Low-temperature Electrodeposition of Aluminium from Lewis Acidic 1-Allyl-3-methylimidazolium Chloroaluminate Ionic Liquids*
ZHENG Yong (郑勇), ZHANG Suojiang (张锁江)**, LÜ Xingmei (吕兴梅) WANG Qian(王倩) ZUO Yong (左勇) and LIU Lian (刘恋) Beijing Key Laboratory of Ionic Liquids Clean Process, State Key Laboratory of Multiphase Complex Systems,Institute of Process Engineering, Chinese Academy of Sciences, Beijing 0090, China
2 College of Chemistry and Chemical Engineering, Graduate University of Chinese Academy of Sciences, Beijing 100049, China
1 INTRODUCTION
As the most abundant metallic element in the earth crust, aluminium has been widely used in the preparation of corrosion-resistant coating owing to its excellent physicochemical properties and decorativeness. The conventional methods for aluminium coating include mainly thermal spraying, hot dipping,sputter deposition, vapor deposition and electrodeposition [1-6]. Among these techniques, the electrodeposition method has more favorable features, such as low energy consumption, high reaction efficiency, mild experimental conditions and easy control of aluminium deposit structure [7]. However, the conventional Hall-Héroult electrolysis process is not applicable to the electrodeposition of aluminium due to the high operating temperature (above the melting point of aluminium). Moreover, since the highly negative standard potential of Al/Al(III) couple vs. normal hydrogen electrode (NHE), aluminium cannot be electrodeposited from aqueous solutions. Consequently, it is necessary to find more appropriate and efficient electrolytes.
In the past century, some organic aprotic solvents were first developed for the electrodeposition of aluminium and high-quality aluminium coatings have been obtained [8-10]. However, these systems suffer from a lot of inherent disadvantages: low electrical conductivities, narrow electrochemical window, low dissolving capability of aluminium salts, high volatility and flammability, which restrict the application of this technique.By contrast, the inorganic electrolytes used show higher chemical stability and better electrochemical properties [11, 12]. Nevertheless, the high experimental temperature always results in hard operation and high energy consumption. From the practical applications point of view, it is still needed to develop novel electrolytes with improved physicochemical features and mild operating conditions.
Labeled as room-temperature molten salts, ionic liquids (IL) are composed entirely of organic cations and organic/inorganic anions [13-15]. Over the past decades, the research on IL has received extensive attention and significant progress in a wide range of research fields [16, 17]. Many studies demonstrate that ILs are promising electrolyte in the electrodeposition of reactive metals [18-20]. The excellent properties of ILs can overcome effectively the drawbacks of traditional electrolytes.
Early in 1950s, Hurley and Wier [21] first found that aluminium could be electrodeposited from the IL consisting of N-ethylpyridinium chloride and AlCl3at low temperature. From then on, extensive and systematical research on the electrodeposition of aluminium and aluminium alloy in ILs has been reported in the literature [22-28]. It shows that improved quality of aluminium deposits and current efficiency can be expected due to the high conductivities and broad electrochemical windows of ILs. The most frequently studied systems are the chloroaluminate ILs generated by the combination of organic chloride salts and AlCl3.Aluminium can be deposited from Lewis acidic chloroaluminate ILs in which the molar percentage of AlCl3is higher than 50%. Among the Al-containing species in these ILs, only [Al2Cl7]-anion is reducible and responsible for the deposition. In recent years,many authors [29-31] reported that the structure of aluminium deposits was greatly influenced by the surface interaction in the interfacial solvation layer. The cation type of IL had a vital effect on the interaction strength of the interfacial layer. In recent years, some bis(trifluoromethylsulfonyl)imide ([NTf2]-) ILs were found to be capable of dissolving AlCl3and nanocrystalline aluminium could be electrodeposited from these solutions [32-34]. Nevertheless, these electrolytes are inferior to the chloroaluminate ones in many key properties, such as conductivity, viscosity and liquid range when they contain the same molar concentration of AlCl3. Moreover, the limited dissolving power of AlCl3and high costs of [NTf2]-ILs hinder their advancement to an industrialization process. Considering the properties and costs, chloroaluminate ILs have wide application potential and are worth of further investigation.
