Effect of Process Parameters on Co-precipitation of Paclitaxel and Poly(L-lactic Acid) by Supercritical Antisolvent Process*
LI Wenfeng (李文锋), LIU Guijin (刘贵金), LI Lixian (李黎仙), WU Juan (伍娟),LÜ Yangxiao (吕扬效) and JIANG Yanbin (江燕斌),**
1 Star Lake Bioscience Co., Inc., Zhaoqing 526040, China
2 School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510640, China
1 INTRODUCTION
Paclitaxel (PTX) is one of the most effective anti-cancer drugs [1, 2], which has an excellent anti-tumor activity against a wide variety of tumors [3],especially for advanced ovarian cancer and breast cancer. However, PTX presents poor solubility in water(<0.004 mg·ml-1) [4], so its dissolution in biological liquids is poor. To solve the solubility problem, the current clinical formulation of PTX contains 1∶1 blend of cremophor EL (polyethoxylated castor oil)and dehydrated ethanol, which leads to low bioavailability, toxicity and hypersensitivity [5]. In order to increase therapeutic efficiency and reduce side-effects of PTX, much effort has been devoted to developing cremophor EL free drug delivery systems, such as micro-spheres of cyclodextrins, liposomes and biodegradable polymer carriers.
Many traditional methods, such as solvent evaporation, spray drying and melting have been developed for preparation of microparticles [6-9]. However, the traditional methods present several drawbacks or limitations, such as relatively large particle size, wide particle size distribution, degradation of the product and difficulties in complete recovery of organic solvents. Supercritical fluid (SCF) techniques have attracted a wide range of concern in the pharmaceutical field, especially for micronization of drug particles and drug delivery systems [10-13], because of their environmentally benign, low organic solvent residue and low operating temperature.
Among various supercritical fluid particle formation methods, supercritical antisolvent (SAS) process has been investigated to manufacture fine particles in various fields. It is possible to control the particle size and particle size distribution and greatly reduce solvent residual in an SAS process. These advantages are significant for the production of pharmaceutical compounds. In an SAS process, mass transfer is critical for particle formation, including carbon dioxide diffusing into organic liquid droplets and the solvent out from the droplets. The process is affected by various parameters such as temperature, pressure, drug concentration,flow rate of organic solution, type of solvents, etc. For example, Kim et al. [14] micronized cilostazol using SAS and indicated that the mean particle size increased slightly as the temperature increased from 40 to 60 °C, and decreased from 2.31 to 0.95µm as the pressure increased from 8 to 15 MPa. Chang et al. [15]reported that pressure had no obvious effect on particle size while temperature showed complicated effects.Poly(L-lactic acid) (PLLA) has been studied as a drug carrier for PTX because of its biodegradability and biocompatibility. Kang et al. [16] prepared PTX loaded PLLA micro-particles using solution enhanced dispersion by supercritical CO2(SEDS) technique,and indicated that the PTX-PLLA formulation significantly potentiated the anticancer activity of PTX.Leeet al. [17] co-precipitated PTX and PLLA by using SAS and SAS with enhanced mass transfer methods separately, and obtained particles with encapsulation efficiencies up to 83.5% and controlled release of PTX for more than 30 days. Although PTX-PLLA formulation has been successfully prepared by using the SAS process in other literatures, the effect of process parameters on the characteristics of particles produced by the SAS process was not discussed in detail. The reasons for the influence of a certain parameter are not clear. For different systems, effects of process parameters on particles are different [18, 19]. The development of an SAS process requires an extensive experimental study with different combinations of process parameters.
The objective of this work is to co-precipitate PTX and PLLA with an SAS process using supercritical CO2as the antisolvent. Single-factor method is adopted to investigate the effects of SAS process parameters on particle morphology, mass median diameter (Dp50) and PTX loading under certain conditions.
2 EXPERIMENTAL
2.1 Materials
PTX with stated mass fraction purity >0.998 was obtained from Jinhe Bio-Technology Co. Ltd., China,the same as that reported in our previous work [20].PLLA (MW=1000) with glass transition temperature of 61.6 °C was purchased from Chengdu Organic Chemistry Co. Ltd., China. CO2with stated mass fraction purity >0.999 was purchased from Guangzhou Shengying Gas Co. Ltd., China. Dichloromethane(DCM), dimethyl Sulphoxide (DMSO), ethanol (EtOH),and acetonitrile (ACN) were of analytical purity and supplied by Guangdong Guanghua Sci. Tech. Co., Ltd.China. Tween80 was purchased from Tianjin Kermel Chemical Reagent Co. Ltd., China. All materials were used without further purification. And ultrapure water was used throughout the study.
2.2 Apparatus and procedure
The equipment of automatic semi-continuous SAS process (SAS50-2-ASSY, Thar Technologies, Inc.,USA) was employed to carry out the co-precipitation experiments, the same as that reported in our previous work [21]. The schematic diagram is shown in Fig. 1,and the SAS process is controlled by a computerized system. Before each experiment, organic solutions containing pharmaceutical were prepared by dissolving certain PTX and PLLA in the mixed solvents. CO2was liquefied with cooler and continuously introduced into the precipitation vesselviaa high-pressure pump.Before entering the injector, the stream of carbon dioxide was pre-heated in a heat exchanger. The pressure was controlled by a backpressure regulating valve. The temperature in the precipitation vessel was regulated to a pre-specified level. When a steady state was achieved, PTX-PLLA solution was charged at a given flow rate by another high pressure pump and sprayed into the precipitation vessel through a nozzle (0.5 mm),where the concentration of PTX was kept at a constant of 2.0 g·L-1. CO2flow rate measured by a flow meter was fed simultaneously at a constant flow rate of 20.0 g·min-1. A metal filter (5 μm) coupled with an ultrafiltration membrane (0.22 μm) was located at the bottom of the precipitation vessel for particle collection. After finishing injection of PTX-PLLA solution, CO2was kept flowing for about 40 min to remove the residual solvent in the precipitation vessel. The precipitation vessel was then depressurized gradually to atmospheric pressure. Finally, the particles were collected from the bottom of the precipitation vessel for further characterization analysis.
