Ethylene Polymerization Using Improved Polyethylene Catalyst
ZHUXiaoheng (朱孝恒), GUO Zifang (郭子芳), CEN Wei (岑为) and MAO Bingquan (毛炳权),
Ethylene Polymerization Using Improved Polyethylene Catalyst
ZHUXiaoheng (朱孝恒)1, GUO Zifang (郭子芳)2, CEN Wei (岑为)1and MAO Bingquan (毛炳权)2,*
1Department of Chemistry, Beijing University of Chemical Technology, Beijing 100029, China2Beijing Research Institute of Chemical Industry, China Petroleum & Chemical Corporation, Beijing 100013, China
The study concerns the use of MgCl2-supported high-activity Ziegler-Natta catalysts for the polymerization of ethylene. In particular, two types of catalysts were investigated, which were N-catalyst (BRICI) and improved polyethylene catalyst. The effects of catalyst structure on kinetic behavior were examined. The distribution of active centers in these catalysts was investigated by energy dispersive analysis by X-rays (EDAX), and morphologies of catalyst particles and polymer products were examined by scanning electron microscope (SEM). Hydrogen response and copolymerization performance were investigated and compared with the two catalysts. The results were correlated with the kinetic behavior of the two catalysts and appropriate models for polymer particle growth were presented. The improved polyethylene catalyst showed higher activity, better hydrogen response and copolymerization performance.
polyethylene catalyst, polyethylene, slurry polymerization process, ethoxy group
1 INTRODUCTION
The Ziegler-Natta catalyst has been used in the olefin polymerization since its discovery in 1950 [1-5]. Heterogeneous Ziegler-Natta catalyst is the main system among polyolefin catalysts and responsible for the production over tens of million tones of polyethylene per year. With well control of polymerization conditions, products with special properties can be obtained with appropriate catalyst, which motivates the continuous study on these catalysts [6-14]. The chemical composition of active sites influences the polymer properties, so the characterization of active sites and the knowledge of their reactivity are very important for design of new materials.
In this work, we improve a polyethylene catalyst in order to achieve high activity in the ethylene polymerization. Two Ziegler-Natta catalysts are used, and the effects of support structure on the kinetic behavior, hydrogen response and copolymerization performance of the catalysts is investigated.
2 EXPERIMENTAL
2.1 Materials
Polymerization grade ethylene was obtained from Beijing Yanshan Petrochemical Co., Ltd. (BYPC), used after passing through 4A molecular sieve. Triethylaluminium (TEA, 95% purity, Ethyl Co.) was used without further purification. Handling of the air and moisture sensitive materials was conducted in a nitrogen- filled dry-box or under nitrogen protection. Titanium tetrachloride, tributyl phosphate, epoxy chloropropane, ethanol,-hexane and anhydrous magnesium chloride were obtained from Beijing Chemical Reagents Co., Ltd. (Beijing, China).
2.2 Preparation of catalysts
Catalyst A: 4.0 g magnesium dichloride, 100 ml toluene, 2.0 ml epoxy chloropropane, 3.4 ml ethanol and 6.0 ml tributyl phosphate were successively added to a reactor. After stirring for 3 h at 80 ºC, the mixture formed a homogeneous solution. 60 ml TiCl4was added to the solution at-25°C. Then, the temperature was raised slowly to 80 °C. After 2 h, the liquid phase was removed, and the solid residue was washed twice with 150 ml toluene and hexane until the liquid phase was colorless. The solid catalyst was dried under N2.
Catalyst B [15]: 4.0 g of magnesium dichloride, 100 ml of toluene treated with molecular sieve, 6.0 ml of epoxy chloropropane, and 12 ml of tributyl phosphate were successively added to a reactor. The mixture was heated to 80 °C with stirring, and after the solid was completely dissolved to form a homogeneous solution, the reaction mixture was cooled to-25 °C. 60 ml of TiCl4was added dropwise, and 15 ml of-hexane was slowly added into the reactor in 15 min. The other steps were the same as those for preparing catalyst A.
2.3 Ethylene polymerization
Ethylene was polymerized in a stainless steel autoclave (2.0 L capacity) equipped with gas ballast through a solenoid valve for continuous feeding of ethylene at constant pressure while using hexane as solvent. 1 L hexane, 1.0 ml AlEt3hexane solution, and a certain amount of the above-prepared solid catalyst (containing 0.25 milligrams of titanium) were added into the autoclave. When the reactor was heated to 75 °C, hydrogen was introduced until the pressure reached 0.28 MPa (gauge pressure). Then, ethylene was introduced until the total pressure in the autoclave reached 0.70 MPa (gauge pressure). Maintaining at 80 °C for 2 h, the polymerization was ended.