In this work, Lewis acidic 1-allyl-3-methylimidazolium chloroaluminate ILs ([Amim]Cl/AlCl3) were first synthesized and used as electrolyte in the low-temperature electrodeposition of aluminium. The properties of [Amim]Cl/AlCl3ILs, especially electrical conductivities have been discussed. Based on electrochemical measurements, the electrodeposition process of aluminium from [Amim]Cl/AlCl3was studied in detail. The effects of deposition parameters,including temperature, current density and molar ratio of [Amim]Cl/AlCl3, on the surface morphology, X-ray diffraction (XRD) pattern and purity of deposits were also investigated. These results were analyzed from the perspective of microcrystalline nucleation and growth. According to above studies, the optimum experimental conditions for aluminium electrodeposition were finally determined.
2 EXPERIMENTAL
2.1 Chemicals
All the chemicals used in this work were purchased commercially with analytic grade from Alfa Aesar and Sinopharm Chemical Reagent Co.N-methylimidazolium, allyl chloride, acetonitrile,1-chlorobutane and acetone were purified by distillation. Anhydrous aluminium chloride was used without further purification. Prior to use, aluminium plate(99.95%), aluminium wire (99.99%), platinum plate(99.95%) and copper foil (99.95%) were polished with emery paper, cleaned with acetone, treated with a dilute hydrochloric acid/sulfuric acid mixture, finally rinsed with deionized water and dried in air.
2.2 Preparation and characterization of ILs
1-Allyl-3-methylimidazolium chloride ([Amim]Cl)This IL was synthesized according to method described in the literature [35]. The final product was obtained as an amber liquid at room temperature. Melting point:290 K. Yield: 96.3%.
1-Butyl-3-methylimidazolium chloride ([Bmim]Cl)It was prepared according to Huddleston et al.’s work[36]. The final product was obtained as a white wax-like solid at room temperature. Melting point: 338 K. Yield:87.4%.
Lewis acidic [Amim]Cl/AlCl3 and [Bmim]Cl/AlCl3ILs 1∶1.5 [Amim]Cl/AlCl3, 1∶1.8 [Amim]Cl/AlCl3, 1∶2.0 [Amim]Cl/AlCl3and 1∶2.0 [Bmim]Cl/AlCl3were prepared by mixing precise molar quantities of [Amim]Cl or [Bmim]Cl with anhydrous AlCl3under dry argon atmosphere at room temperature. The resulting liquids were filtered through G4 glass frit and purified by pre-electrolysis before use. The final products were obtained as pale yellow liquids.
[Bmim][NTf2] (1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide)The IL was prepared and purified as a yellow liquid via an early method [37]. Yield: 95.5%.
All ILs prepared have been characterized by nuclear magnetic resonance (NMR). The peaks and corresponding chemical shifts obtained confirmed the structures of these ILs, and no impurity peaks were found in the NMR spectra.
2.3 The measurement of physicochemical properties
The NMR experiments of ILs were performed on a NMR spectrometer (av-400 MHz, Bruker, Switzerland) at 298.2 K. In the measurement of NMR for Lewis acidic [Amim]Cl/AlCl3and [Bmim]Cl/AlCl3ILs, acetone-D6was used as an external standard to suppress the interference from other solvents. The surface morphology and compositional analysis of aluminium deposits were examined with emission scanning electron microscope (SEM) (JSM-6700F,JEOL Ltd., Japan) and energy dispersive analysis X-ray (EDAX) (XL30 S-FEG, FEI Co., USA) respectively. And the X-ray diffraction (XRD) patterns of aluminium deposits were collected on a X-ray diffractometer (X’Pert Pro MPD, PANalytical Co., Netherlands) with Cu Kαradiation (λ=0.15405 nm). A density meter (Anton Paar DMA5000, Anton Paar Co.,Austria) with a resolution of 1 µg was employed to determine the densities of ILs.
2.4 Electrochemical experiments
All the electrochemical experiments were carried out in an argon-filled glove box (Universal, MIKROUNA Co., China) where water and oxygen content was both kept below 1×10-6. The data of electrical conductivities were obtained through a conductivity meter (DDS-307, Shanghai Precision & Scientific Instrument Co., China) from 298.2 to 373.2 K. The other electrochemical measurements were performed using an electrochemical workstation (CHI1140A, CH Instrument, USA) controlled by CHI1140A software in a three-electrode cell. In the determination of cyclic voltammograms, platinum working electrode and platinum plate counter electrode were used. A non-aqueous Ag+/Ag electrode (CHI112, CH Instrument, USA)containing the 0.1 mol·L-1solution of AgNO3in[Bmim][NTf2]/acetonitrile (298.2 K) was employed as reference electrode. As for the electrodeposition experiments, the working, counter and reference electrodes were copper foil, aluminium plate and aluminium wire, respectively. And the electrodeposition of aluminium on copper substrates was conducted by galvanostatic method. All the ILs were heated in an oil bath and the accuracy of temperature was ±0.5 K(RET basic, IKA, Germany). After electrodeposition,all the deposits were washed by acetonitrile, deionized water and finally dried in air.