2.3 Characterization of products
Dp50and particle size distribution of samples were measured by a laser diffraction particle size analyzer (Mastersizer 2000, Malvern, UK). Before each measurement, the micronized particles were suspended in pure water. To avoid the aggregation of particles,two drops of Tween80 were added into the sample,with stirring by ultrasonic for 15 min in order to disperse effectively [21, 22]. Every measurement was repeated at least three times.

Figure 1 Schematic diagram for the apparatus of SAS process [21]1—CO2 cylinder; 2—cooler; 3—CO2 pump; 4—heat exchanger; 5—injector; 6—precipitation vessel; 7—filter; 8—back pressure regulator; 9—depressure vessel; 10—solvent; 11—vent CO2; 12—solution supply; 13—solution pump; 14—valve switching
In order to characterize powder products, X-ray diffractometer (D8 ADVANCE, Bruker AXS, German)was used for attaining X-ray powder diffraction patterns of products. Scanning electron microscopy(SEM) (S-3700N, HITACHI, JP) was used for imaging the particle surface and analyzing the morphology of products. When preparing the samples for SEM measurement, particles were golden sputtered under vacuum 66.661×10-4Pa.
High performance liquid chromatography (HPLC)(HP1100, Agilent, USA) with a reverse-phase Hypersil ODS column (4.6 mm×250 mm i.d., 5 µm) was used to determine the PTX loading [23]. The mobile phase was the mixture of water and ACN (50/50, by volume). The PTX loading is defined as the content of PTX in the final product and measured as follows:accurately weight 1 mg of sample and dissolve it in 1 ml DCM to release PTX from co-precipitation particles. In order to eliminate the influence of solvent peak, DCM was evaporated at room temperature, and then 5 ml mobile phase was added to extract PTX under magnetic stirring. The solution was filtered using 0.22 μm membrane before 10 μl solution was injected by an auto-injector into the HPLC apparatus. PTX loading is calculated using the following equation,similar to the method used by Jinet al[24].

3 RESULTS AND DISCUSSION
A critical factor in the SAS process is the selection of the correct combination of a suitable organic solvent and a supercritical fluid as antisolvent. Supercritical fluid miscible organic solvents are suitable to dissolve the biological molecules [25]. For example,Kang [16] and Lee [17]et al. have successfully co-precipitated PTX and PLLA using DCM as the solvent, with supercritical CO2as the antisolvent.However, when these compounds are dissolved in DCM, irreversibly change of the molecules structure might occur. A suitable cosolvent, such as ethanol, can be used to enable overcome this setback [26].Therefore,in this study, the mixtures of DCM/EtOH or DCM/DMSO has been used as the solvent to investigate the effect of solvent and solvent ratio on the characteristics of the particle produces in the SAS process.
The effect of other four operating conditions on particle morphology,Dp50and PTX loading were investigated by using single-factor method. The four parameters are temperature (30-45 °C), pressure (8-14 MPa),PLLA concentration (5-12 g·L-1) and solution flow rate (0.2-1.0 ml·min-1), with PTX concentration and CO2flow rate kept at a constant of 2 g·L-1and 20 g·min-1, respectively. All experimental conditions and some results are listed in Table 1, and Fig. 2 shows the particle size distribution of typical samples. Fordifferent solvents, fine particles with a narrow particle size distribution were obtained as shown in Fig. 2,also it can be found that the microparticles gained by using DCM as solvent has smaller particle size and narrower particle size distribution than that by using mixture of DCM/EtOH or DCM/DMSO as solvent.

Table 1 Summary of operating conditions and experimental results for SAS processing

Figure 2 Particle size distribution of PTX-PLLA microparticles prepared by SAS process using different solvents
The XRD analysis was performed to elucidate the change in crystalline structure of different samples.Fig. 3 shows the XRD patterns of PTX, PLLA and PTX-PLLA microparticles prepared by SAS process using different solvents (Nos. 1, 4 and 8 in Table 1).As shown in Fig. 3, almost all of the crystalline peaks of PTX disappeared for the PTX-PLLA microparticles,besides a weak characteristic peak at 2θ=12.62° can be observed, which implies that the PTX is most likely in an amorphous state in the PLLA polymer matrix.

Figure 3 X-ray powder diffraction spectra of raw materials and typical samples1—PTX; 2—PLLA; 3—sample No.1; 4—sample No. 4; 5—sample No. 8
And the effects of parameters on characteristics of samples are discussed in detail as follows.
3.1 Effects of parameters on particle morphology
3.1.1Effect of solvent and solvent ratio
The influence of the solvent type and solvent ratio on particle morphology is summarized in Fig. 4,while other parameters were kept at suitable constants.
Figures 4 (a) to 4 (e) show the effect of DCM/DMSO volume ratio in mixed solvents on particles morphology. Figs. 4 (a) and 4 (b) suggest that with DMSO added to DCM, particles present non-spherical shape and rough surface, since the higher viscosity of DMSO reduces the mass transfer in the system and DMSO is not extracted quickly enough by CO2, resulting in particle-hole when particles fall. Moreover,particles are less spherical and slightly agglomerate with the increase of volume ratio of DMSO, as shown in Figs. 4 (c) to 4 (e). The agglomeration phenomenon can be attributed to the residual DMSO on the filter,so the drying of particles is not sufficient and they are aggregated to each other.
Figures 4 (a), 4 (f) to 4 (h) show the effect of DCM/EtOH volume ratio in mixed solvents on particle morphology. Particles present better morphology compared to those with DCM/DMSO as solvent. At DCM/EtOH of 50/50 (by volume) [Fig. 2 (h)], fibers appear. First, EtOH just dissolves PTX slightly, so the mixture of DCM/EtOH=50/50 (by volume) has lower solvent power for PTX, which leads to higher supersaturation and fast precipitation of PTX. Second,EtOH is more easily extracted from droplets, as a result, particles grow quickly and fibers are generated.