Table 1 Characteristics of catalysts in ethylene polymerization①

2.4 Characterization of catalysts and polymers

3 RESULTS AND DISCUSSION
3.1 Properties of catalysts
As shown in Table 1, ethoxy groups markedly influence the surface area and porosity of the catalyst. With ethoxy groups, catalyst A shows very high surface area and large pore volume. Catalyst B, without ethoxy group, has lower activity, while catalyst A, with some ethoxy groups, presents higher activity for ethylene polymerization. The activity of catalyst A achieves to 3.349×104(g PE)·(g cat)-1, higher than that of domestic polyethylene slurry catalyst BCS01 [(2.5×104)-(2.8×104) (g PE)·(g cat)-1] [16].
3.2 Hydrogen response of catalysts
Hydrogen is most widely used as chain-transfer agent to control molecular weight (W) of the polymer, and is the only commercially applicable chain-transfer agent in the low-pressure olefin polymerization process over Ziegler-Natta catalysts. The effects of H2concentration on ethylene polymerization are showed in Table 2. Under the same polymerization conditions, the hydrogen response of the catalysts is strongly dependent on ethoxy groups. As the ratio of hydrogen/ ethylene increases, the polymerWdecreases, so that the polymerWwith catalyst A is lower than that with catalyst B. The rate of chain-transfer reaction increases with the increase of hydrogen, resulting in a decrease ofW. Thus, catalyst A has better hydrogen response than catalyst B. As the ratio of hydrogen/ethylene increases, the activities of both catalysts decrease.

Table 2 Evaluation of hydrogen modulation forthe catalysts①

3.3 Copolymerization performance of catalysts
In order to evaluate copolymerization performance of the two catalysts, different amount of 1-hexene was added to the reactor and the results are shown in Table 3.The copolymerization performance of 1-hexene is dependent on ethoxy groups. The activity of the catalysts increases with the increase of 1-hexene, but for the same amount of 1-hexene, catalyst A presents better copolymerization performance. The reason may be that catalyst A has larger pore volume and higher surface area, so that 1-hexene is easier to contact with Ti+-olefin centers.

Table 3 Copolymerization of ethylene and 1-hexene withtwo catalysts①


Figure 1 Ethylene monomer absorption curves of catalysts
(Polymerization conditions: 2 L autoclave, 1 L hexane, 2 h, 80 °C, pressure ratio of hydrogen/ethylene of 0.28MPa/0.45MPa)
■ catalyst B;● catalyst A
3.4 Kinetic behavior of catalysts
The rate-time profiles are shown in Fig. 1 for the two catalysts. An induction period is observed for catalyst B, whereas no induction period for catalyst A. Under the conditions, catalyst A exhibits higher activityand its average rate is 300% higher than that of catalyst B. It can be concluded that the porosity of the support used to prepare a catalyst evidently controls both the rate-time profiles and the ultimate catalyst activity.
3.5 Mechanism of polymer growth
The two catalysts and the polymers produced were subjected to morphological examinations. The internal structures of catalyst particles A and B were investigated by SEM, after slicing the catalyst particle using a microtome, and the resulting pictures are shown in Fig. 2. Catalyst A has higher porosity and more meso-pores, and a number of voids (empty spaces) can be identified. The internal structure of catalyst B shows tiny porosity, which explains its induction period in Fig. 1, and the lower average activity is owing to its close-grained internal structure.

Figure 2 Internal structure of catalyst particles A and B
The elemental distribution of titanium and magnesium atoms was determined by EDAX (energy dispersive analysis by X rays), and the result is showed in Fig. 3. In the two catalysts, titanium atoms and magnesium atoms distribute uniformly throughout the catalyst particle. The mass content of titanium atoms in catalyst A is more than that in catalyst B, while the mass contents of magnesium atoms in the two catalysts are the same.