2.5 Calculation of current efficiency
The current efficiency (CE) in electrodeposition was calculated by
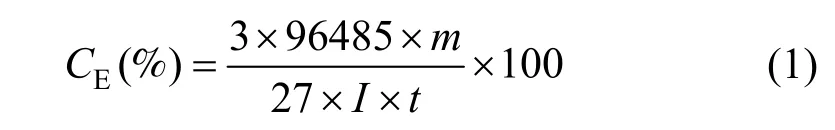
where the yield of aluminium (m) was determined from the mass difference of cathode before and after deposition.
3 RESULTS AND DISCUSSION
Compared with the conventional ILs applied in the low-temperature electrodeposition of aluminium,[Amim]Cl/AlCl3and [Amim]Cl have more attractive properties. As Fig. 1 illustrates, the electrical conductivity of 1∶2.0 [Amim]Cl/AlCl3is significantly higher than that determined in the well-known 1∶2.0[Bmim]Cl/AlCl3[22]. It indicates that lower cell voltage, higher current efficiency and higher deposition rate can be expected in [Amim]Cl/AlCl3. Considering the relationship between ionic conduction and molecular features of ILs, the high electrical conductivities of [Amim]Cl/AlCl3are probably ascribed to the relatively weak cation-anion interaction, high structural symmetry of [Amim]+cation and high molar concentration of ions (8.89 mol·L-1, 298.2 K) [38, 39].Besides, [Amim]Cl and [Amim]Cl/AlCl3are much easier to prepare and purify. The final IL products were obtained with good purity, which paved the way for low-temperature electrodeposition of aluminium.In the following work, Lewis acidic [Amim]Cl/AlCl3ILs with molar ratio of 1∶2.0, 1∶1.8 and 1∶1.5 were used as electrolytes.
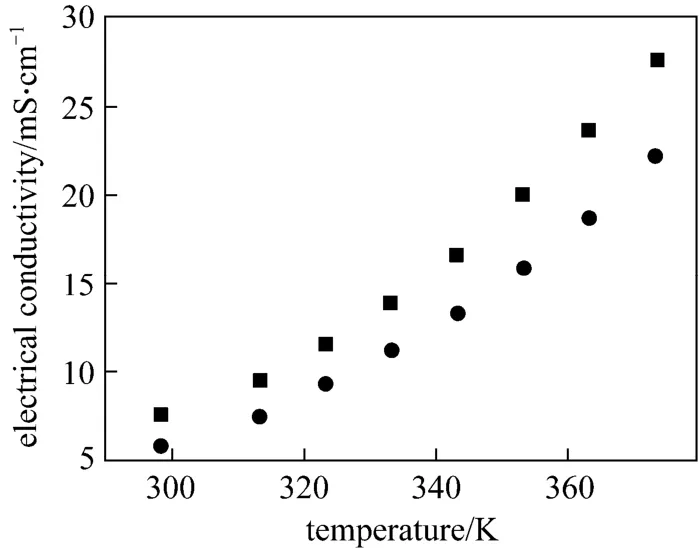
Figure 1 The electrical conductivities of 1∶2.0 [Amim]Cl/AlCl3 and 1∶2.0 [Bmim]Cl/AlCl3 from 298.2 to 373.2 K
3.1 Results of cyclic voltammetry
To probe into the eletrodeposition behavior of aluminium, cyclic voltammetry has been used in this work. Fig. 2 shows the cyclic voltammograms of Lewis acidic [Amim]Cl/AlCl3ILs recorded on a Pt electrode at 303.2 K. The potential range was controlled between -2.0 and 0.2 V to avoid the interference from the oxidation of [AlCl4]-anions and reduction of [Amim]+cations. It can be seen that a pair of peaks is obtained between -0.1 and -2.0 V in each sample. The reduction peaks at -2.0 V on the negative scan are ascribed to the bulk electrodeposition of aluminium, while the oxidation peaks around -0.4 V on the reverse scan reflect the stripping process of deposited aluminium:


Figure 2 Cyclic voltammograms recorded on a Pt electrode in Lewis acidic [Amim]Cl/AlCl3 ILs at 303.2 K (potential scan rate: 0.1 V·s-1)
In these ILs, the reduction of Al(III) begins to occur around -1.2 V, which is much more positive than the standard potential of Al/Al(III) couple in aqueous solutions (-2.47 V,vs. Ag+/Ag). The difference in deposition potential of aluminium is probably attributed to the different coordination pattern of Al(III) in [Amim]Cl/AlCl3and aqueous solutions. As Fig. 2 shows, it can also be found that the reduction potential of Al(III) in ILs decreases in the order 1∶2.0[Amim]Cl/AlCl3>1 ∶ 1.8 [Amim]Cl/AlCl3>1 ∶ 1.5[Amim]Cl/AlCl3. According to the values of molar ratio and density, the molar concentration of [Al2Cl7]-in above systems follows the same order. The predominant presence of anion can be expressed as [Al2Cl7]-in 1∶2.0 [Amim]Cl/AlCl3and 1∶2.0 [Bmim]Cl/AlCl3based on the analysis of27Al NMR results (see Table 1) [29]. Therefore, it is proper to state that aluminium is more prone to be electrodeposited from Lewis acidic [Amim]Cl/AlCl3ILs with higher molar concentration of [Al2Cl7]-anions under the same conditions.