3.1.2Effect of temperature
Higher temperature improves both mass transfer and solvent power of fluids. Fig. 5 shows the complicated effect of temperature on particle morphology,with other parameters kept at constants. In Figs. 5 (a)to 5 (c), particle morphology does not change as the temperature increases from 30 °C to 35 °C. At 40 °C,particles tend to aggregate as shown in Fig. 5 (d). Elvassoreet al. [27] suggested that particles tended to aggregate at higher temperatures. This is attributed to the glass transition temperature of PLLA in the compressed CO2system, since PLLA is easily plasticized at higher temperatures and aggregated particles are obtained. Moreover, at higher temperatures, the saturation decreases and particle growth is dominative, so that fibers and bigger particles form (e.g., at 40 °C).
In order to identify the particle aggregation at higher temperature, an experiment at 45 °C was conducted, in which film appeared [Fig. 6 (b)], which provides a further evidence for the fact that particles tend to aggregate at higher temperatures. The reason is that supercritical CO2decreases the glass transition temperature of biodegradable polymers, acting like a plasticizer. Therefore, these polymers with a low glass transition temperature tend to form sticky and aggregated particles [25, 28].
3.1.3Effect of pressure

Figure 4 The effect of solvent and solvent ratio on particle morphology(35 °C, 12 MPa, PLLA 5.0 g·L-1 and solution flow rate 0.5 ml·min-1)
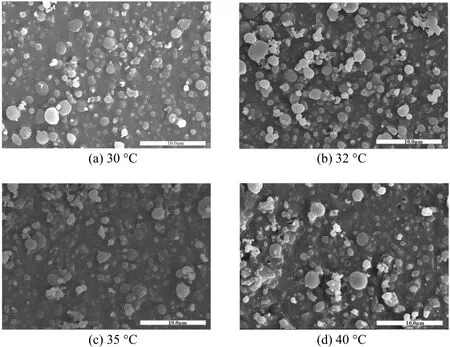
Figure 5 The effect of temperature on particle morphology[12 MPa, PLLA 5.0 g·L-1, DCM/EtOH (50/50, by volume) and solution flow rate 0.5 ml·min-1]
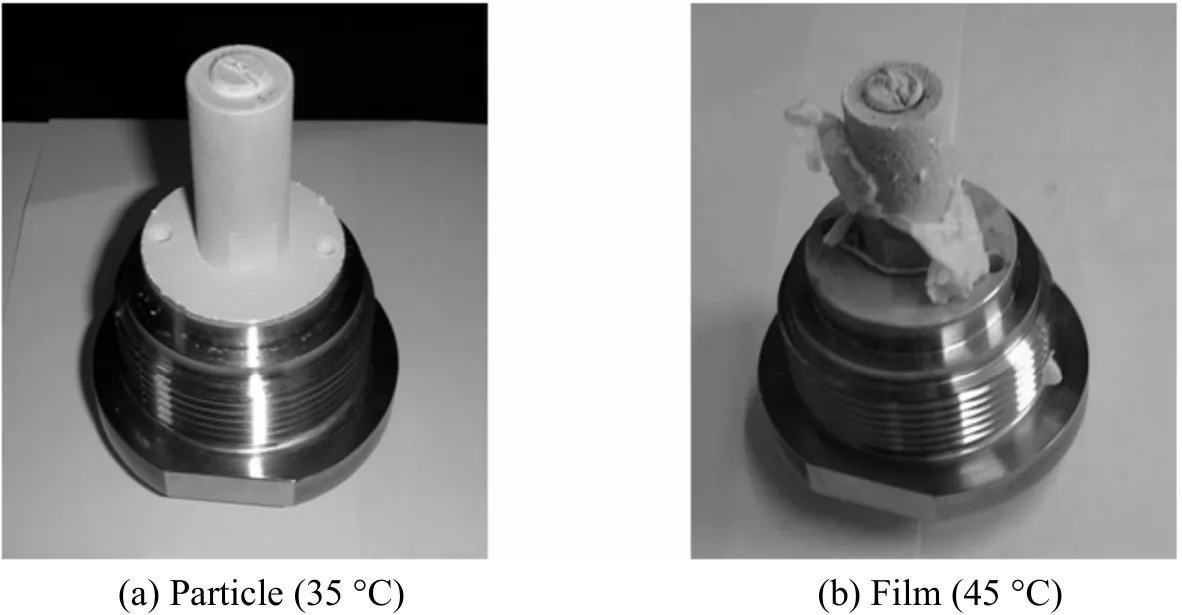
Figure 6 Photos of the nozzle at different temperatures
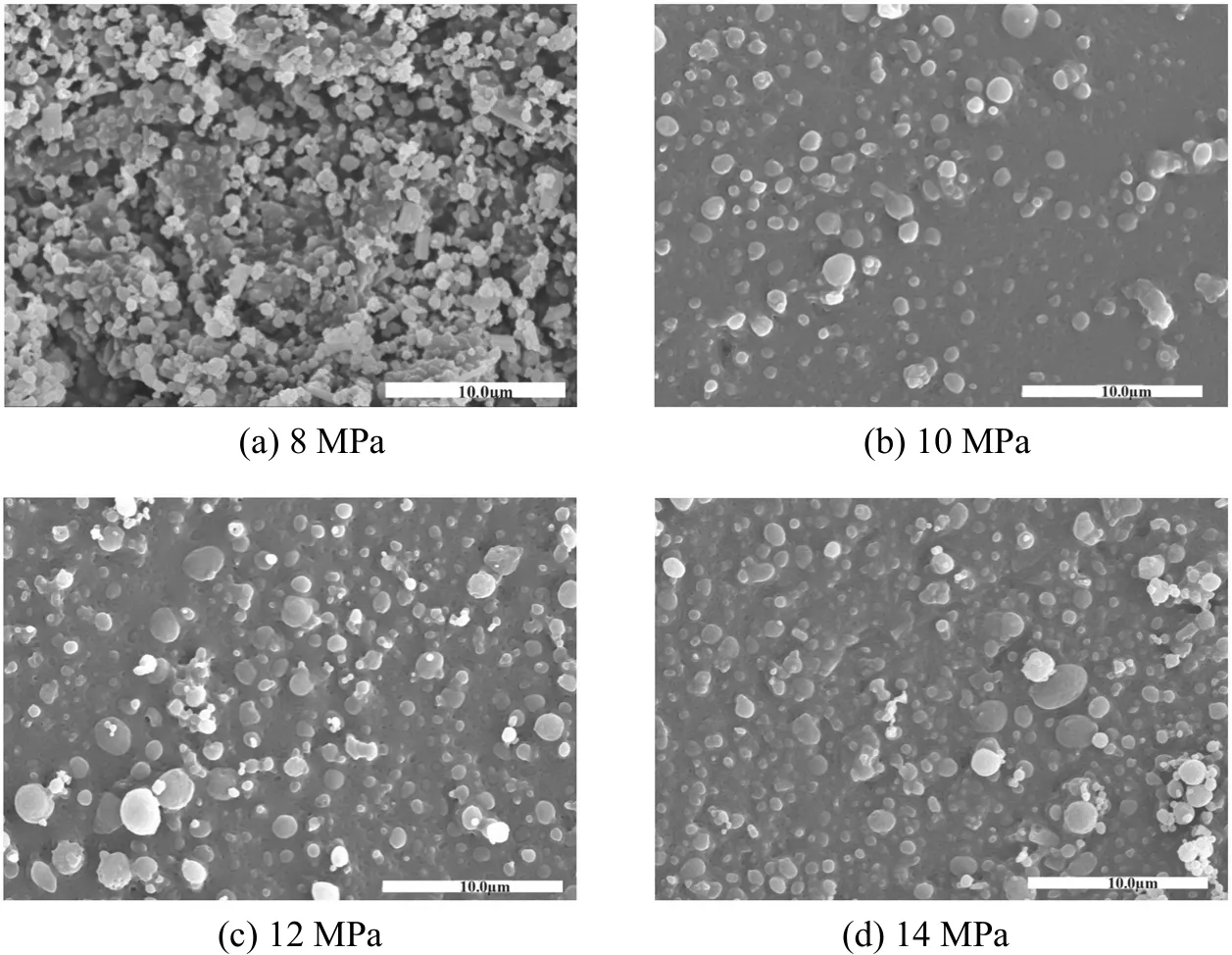
Figure 7 The effect of pressure on particle morphology[30 °C, PLLA 5.0 g·L-1, DCM/EtOH (50/50, by volume) and solution flow rate 0.5 ml·min-1]
Figure 7 shows the effect of pressure on particle morphology. Particles are slightly aggregated at 8 MPa,since the mass transfer is slow at low pressure, particles are not dried fast enough and tend to aggregate during the falling. At the same time, the solvent is not brought out of the vessel quickly and deposits at the bottom, also causing re-dissolution and aggregation of particles. At 10 MPa, the aggregation is less and particle morphology is improved remarkably. The particle morphology does not change when the pressure increases from 10 to 14 MPa, so the pressure has little effect on particle morphology in this range.