Figure 3 Elemental distribution within catalysts A and B

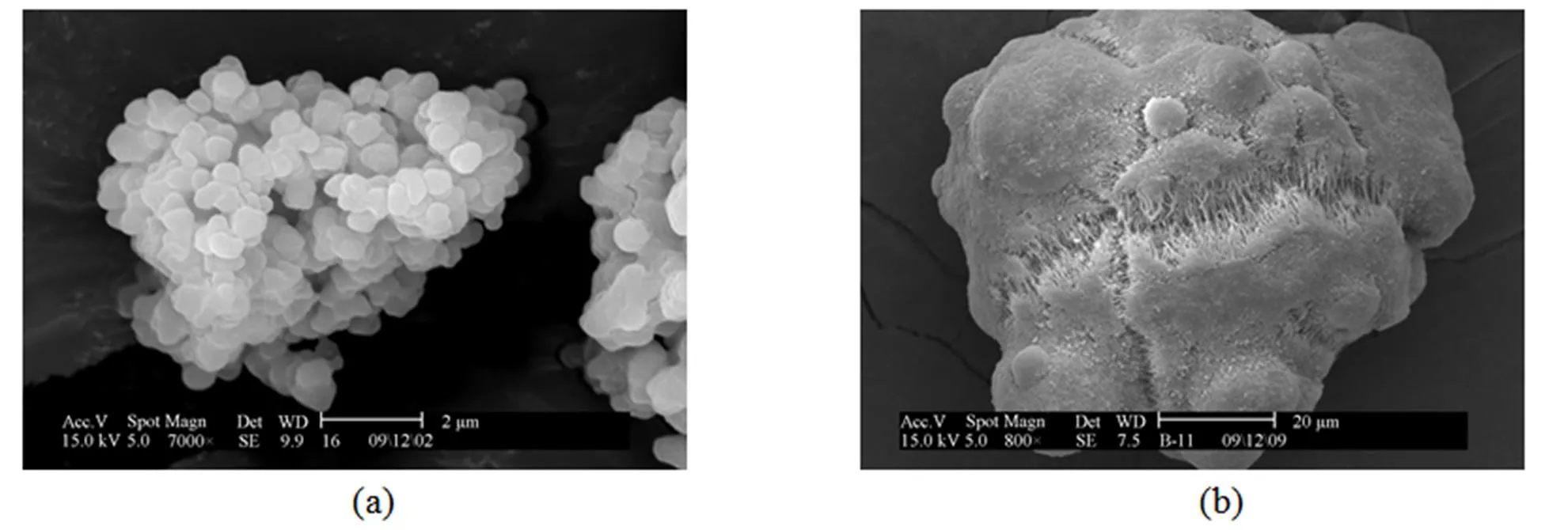
Figure 4 SEM micrograph of catalyst A (a) and polymer produced by catalyst A (b)
Figure 5 SEM micrograph of catalyst B (a) and polymer produced by catalyst B (b)

catalyst A
catalyst B
Figure 6 Models for polymer particle growth
O—catalyst pore; x—catalyst active site
Representative samples of polymer produced by both catalysts and catalyst particles were also studied by SEM, as shown in Figs. 4 and 5. The particle shapes are similar in both polymerization systems, but more small particles are produced with catalyst B. Thus a more uniform and rapid fragmentation occurs by using catalyst A. In the fragmentation process, growing micro-reactors [17] are produced, leading to excellent catalyst particle replication. The internal fragmentation of catalyst A also allows the retention of porosity, since not all the pores have to be filled with polymer before fragmentation occurs [18, 19].
The mechanism for polymer growth is proposed in Fig. 6. A polymer layer formed around catalyst particle B is envisaged, which starts the polymerization. Diffusion effects of reactants through this polymer layer determine the shapes of the rate-time profiles. The polymer growth with catalyst A takes place within micro-reactors and the growth process can be described by the multigrain model proposed by Harmon. [20]. The onion-skin type of model for MgCl2-supported Ziegler-Natta catalysts [16] has not been validated, though Ti atoms in the outer surface layers are firstly activated as polymerization centers.
4 CONCLUSIONS
In conclusion, these results show very clearly that the improved polyethylene catalyst showed higher activity, better hydrogen response and copolymerization performance for ethylene polymerization and copolymerization than the commercial catalyst N-catalyst (BRICI).
1 Flisak, Z., “Multidentate tetrahydrofurfuryloxide ligand in a Ziegler- Natta catalyst studied by molecular modeling”,, 41 (19), 6920-6924 (2008).
2 Stukalov, D.V., Zakharov, V.A., “Active site formation in MgCl2-supported Ziegler-Natta catalysts. A density functional theory study”,..., 113 (51), 21376-21382 (2009).
3 Paolo, C., Gaetano, G., Luigi, C., “Do new century catalysts unravel the mechanism of stereocontrol of old Ziegler-Natta catalysts?”,..., 37 (4), 231-241 (2004).
4 Andrea, C., Fabrizio, P., Giampiero, M., Luigi, C., “Key elements in the structure and function relationship of the MgCl2/TiCl4/Lewis base Ziegler-Natta catalytic system”,, 40 (25), 9181-9189 (2007).
5 Michael, S., Tom, Z., “Theoretical study of the copolymerization of ethylene and propylene by a heterogeneous Ziegler-Natta catalyst”,, 37 (24), 9191-9200 (2004).