Table 1 27Al NMR chemical shifts of 1∶2.0[Amim]Cl/AlCl3 and 1∶2.0 [Bmim]Cl/AlCl3
Additionally, cyclic voltammograms of [Amim]Cl/AlCl3ILs at different scan rates have been determined.The cathodic limit was set to -2.1 V so that the current density (ipc) of bulk deposition peak could be achieved.The experimental results for 1∶2.0 [Amim]Cl/AlCl3are presented in Fig. 3. It is evident that bothipcand the corresponding deposition peak potential (pcφ) of aluminium shift more negative when the scan rate (v)increases. As shown in the inset of Fig. 3, the plots ofipcagainst the square root of scan rate (v1/2) display good linearity. However, the straight line obtained by plottingipcvs.v1/2does not pass through the origin as expected for a simple linear diffusion controlled process.It can be inferred that the electrodeposition of aluminium from 1∶2.0 [Amim]Cl/AlCl3is mainly influenced by mass diffusion and kinetic limitations,such as electron transfer or surface chemical reaction steps [23]. Similar conclusion has also been drawn from other Lewis acidic [Amim]Cl/AlCl3ILs.
3.2 Nucleation/growth process for electrodeposition of aluminium
Chronoamperometric experiments have been conducted to investigate the nucleation/growth process of aluminium, which is critical to the study of electrocrystallization mechanism and surface morphology of aluminium deposits on copper substrates.For each experiment, the potential of working electrode was stepped from an initial value without reduction of Al(III) taking place to sufficiently negative potentials to initiate the nucleation/growth process. A series of typical current-time transients derived from different step potentials for the electrodeposition of aluminium from Lewis acidic [Amim]Cl/AlCl3ILs at 313.2 K are shown in Figs. 4 and 5. Apparently, all the transients exhibit typical shape for a nucleation process. Owing to the charging of electrode double layer, a sharp decay of current can be observed after the application of step potential, especially at higher potentials. The following increase in current is attributed to the nucleation and growth of aluminium nuclei on copper electrode. The rising current finally reaches a maximum (im) at time (tm), when the discrete diffusion zones of growing crystallites start to overlap [22]. After that, the current transients decay as the deposited
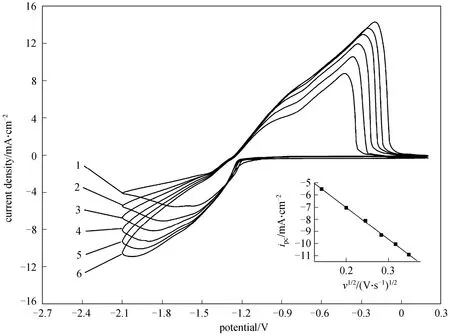
Figure 3 Cyclic voltammograms recorded on a Pt electrode in 1∶2.0 [Amim]Cl/AlCl3 at 303.2 K potential scan rate/V·s-1: 1—0.02; 2—0.04; 3—0.06; 4—0.08; 5—0.10; 6—0.12