3.1.4Effect of solution flow rate
Figure 8 shows the effect of solution flow rate on particle morphology. From 0.2 ml·min-1to 1.0 ml·min-1,the solution flow rate does not have significant effect,and the particles have smooth surface and well dispersed. However, fibers appear at the flow rate of 1.5 ml·min-1. The amount of organic solvent in the system increases with the increase of solution flow rate, as a result, the diffusion time of total organic solvent into CO2is longer, which is propitious to the growth of particles. Meanwhile, the droplets appear to be fibers under the effect of gravity.
3.2 Effects of parameters on Dp50
It can be found from Table 1 that PTX and PLLA have been successfully micronized using the SAS process.Dp50of particle samples varies from 832.4 nm to 1432.9 nm, which is suitable for drug delivery system.
Figure 9 shows the effect of process parameters onDp50of particles. From Fig. 9 (a), it is noted thatDp50of particles changes little with DMSO added. In Table 1,Dp50of particles increases slightly from 836.2 nm to 886.2 nm when volume ratio of DCM/DMSO increases from 50/50 to 60/40. As discussed above,higher viscosity of DMSO makes the mass transfer more difficult and produces hole-particles, so thatDp50is larger. On the other hand, higher surface tension leads to form smaller droplets while the solution is sprayed into supercritical CO2. As a result,Dp50of particles changes slightly.
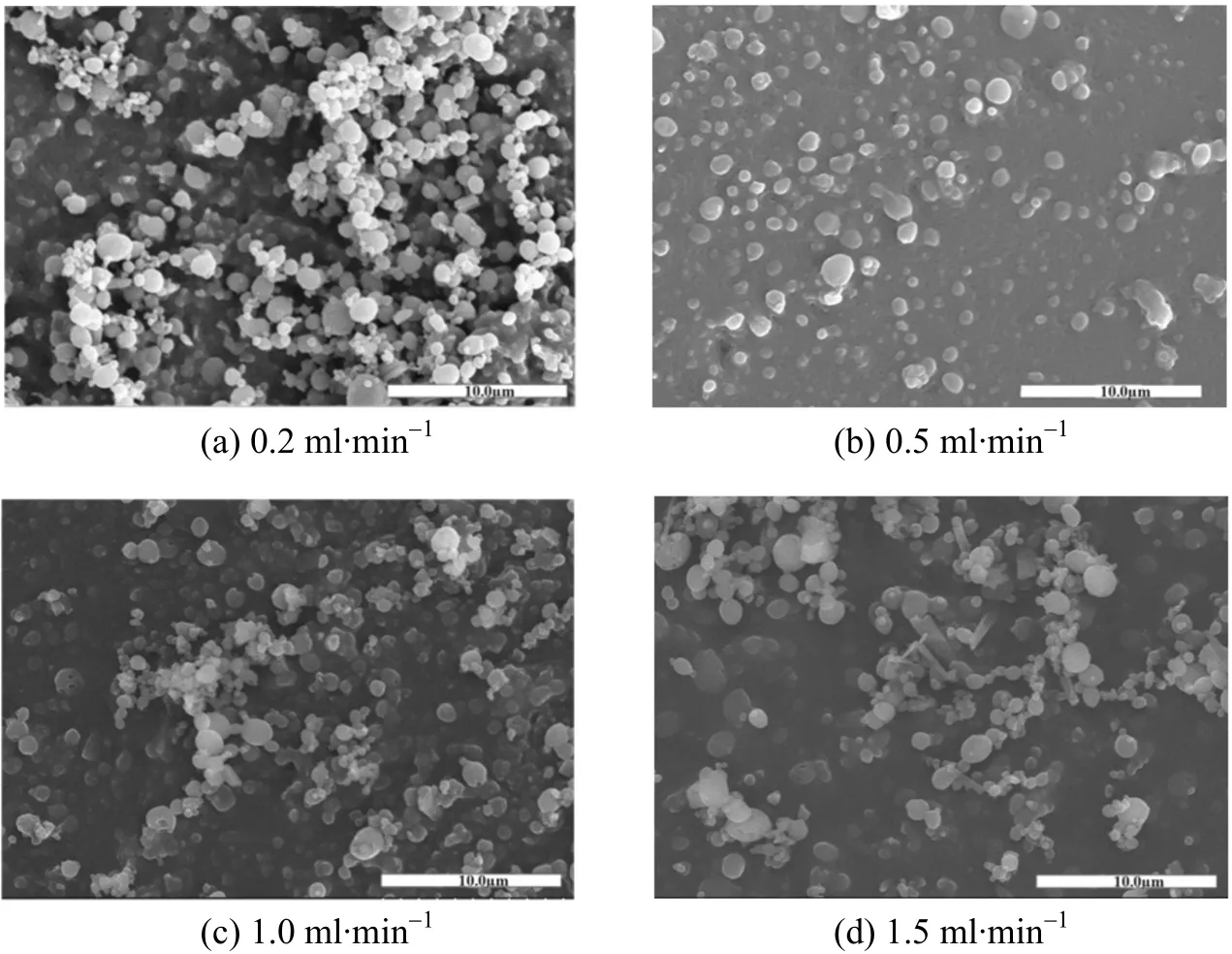
Figure 8 The effect of solution flow rate on particle morphology[30 °C, 12 MPa, PLLA 5.0 g·L-1 and DCM/EtOH (50/50, by volume)]
Figure 9 (b) indicates thatDp50of particles increases greatly with the addition of EtOH. Because EtOH presents lower surface tension, bigger droplets will be produced when the solution is sprayed into supercritical CO2. Further more, EtOH is easy to be extracted, so that bigger particles are generated.Therefore, solvent has a distinct effect onDp50because of their different physical properties, and DCM/EtOH produces bigger particle size than DCM/DMSO under the same conditions, which is beneficial to increase the drug release time. Fig. 9 (c) shows thatDp50of particles increases with temperature. At higher