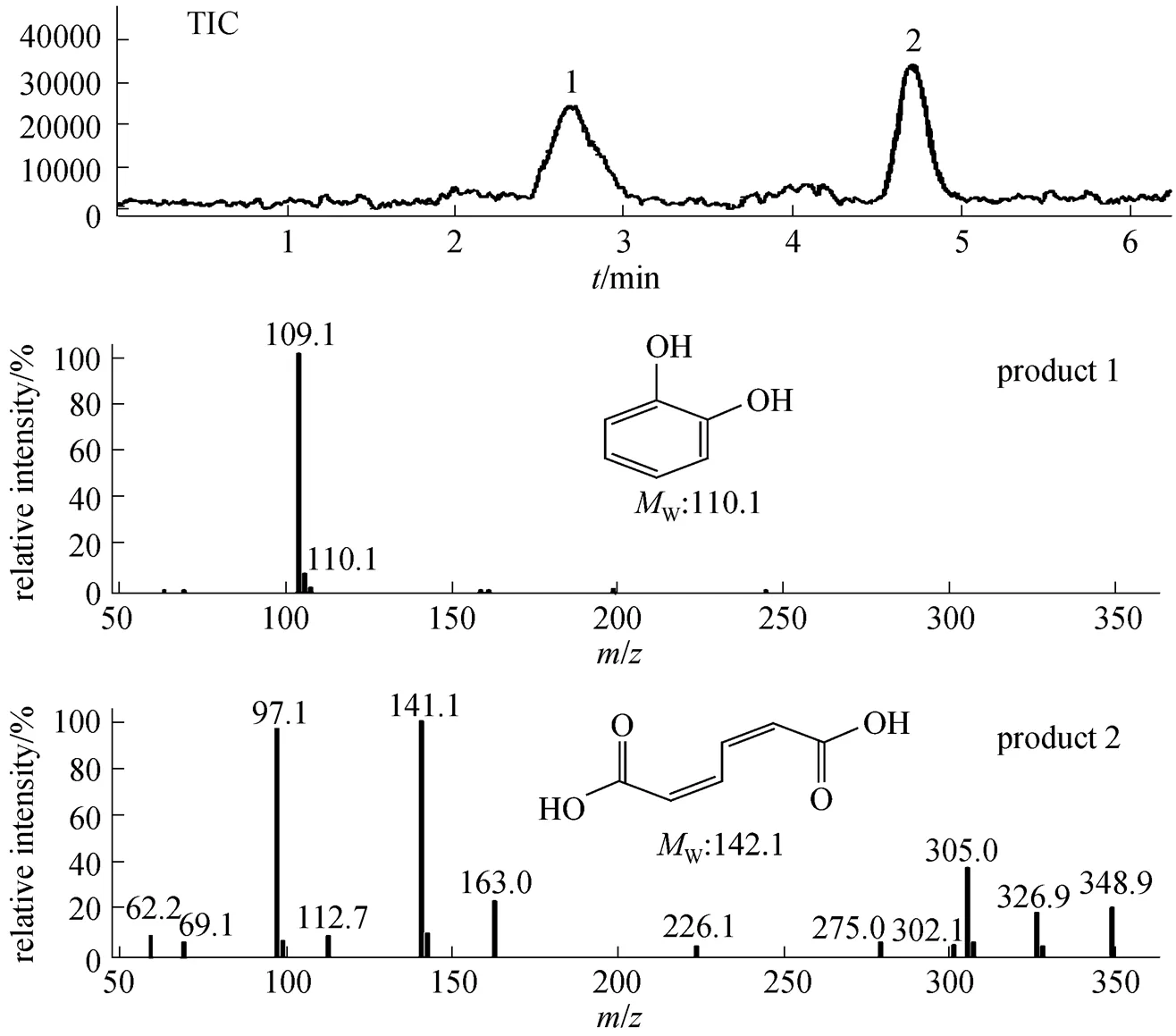
Figure 6 Total ion current chromatogram (TIC) and mass spectra of the products of aniline biodegradation 1—catechol; 2—ccMA
Figure 7 Proposed pathway for the biodegradation of aniline bysp. XYJ6
The above results indicated that the first intermediate product of aniline biodegradation bysp. XYJ6 was catechol, and the amino-group rather than benzene ring of aniline was firstly attacked bysp. XYJ6, which was in agreement with the earlier reports [8, 17]. The intermediate product of catechol biodegradation bysp. XYJ6 was ccMA. It reported that catechol 2,3-dioxygenase played a main role in aniline biodegradation, which was responsible for the conversion of catechol to 2-hydroxymuconic semialdehyde by[8]. However, this product could not be found in our study, which showed that the different pathways for the biodegradation of aniline might be used in different bacterial strains.
As for the pathway of aniline biodegradation bysp. XYJ6 (Fig. 7), it was concluded that aniline was converted to catechol as a first product catalyzed by aniline dioxygenase ofsp. XYJ6, and then catechol was further biodegraded to ccMA catalyzed by catechol 1,2-dioxygenase ofsp. as the second product. It was also indicated that ccMA could also be further biodegraded to some other small compounds.
4 CONCLUSIONS
A promising bacterial strain for the biodegradation of aniline was successfully isolated and identified assp. XYJ6 based on the morphological and physiological traits as well as the phylogenetic analysis of 16S rDNA sequences. The initial aniline of 2000 mg·L-1could be completely removed by the strain at 22 h. The optimal temperature and pH for both the growth ofsp. XYJ6 and biodegradation of aniline were 30°C and 7.0, respectively. The pathway for the biodegradation of aniline bysp. XYJ6 could be suggested as follows: aniline was converted to catechol as a first product catalyzed by aniline dioxygenase ofsp. XYJ6, and then catechol was further biodegraded to ccMA catalyzed by catechol 1, 2-dioxygenase ofsp. XYJ6. CcMA could also be further biodegraded to other small molecular compounds.