Figure 4 Current-time transients resulting from potential step experiments on Cu electrodes in 1∶2.0 [Amim]Cl/AlCl3 at 313.2 K
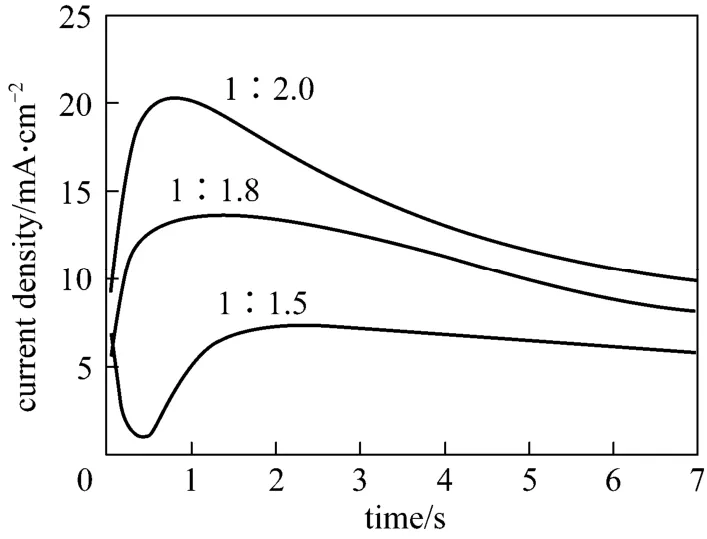
Figure 5 Current-time transients resulting from potential step experiments on Cu electrodes in 1∶2.0, 1∶1.8, 1∶1.5[Amim]Cl/AlCl3 ILs at -0.2 V and 313.2 K
layer becomes thicker. The time required to reachimis shorten when the applied potential is more negative in the same IL (see Fig. 4). Meanwhile, Fig. 5 indicates thatimincreases in the order 1∶1.5 [Amim]Cl/AlCl3<1∶1.8 [Amim]Cl/AlCl3<1∶2.0 [Amim]Cl/AlCl3, whiletmchanges in an opposite trend. These results suggest that the nucleation density and rate of aluminium are enhanced as the molar concentration of Al(III) increases in Lewis acidic [Amim]Cl/AlCl3ILs.The bulk electrodeposition of aluminium on foreign substrates often involves a three-dimensional nucleation/growth process. Some classic models have been proposed to describe this process, typically as hemispherical diffusion-controlled growth of the nuclei
[40]. This model has two limiting cases: instantaneous nucleation and progressive nucleation. In the first limiting case, all the nuclei are instantaneously formed at the beginning of chronoamperometric experiments.The second case refers to a situation where the nuclei are generated gradually as the deposition experiment proceeds. They are expressed respectively by [41]

To identify the nucleation model, a common method is applied by the comparison between dimensionless experimental current-time experiments and dimensionless theoretical transients for each nucleation mechanism. Before an effective comparison can be made, the experimental time has been corrected for the potential-dependent induction time (t0), by refining the time axis ast′ =t-t0andtm′ =tm-t0. Estimation oft0can be made according to the method described in [42]. The dimensionless plots of (i/im)2vs. (t′/tm′)2,along with theoretical curves obtained from Eqs. (4)and (5) are presented in Fig. 6. As can be seen, the electrodeposition of aluminium on copper substrates fits well to the theoretical model for three-dimensional instantaneous nucleation with diffusion-controlled growth.

Figure 6 Comparison of the dimensionless experimental current-time transients, derived from the results in Fig. 4,with the theoretical curves for instantaneous nucleation and progressive nucleationinstantaneous; progressive; ● -0.15 V; ▽ -0.20 V;◆ -0.25 V
3.3 Surface morphology of aluminium deposits
It is necessary to explore optimum experimental conditions of aluminium deposition on copper substrates for industrial application. Consequently, the effects of main experimental parameters, including temperature(313.2-373.2 K), current density (5-25 mA·cm-2) and molar ratio of [Amim]Cl to AlCl3[(1∶1.5)- (1∶2.0)]on the surface morphology of aluminium deposits and current efficiency have been investigated. The deposition time and stirring speed were kept as 30 min and 300 r·min-1, respectively.