temperature, solvent power is higher and the supersaturation of the system is lower. Particles tend to grow instead of nucleation at lower saturation, as a result,particles are larger. Fig. 9 (d) shows thatDp50of particles increases with pressure, which is different from the result reported by Matteaet al[19]. At higher pressures, the solubility of solutes in the mixed solvent is higher, resulting in lower supersaturation, so that the growth of particles is dominative. Fig. 9 (e)shows thatDp50of particles increases with the increase of polymer concentration. Similar result has been reported by Elvassoreet al[27]. According to the formation mechanism “one droplet one particle” under the critical condition, the PLLA content in droplets increases with increasing PLLA in the solution, supplying sufficient solute for the growth of particles. As a result, bigger size particles are produced. Fig. 9 (f)shows thatDp50increases with increasing solution flow rate, because the collision opportunity of droplets increases and the growth dominates the falling process,producing bigger particles.
3.3 Effects of parameters on PTX loading
Figure 10 shows the effect of process parameters on PTX loading of the particles. As shown in Fig. 10(a), with DCM/DMSO as the solvent, the PTX loading on the produced particles increases at the beginning when DMSO is added to DCM, but decreases when the DCM/DMSO volume ratio is equal to or higher than 50/50. Similar tendency was previously reported[18], in which the PTX loading dropped with the increase of the ratio of DMSO in the mixed solvent at 302.15 K. The mass transfer rate in the process decreases as the volume ratio of DMSO increases, so that the solutes precipitate thoroughly. However, the mass transfer resistance becomes larger with the increase of DMSO ratio in the solvent and DMSO will be rich on the filter, so that the solute re-dissolves and does not precipitate completely, and low PTX loading particles are obtained. However, for DCM/EtOH system, as shown in Fig. 8 (b), the PTX loading changes little at different EtOH volume ratios in the mixed solvent. The saturation of PTX in the fluids increases with EtOH added, so some of PTX precipitates alone and does not co-precipitate with PLLA, decreasing the PTX loading. On the other hand, EtOH dries quickly,improving the co-precipitation of PTX and PLLA.Consequently PTX loading changes little.
