The Acute Toxicity Test of Neo-enfx in Mice
College of Veterinary Medicine of Nanjing Agricultural University
Enrofloxacin,a third-generation quinolone,is a broad-spectrum antibacterial drug.It has activity against some bacteria,which include E.coli,klebsiella,salmonella,vibrio parahaemolyticus,staphylococcus aureus,pseudomonas aeruginosa,hemophilus and pasteurella multocida.Enrofloxacin has the special effect on mycoplasma.Enrofloxacin is rapidly and completely absorbed by oral administration or intramuscular injection and reaches the peak blood concentration within 0.5~2 h and is widely distributed in vivo animal.After application of enrofloxacin,almost all tissue drug concentrations in livestock are higher than plasma except central nervous system,which is beneficial to cure general and deep tissue infection.Neo-enfx untoward effect is a little and the drug 15%~50%is excreted by urine as well as the original form.
At present,enrofloxacin with bitter taste and alkalinity,after directly using,animals refuse to feed and water,the feed intake and drinking amount decrease,especially causes vomit because of its stimulation to stomach,ingestion and curative effect drop.In order to improve these shortcomings of enrofloxacin,Zhejiang King Techina Technology Co.,Ltd.developed the coated enrofloxacin named Neo-enfx.The Neo-enfx without bitter,it could gradually release and target release.The objective of this study is to research the pharmacokinetics of the Neo-enfx in vivo swine by oral administration and investigate the relative bioavailability of the Neo-enfx.
MATERIALS AND METHODS
1 Experimental medicine
The coated enrofloxacin(the content of enrofloxacin is 10%,batch number is 100114)was supplied by Zhejiang King Techina Technology Co.,Ltd.The trade name is Neo-enfx.
2 Experimental animal
A total of 100 healthy white mice(ICE,half male and half female,18~22g of BW)were supplied by Shanghai SLAC Laboratory Animal Co.,Ltd.The certificate number is scxk 2007-005.
3 Experimental method
3.1 Trial test
A total of 50 healthy white mice(ICE,half male and half female)were randomly divided into ten groups.The LD100 and LD0 were ascertained in trial test,and as the basis for formal test of the dose gradient.The mice took orally Neo-enfx repeatedly by gavage within two hours because of Neo-enfx with lesser toxicity and higher concentration.The LD100 and LD0 in trial test were 4,500mg/kg and 10,500mg/kg respectively,so the dose range of formal test was 4,500~10,500mg/kg.
3.2 Formal test
A total of 50 healthy white mice(ICE,half male and half female)were randomly divided into five groups.The mice were fasted for 12 h before oral gavage and water was freely available.The mice took orally Neo-enfx 0.5 ml/10 g body weight by gavage,according to the dose gradient(5,063;6,076;7,292;8,750 and 10,500mg/kg).The health condition,poisoning symptom and death process of mice were observed after oral drug,the observation time was two weeks,and the death of the mouse was dissected.
3.3 Data processing
The LD50 and 95%credible limit were calculated by the modified Karber's method.
RESULTS
1 The observation of clinical symptom
The high dose of Neo-enfx can make mice poisoning,appeared poisoning symptom after poisoning for 20 min.The major clinical manifestations of mice as follows:
The mice didn’t move,didn’t eat and drink,depressed spirit,poisoning severity,suddenly restless excitement,tachypnea,crowhop and failure to death.The mice got to death about 40 min with high a dose of Neo-enfx,poisoning within 1~3 h of the death peak.There was no abnormal pathological change when the dead mice were dissected.The live mice began toeat and drink after 5~6 h,later the symptom disappeared basically and activity to normal,all survived when the test finished.
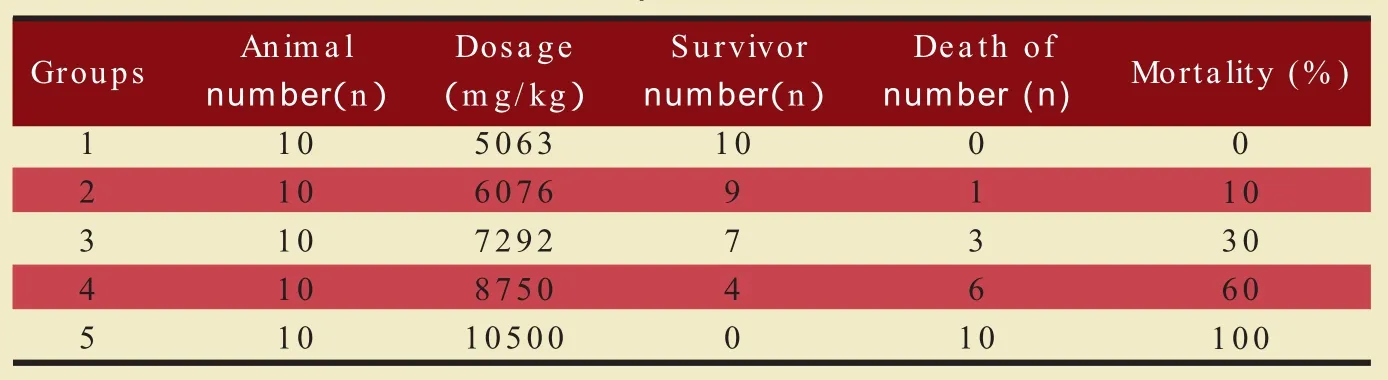
Table 1 the acute of toxicity test result of Neo-enfx in mice
2 The calculation of LD50 and 95%credible limit
The LD50 and 95%credible limit were calculated by the modified Karber's method.The LD50 and 95%credible limit of Neo-enfx were 7,642.2mg/kg and 6,939.6~8,416.0mg/kg respectively.The death condition of mice in each group was shown in table 1.
CONCLUSION
According to toxicity grading standard for foreign exogenous chemical compound recommended by the World Health Organization,the toxicity of compound more than 5,000mg/kg belongs to practically non-toxic,so Neo-enfx belongs to practically non-toxic compounds.
- 饲料工业的其它文章
- Muyang Group Gained the Largest Order in the Middle East,Causing the Concerns of CCTV
- SCIENTISTS HAVE SUCCESSFULLY DRAWN THE COMPLETE GENOME SEQUENCE MAP OF CARP
- ADindex
- China Can Endure Inflation Up to 6 Percent
- Chinese Police Target Illegal Pork Additives
- China Becomes Major Importer of Argentine Soy Products