In this work, it is found that temperature has a significant impact on the surface morphology of aluminium coatings. Bright, smooth and dense deposits were obtained in the temperature range from 313.2 to 353.2 K. However, the deposits turned dark and poorly adherent at the temperature higher than 353.2 K,particularly at 373.2 K. For illustration, the SEM micrographs of aluminium deposits prepared from 1∶2.0 [Amim]Cl/AlCl3under different temperatures are shown in Fig. 7. When the temperature rises, the average grain size of crystallites increases from 1 to 10 µm approximately. The deposits produced at 313.2,333.2 and 353.2 K all display dense and homogeneous microstructures with spherical particles. By contrast, a relatively rough and incompact surface with large clusters of platy-shaped crystals is observed on the sample obtained at 373.2 K. The difference in microstructures of aluminium deposits probably results from the variation of electrical conductivities and diffusion rate of ions in IL at different temperatures. A higher temperature followed by nonuniform distribution of current density and high deposition rate is prone to promote the dendritic growth, which often leads to rough coatings.
Searching for appropriate current density is key to the electrodeposition of aluminium. Figs. 8 and 7 (a)show the SEM images of deposits prepared from 1∶2.0 [Amim]Cl/AlCl3with different current densities at 313.2 K. It is apparent that the grain size of aluminium crystallites decreases from about 3 to 1 µm as current density increases in the range of 5-20 mA·cm-2. Nevertheless, the deposited crystals grow to a larger size when the current density increases to 25 mA·cm-2. Among these samples, the deposits obtained at 15 and 20 mA·cm-2have the most smooth and compact structures. It can be