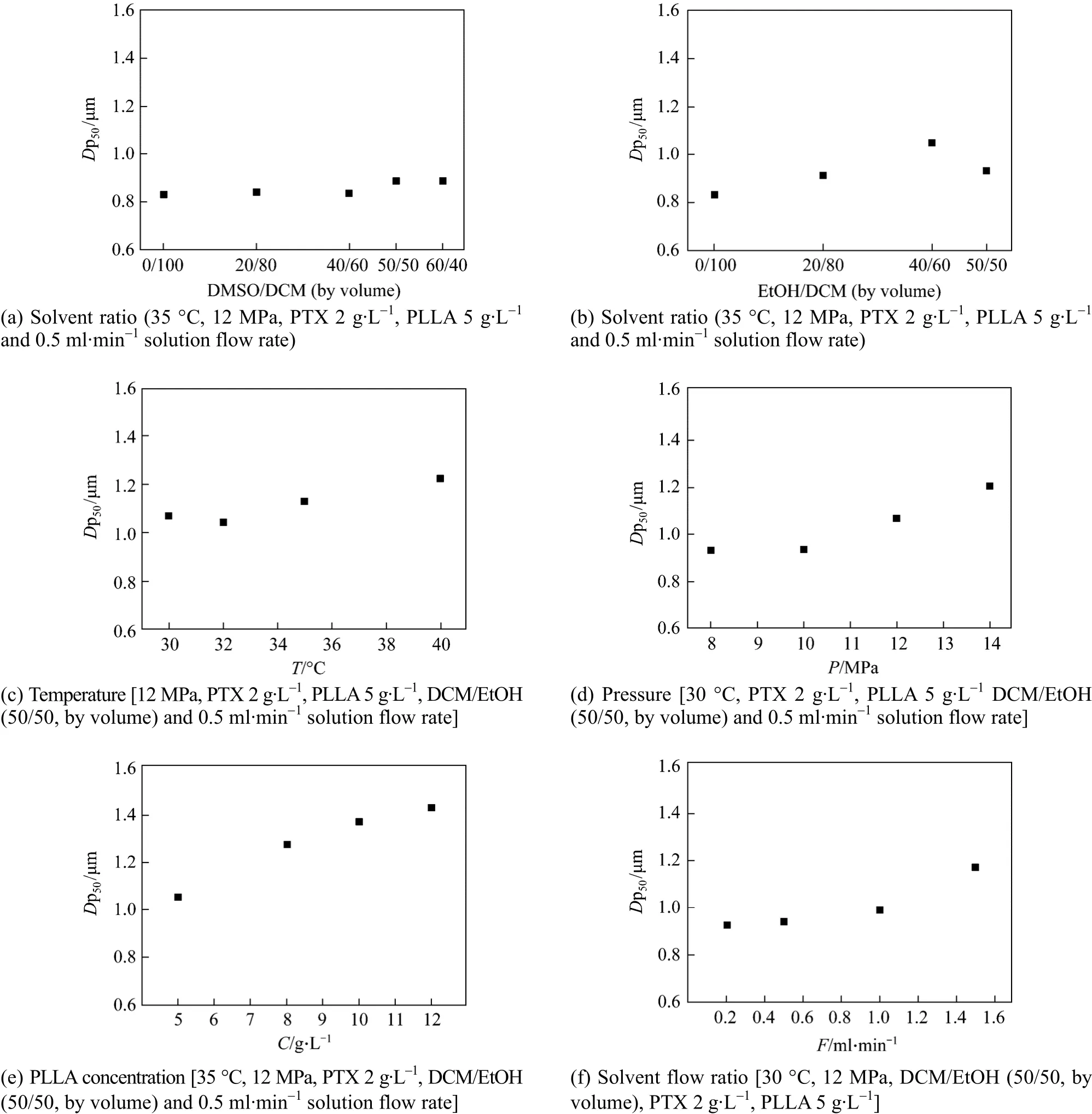
Figure 9 Effects of process parameters on Dp50
Figure 8 (c) shows the effect of temperature on particle PTX loading. Temperature affects the solubility of solute in the solvent and the inter-solubility of solvent and antisolvent, influencing PTX loading.Under the experimental conditions, the solubility of solute in the solvent and the expansion of solvent into CO2increase with temperature, but PTX loading change little due to the interaction of expansion coefficient and solubility. Fig. 8 (d) shows the effect of pressure on PTX loading. The PTX loading increases remarkably as pressure increases for DCM/EtOH(50/50, by volume) system. This is mainly attributed to the improved mass transfer under higher pressure,so that PTX-PLLA co-precipitated efficiently. Fig. 8(e) shows that PTX loading decreases with the increase of PLLA concentration in the solution. The definition of PTX loading shows that PTX loading decreases with the increase of the amount of PLLA while keeping the amount of PTX constant during co-precipitation. Fig. 8 (f) shows that the solution flow rate has no significant effect on PTX loading,though it will affect particle size and morphology. In this study, good morphology particles were obtained when the solution flow rate was 0.5 ml·min-1.
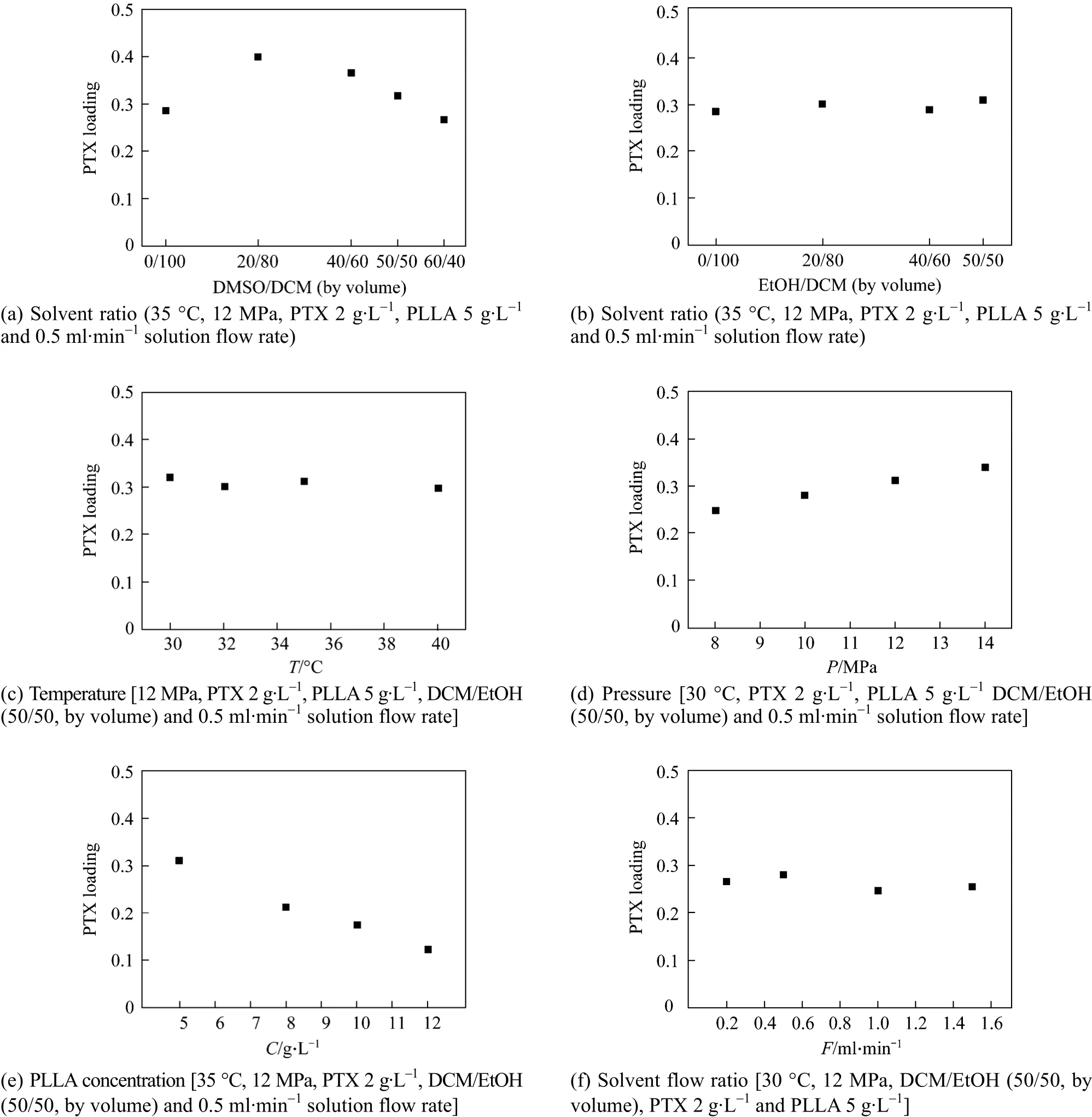
Figure 10 Effects of process parameters on PTX loading of the particles
The effect of five process parameters, i.e., solvent and solvent ratio, temperature, pressure, PLLA concentration and solution flow rate on particle morphology, Dp50and PTX loading are summarized as follow.