7 Denis, V.S., Igor, L.Z., Vladimir, A.Z., “Surface species of titanium(IV) and titanium(III) in MgCl2-supported Ziegler-Natta catalysts. A periodic density functional theory study”,, 42 (21), 8165-8171 (2009).
8 Guo, Z.F., Chen, W., Zhou, J., Yang, H., “Novel high performance Ziegler-Natta catalyst for ethylene slurry polymerization”,...., 17 (3), 530-534 (2009).
9 Eisch, J.J., Caldwell, K.R., “Active sites in soluble Ziegler polymerization catalysts generated from titanocene halides and organoaluminum Lewis acids”, In: Homogeneous Transition Metal Catalyzed Reactions, Moser, W.R., Slocum, D.W., eds., the American Chemical Society, Washington, 575-590 (1992).
10 Jin, S.C., Jeong, H.C., “Effect of ethanol treatment in the preparation of MgCl2support for the propylene polymerization catalyst”,, 28 (5), 1717-1718 (1995).

12 Sozzani, P., Bracco, S., Comotti, A., “Stoichiometric compounds of magnesium chloride with ethanol for the supported Ziegler-Natta catalysis: First recognition and multidimentional MAS NMR study”,....,125, 12881-12893 (2003).
13 Chirinos, J., Fernandez, J., Perez, D., Rajmankina, T., Parada, A., “Effect of alkoxysilanes formedon the properties of Ziegler-Natta catalysts for olefin polymerization”,...,:, 231, 123-127 (2005).
14 Abboud, M., Denifl, P., Reichert, K., “Advantages of an emulsion- produced Ziegler-Natta catalyst over a conventional Ziegler-Natta catalyst”,..., 201, 1220-1226 (2005).
15 Mao, B.Q., Yang, A.N., Yang, Y., “Catalyst System for Use in Olefinic Polymerization”, U. S. Pat., 4861847 (1989).
16 Yang, P., Zeng, F., “Industrial application of domestic polyethylene slurry catalyst”,, 21 (3), 29-32 (2004). (in Chinese)
17 Tait, P.T., Zohuri, G.H., Kell, A.M., McKenzie, I.D., Ziegler Catalysts, Fink, G., Bruntzunger, H.H., eds., Springer, Berlin, 343 (1995).
18 Zheng, X., Madri, S., John, C.C., Joachim, L., “Fragmentation behavior of silica-supported metallocene/MAO catalyst in the early stages of olefin polymerization”,, 38 (11), 4673-4678 (2005).
19 McDaniel, M.P., “Controlling polymer properties with the Phillips chromium catalysts”,...., 27, 1559-1564 (1988).
20 Jin, S.Y., Harmon, R.W., “Simple mechanistic model for the kinetics and catalyst activity decay of propylene polymerization over titanium trichloride catalyst with DEAC cocatalyst”,...., 45 (3), 5932-5938 (2004).
* To whom correspondence should be addressed. E-mail: maobingquan@brici.ac.cn
2010-10-11,
2010-11-11.
 Chinese Journal of Chemical Engineering2011年1期
Chinese Journal of Chemical Engineering2011年1期
- Chinese Journal of Chemical Engineering的其它文章
- Effect of Boundary Layers on Polycrystalline Silicon Chemical Vapor Deposition in a Trichlorosilane and Hydrogen System*
- Experimental and CFD Study on the Role of Fluid Flow Pattern onMembrane Permeate Flux
- Separation of Eu3+ Using a Novel Dispersion Combined LiquidMembrane with P507 in Kerosene as the Carrier*
- Fabrication of SPES/Nano-TiO2 Composite Ultrafiltration Membrane and Its Anti-fouling Mechanism*
- Adsorption and Ozonation Kinetic Model for PhenolicWastewater Treatment*
- Properties of Bio-oil from Fast Pyrolysis of Rice Husk*