inferred from above phenomena that the nucleation density on copper electrode surface increased with current density rising between 5 and 20 mA·cm-2. As a result, more aluminium nuclei were generated so that the deposits displayed finer microstructures. After the nucleation density reached a maximum value, the crystallites experienced a rapid overlapping growth at 25 mA·cm-2, which eventually resulted in larger grain size and rougher deposits.
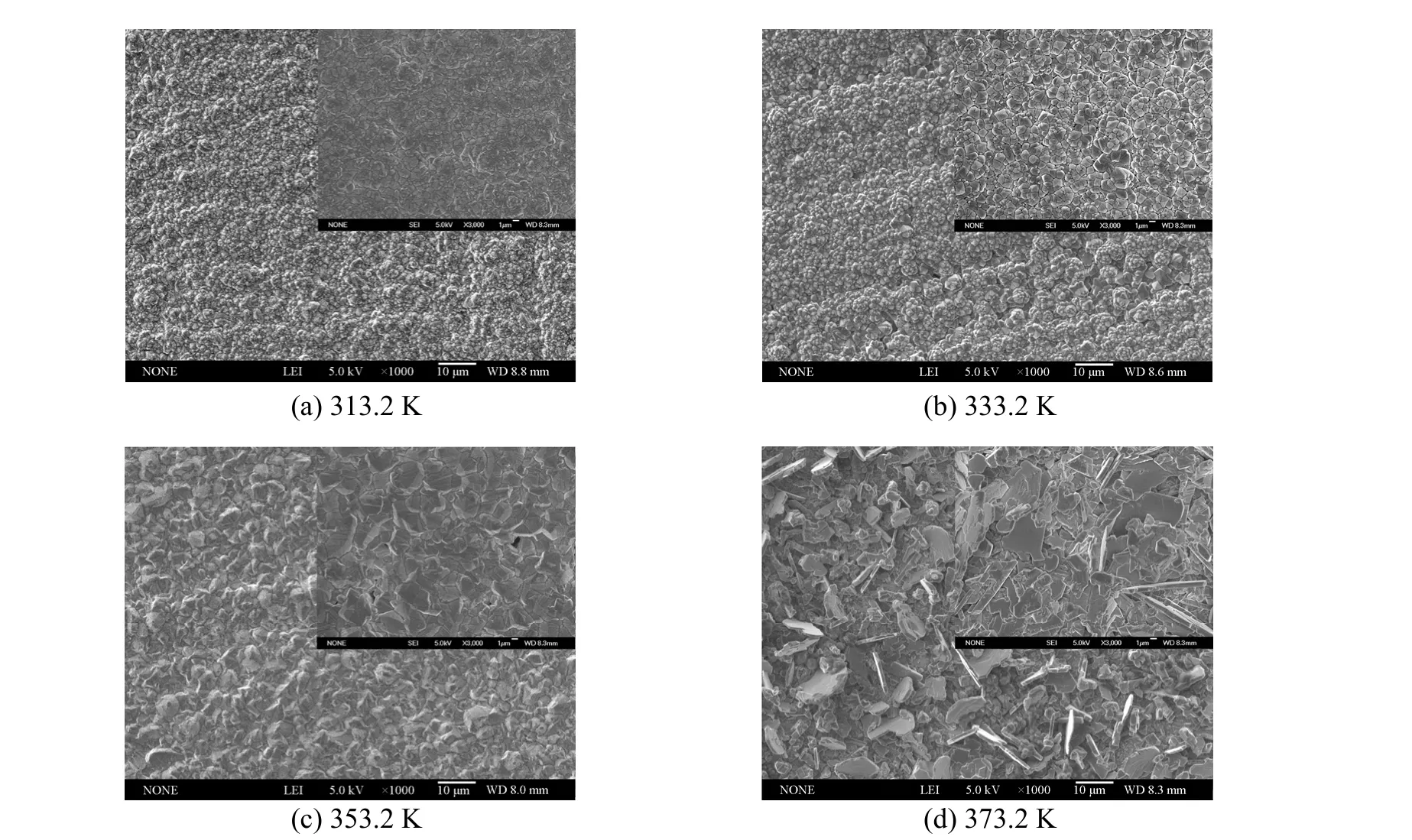
Figure 7 SEM micrographs of aluminium electrodeposits prepared from 1∶2.0 [Amim]Cl/AlCl3 at 20 mA·cm-2 and different deposition temperatures (The inset images show the higher magnification micrographs of deposits)
As is well-known, the content of AlCl3in chloroaluminate ILs is another important parameter in electrodeposition of aluminium. Herein, experimental results reveal that the deposits increase in grain size and become less compact when the molar ratio of[Amim]Cl to AlCl3[(1∶1.5)- (1∶2.0)] rises under the same conditions. For example, the particle size of resulting deposits in the studied ILs follows the order 1∶1.5 [Amim]Cl/AlCl3>1∶1.8 [Amim]Cl/AlCl3>1∶2.0 [Amim]Cl/AlCl3at 20 mA·cm-2and 313.2 K.This trend of variation of surface morphology can be seen clearly from the SEM micrographs of aluminium deposits shown in Figs. 9 and 7 (a). Based on the discussion above, it is proposed that the molar concentration of reducible [Al2Cl7]-anions and corresponding nucleation density of aluminium on copper surface decrease as the molar content of AlCl3increases in[Amim]Cl/AlCl3ILs. Consequently, uniform, compact and dense aluminium coatings have been obtained from 1∶2.0 [Amim]Cl/AlCl3more easily than 1∶1.5 and 1∶1.8 [Amim]Cl/AlCl3.
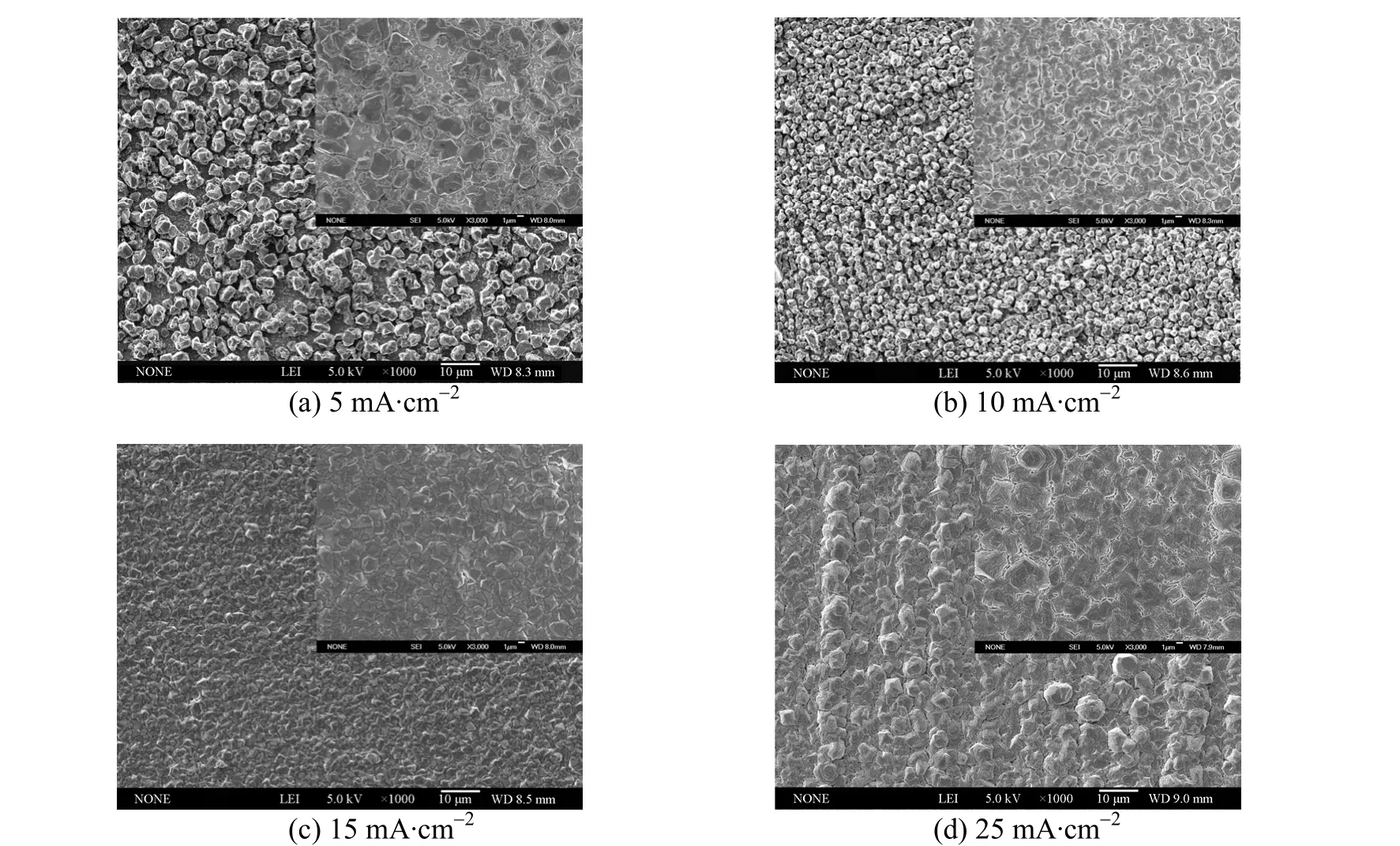