Solvent and solvent ratio plays an important role in the SAS process. For different solvents, the volatility and strength of solvent, as well as the solubility of both the biodegradable polymer and drug in the organic solvent, are different. Usually, the stronger solvents with higher volatility result in reduced particle size. And for effective encapsulation of the drug, it is essential that the solubility of the biodegradable polymer in the organic solvent is higher than the solubility of its drug content. For the co-precipitation of PTX and PLLA by the SAS process, the results indicated that the mixed DCM/EtOH solvent was more suitable for encapsulating PTX into PLLA with good particle morphology. Although PTX loading increased while using the mixed DCM/DMSO solvent, but the particles became agglomerated. It has been found that particles with good morphology, suitable Dp50, narrow distribution and high PTX loading were obtained when DCM/EtOH=50/50 (by volume).
Temperature and pressure have effects on the density of supercritical fluid and the viscosity of solution,which further affect the solubility of high-molecular-mass drugs and polymers in the supercritical fluid and the mass transfer between organic solvent and supercritical fluid during precipitation. Generally, the temperature and pressure have the competing effects for the SAS process. At a fixed temperature, increase of pressure might cause a higher volume expansion, but reduce the diffusion and evaporation rate between CO2and the organic solvent. In addition, at a fixed pressure,although increasing temperature might result in increasing the nucleation rate of solute, the degree of supersaturation might decrease with increasing temperature. For our experimental results, when using the mixed DCM/EtOH as solvent, the Dp50increased with increasing temperature and pressure, and PTX loading increased with the increase of pressure but changed slightly with increasing temperature. When temperature was 35 °C and pressure changed between 10-12 MPa,the PTX-PLLA microparticles with good morphology,suitable Dp50and high PTX loading were achieved.
PLLA concentration and the solution flow rate affect the degree of solute supersaturation. At lower concentrations and solution flow rate, the supersaturation of the drug occurs rather slowly. Therefore the precipitation delays and nucleation dominate growth.Besides, increasing the concentration and solution flow rate enhances the viscosity and surface tension of the solution. Therefore, particles with better morphology, higher PTX loading and smaller particles with narrower particle size distribution would be obtained by adjusting the initial concentration and solution flow rate. In this study, Dp50increased while increasing the PLLA concentration and solution flow rate. However, PTX loading decreased with the increase of PLLA concentration, and solution flow rate only had slight effect on PTX loading. The suitable operating conditions were that PLLA concentration 5 g·L-1and solution flow rate 0.5 ml·min-1.
4 CONCLUSIONS
PTX and PLLA were successfully micronized and co-precipitated by the SAS process using DCM and the mixtures of DCM/EtOH or DCM/DMSO as the solvent, with supercritical CO2as the antisolvent.For different solvents, fine particles with a narrow particle size distribution were obtained. And XRD results suggested that the micronized PTX was dispersed into the PLLA polymer matrix in an amorphous form.
The effects of five process parameters on co-precipitation of PTX and PLLA by SAS process were experimentally investigated. The results indicate that particle morphology, Dp50and PTX loading can be manipulated by adjusting these parameters, which affect the fluid mechanics, mass transfer, and particle formation and growth. And the suitable operating conditions for the experimental system are as follow:DCM/EtOH=50/50 (by volume), 35 °C, 10-12 MPa,PLLA concentration 5 g·L-1and solution flow rate 0.5 ml·min-1.
1 Orr, G.A., Verdier-Pinard, P., McDaid, H., Horwitz, S.B., “Mechanisms of taxol resistance related to microtubules”, Oncogene, 22(47), 7280-7295 (2003).
2 Singla, A.K., Garg, A., Aggarwal, D., “Paclitaxel and its formulations”, Int. J. Pharm., 235 (1-2), 179-192 (2002).
3 Rowinsky, E.K., Cazenave, L.A., Donehower, R.C., “Taxol: A novel investigational antimicrotubule agent”, J. Natl. Cancer I., 82 (15),1247-1259 (1990).
4 Lee, J., Lee, S.C., “Hydrotropic solubilization of paclitaxel: Analysis of chemical structures for hydrotropic property”, Pharm. Res., 20 (7),1022-1030 (2003).
5 Greenwald, R.B., Pendri, A., Bolikal, D., “Highly water soluble taxol derivatives: 7-polyethylene-glycol carbamates and carbonates”,J. Org. Chem., 60 (2), 331-336 (1995).
6 Kang, B.K., Chon, S.K., Kim, S.H., Jeong, S.Y., Kim, M.S., Cho,S.H., Lee, H.B., Khang, G., “Controlled release of paclitaxel from microemulsion containing PLGA and evaluation of anti-tumor activity in vitro and in vivo”, Int. J. Pharm., 286 (1-2), 147-156 (2004).
7 Gupte, A., Ciftci, K., “Formulation and characterization of paclitaxel,5-FU and paclitaxel+5-FU microspheres”, Int. J. Pharm., 276 (1-2),93-106 (2004).