1 O’Neill, F.J., Bromley-Challenor K.C.A., Greenwood R.J., Knapp, J.S., “Bacterial growth an aniline: implications for the biotreatment of industrial wastewater”,.., 34, 4397-4409 (2000).
2 Kearney, P.C., Kaufmann, D.D., Herbicides: Chemistry, Degradation and Mode of Action, Marcel Dekker, New York (1975).
3 Emtiazi, G., Satarii, M., Mazaherion, F., “The utilization of aniline, chlorinated aniline, and aniline blue as the only source of nitrogen by fungi in water”,.., 35, 1219-1224 (2001).
4 Gheewala, S.H., Annachhatre, A.P., “Biodegradation of aniline”,..., 36, 53-63 (1997).
5 Lyons, C.D., Katz, S., Bartha, R., “Persistence and mutagenic potential of herbicide-derived aniline residues in pond water”,...., 35, 696-703 (1985).
6 Khan, M.F., Wu, X.H., Kaphalia, B.S., Boor, P.J., Ansari, G.A.S., “Nitrotyrosine formation in splenic toxicity of aniline”,, 194, 95-102 (2003).
7 Wang, L., Barrington, S., Kim, J.W., “Biodegradation of pentyl amine and aniline from petrochemical wastewater”,...,83, 191-197 (2007).
8 Urata, M., Uchida, E., Nojiri, H., Omori, T., Obo, R., Miyaura, N., Ouchiyama, N., “Genes involved in aniline degradation byacidovorans strain 7N and its distribution in the natural environment”,..., 68 (12), 2457-2465 (2004).
9 Wen, J.P., Li, H.M., Bai, J., Jiang, Y., “Biodegradation of 4-chlorophenol byPDY-07 under anaerobic conditions”,...., 14 (6), 790-795 (2006).
10 Jiang, Y., Ren, N.Q., Cai, X., Wu, D., Qiao, L.Y., Lin, S., “Biodegradation of phenol and 4-chlorophenol by the mutant strain CTM 2”,...., 16 (5), 796-800 (2008).
11 Konopka, A., Knight, D., Turco, R.F., “Characterization of asp. capable of aniline degradation in the presence of secondary carbon sources”,..., 48, 491-496 (1989).
12 Zeyer, J., Wasserfallen, A., Timmis, K.N., “Microbial mineralization of ring-substituted anilines through an-cleavage pathway”,..., 50, 447-453 (1985).
13 Parales, R.E., Ontl, T.A., Gibson, D.T., “Cloning and sequence analysis of a catechol 2,3-dioxygenase gene from the nitrobenzene-degrading strainsp. JS765”,...., 19, 385-391 (1997).
14 Schukat, B., Janke, D., Krebs, D., Fritsche, W., “Cometabolic degradation of 2- and 3-chloroaniline because of glucose metabolism bysp. AN117”,.., 9, 81-86 (1983).
15 Aoki, K., Ohtsuka, K., Shinke, R., Nishira, H., “Rapid biodegradation of aniline byANA-18”,..., 48, 865-872 (1984).
16 Bachofer, R., Lingens, F., Schafer, W., “Conversion of aniline into pyrocatechol by asp.: Incorporation of oxygen-18”,., 50, 288-290 (1975).
17 Aoki, K., Ohtsuka, K., Shinke, R., Nishira, H., “Rapid Biodegradation of aniline by Frateuria species ANA-18 and its aniline metabolism”,..., 48 (4), 865-872 (1984).
18 Kahng, H.Y., Kukor, J.J., Oh, K.H., “Characterization of strain HY99, a novel microorganism capable of aerobic and anaerobic degradation of aniline”,.., 190, 215-221 (2000).
19 Liu, Z., Yang, H., Huang, Z., Zhou, P., Liu, S.J., “Degradation of aniline by newly isolated, extremely aniline-tolerantsp. AN3”,..., 58, 679-682 (2002).
20 Kahng, H.Y., Kukor, J.J., Oh, K.H.J., “Physiological and phylogenetic analysis ofsp. HY1 capable of aniline degradation”,..., 10, 643-650 (2000).
21 Zhang, T., Zhang, J.L., Liu, S.J., Liu, Z.P., “A novel and complete gene cluster involved in the degradation of aniline bysp. AN3”,..., 20, 717-724 (2008).
22 Sheludchenko, M.S., Kolomytseva, M.P., “Degradation of aniline by14S in batch and continuous processes”,..., 41 (5), 465-468 (2005).
23 Radianingtyas, H., Robinson, G.K., Bull, A.T., “Characterization of a soil-derived bacterial consortium degrading 4-chloroaniline”,, 149, 3279-3287 (2003).
24 Bi, H.K., Huang, Y., Wu, B., “Isolation, identification and characterization of aniline-degrading strain GXA7”,., 28, 309-313 (2003). (in Chinese)
2008-10-06,
2009-02-28.
the National Natural Science Foundation of China (20777008) and the Education Committee of Beijing.
** To whom correspondence should be addressed. E-mail: haiyan@sas.ustb.edu.cn
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Combination of Supercritical Fluid Extraction with Ultrasonic Extraction for Obtaining Sex Hormones and IGF-1 from Antler Velvet*