8 Dong, Y., Feng, S.S., “Poly(D, L-lactide-co-glycolide) (PLGA)nanoparticles prepared by high pressure homogenization for paclitaxel chemotherapy”, Int. J. Pharm., 342 (1-2), 208-214 (2007).
9 Kim, S.C., Kim, D.W., Shim, Y.H., Bang, J.S., Oh, H.S., Kim, S.W.,Seo, M.H., “In vivo evaluation of polymeric micellar paclitaxel formulation: Toxicity and efficacy”, J. Control. Release, 72 (1-3),191-202 (2001).
10 Pasquali, I., Bettini, R., Giordano, F., “Solid-state chemistry and particle engineering with supercritical fluids in pharmaceutics”, Eur. J.pharm. Sci., 27 (4), 299-310 (2006).
11 Mishima, K., “Biodegradable particle formation for drug and gene delivery using supercritical fluid and dense gas”, Adv. Drug Delivery Rev., 60 (3), 411-432 (2008).
12 Davies, O.R., Lewis, A.L., Whitaker, M.J., Tai, H., Shakesheff, K.M.,Howdle, S.M., “Applications of supercritical CO2in the fabrication of polymer systems for drug delivery and tissue engineering”, Adv.Drug Delivery Rev., 60 (3), 373-387 (2008).
13 Kang, Y.Q., Yin, G.F., Ouyang, P., Huang, Z.B., Yao, Y.D., Liao,X.M., Chen, A.Z., Pu, X., “Preparation of PLLA/PLGA microparticles using solution enhanced dispersion by supercritical fluids(SEDS)”, J. Colloid Interface Sci., 322 (1), 87-94 (2008).
14 Kim, M.S., Lee, S., Park, J.S., Woo, J.S., Hwang, S.J., “Micronization of cilostazol using supercritical antisolvent (SAS) process: Effect of process parameters”, Powder Technol., 177 (2), 64-70(2007).
15 Chang, Y.P., Tang, M., Chen, Y.P., “Micronization of sulfamethoxazole using the supercritical antisolvent process”, J. Mater. Sci., 43(7), 2328-2335 (2008).
16 Kang, Y.Q., Wu, J., Yin, G.F., Huang, Z.B., Liao, X.M., Yao, Y.D.,Ouyang, P., Wang, H.J., Yang, Q., “Characterization and biological evaluation of paclitaxel-loaded poly(L-lactic acid) microparticles prepared by supercritical CO2”, Langmuir, 24 (14), 7432-7441(2008).
17 Lee, L.Y., Wang, C.H., Smith, K.A., “Supercritical antisolvent production of biodegradable micro- and nanoparticles for controlled delivery of paclitaxel”, J. Control. Release, 125 (2), 96-106 (2008).
18 Kalogiannis, C.G., Pavlidou, E., Panayiotou, C.G., “Production of amoxicillin microparticles by supercritical antisolvent precipitation”,Ind. Eng. Chem. Res., 44 (24), 9339-9346 (2005).
19 Mattea, F., Martin, A., Cocero, M.J., “Co-precipitation of β-carotene and polyethylene glycol with compressed CO2as an antisolvent: Effect of temperature and concentration”, Ind. Eng. Chem. Res., 47(11), 3900-3906 (2008).
20 Luo, N., Lu, Y.M., Jiang, Y.B., “Solubility of paclitaxel in mixtures of dichloromethane and supercritical carbon dioxide”, Chin. J. Chem.Eng., 19 (4), 558-564 (2011)
21 Jiang, Y.B., Sun, W.L., Wang, W., “Recrystallization and micronization of 10-hydroxycamptothecin by supercritical antisolvent process”, Ind. Eng. Chem. Res., 51 (6), 2596-2602 (2012).
22 Liu, J., Meisner, D., Kwong, E., Wu, X.Y., Johnston, M.R., “A novel trans-lymphatic drug delivery system: Implantable gelatin sponge impregnated with PLGA-paclitaxel microspheres”, Biomaterials, 28(21), 3236-3244 (2007).
23 Wang, Y.M., Sato, H., Adachi, I., Horikoshi, I., “Preparation and characterization of poly(lactic-co-glycolic acid) microspheres for targeted delivery of a novel anticancer agent, taxol”, Chem. Pharm.Bull., 44 (10), 1935-1940 (1996).
24 Jin, H., Xia, F., Jiang, C., Zhao, Y., He, L., “Nanoencapsulation of lutein with hydroxypropylmethyl cellulose phthalate by supercritical antisolvent”, Chin. J. Chem. Eng., 17 (4), 672-677 (2009).
25 Kalani, M., Yunus, R., “Application of supercritical antisolvent method in drug encapsulation: A review”, Int. J. Nanomed., 6,1429-1442 (2011).
26 Davies, O.R., Lewis, A.L., Whitaker, M.J., Tai, H., Shakesheff, K.M.,Howdle, S.M., “Applications of supercritical CO2in the fabrication of polymer systems for drug delivery and tissue engineering”, Adv.Drug Delivery Rev., 60 (3), 373-387 (2008).
27 Elvassore, N., Bertucco, A., Caliceti, P., “Production of protein-loaded polymeric microcapsules by compressed CO2in a mixed solvent”, Ind. Eng. Chem. Res., 40 (3), 795-800 (2001).
28 Kikic, I., “Polymer-supercritical fluid interactions”, J. of Supercritical Fluids, 47 (3), 458-465(2009).
 Chinese Journal of Chemical Engineering2012年4期
Chinese Journal of Chemical Engineering2012年4期
- Chinese Journal of Chemical Engineering的其它文章
- Phenol Oxidation by Combined Cavitation Water Jet and Hydrogen Peroxide*
- Venting Design for Di-tert-butyl Peroxide Runaway Reaction Based on Accelerating Rate Calorimeter Test
- Effect of Return Sludge Pre-concentration on Biological Phosphorus Removal in a Novel Oxidation Ditch*
- Separation of α-Tocopherol with a Two-Feed Simulated Moving Bed*
- Experimental and CFD Studies on the Performance of Microfiltration Enhanced by a Turbulence Promoter*
- Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane*