Figure 8 SEM micrographs of aluminium electrodeposits prepared from 1∶2.0 [Amim]Cl/AlCl3 at 313.2 K and different deposition current densities (The inset images show the higher magnification micrographs of deposits)

Figure 9 SEM micrographs of aluminium electrodeposits obtained at 313.2 K and 20 mA·cm-2 from different[Amim]Cl/AlCl3 ILs (The inset images show the higher magnification micrographs of deposits)
In summary, the aluminium deposits prepared from Lewis acidic [Amim]Cl/AlCl3ILs have finer microstructures than those produced in many conventional chloroaluminate-based ILs at the studied experimental conditions [22-24]. Moreover, most of the deposited films adhered well to the substrates without obvious crevices in between (see Fig. 10). The aluminium coatings with optimum surface morphology were obtained from 1∶2.0 [Amim]Cl/AlCl3at 313.2-353.2 K and 10-20 mA·cm-2. It can be concluded that the morphology of deposits changes as a function of experimental parameters (temperature, current density and molar ratio of [Amim]Cl/AlCl3), which have a significant effect on the nucleation and crystal growth process of aluminium. Therefore, appropriate nucleation density and growth rate of crystallites are critical to the generation of favorable coatings.
3.4 Current efficiency in the electrodeposition of aluminium
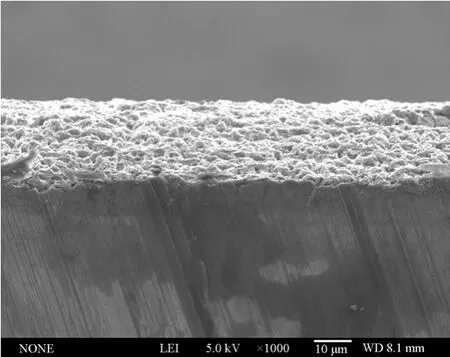
Figure 10 SEM micrograph of the cross section for an about 20 µm aluminium coating (bright region) obtained from 1∶2.0 [Amim]Cl/AlCl3 at 20 mA·cm-2 and 313.2 K on Cu substrate (underneath the bright region)
In this work, more than 95% current efficiency has been achieved for all the aluminium deposits. The optimal efficiency was obtained as 99.6% from 1∶2.0[Amim]Cl/AlCl3at 313.2 K and 20 mA·cm-2, while the minimum value was 95.3% for the deposition conducted in 1∶1.5 [Amim]Cl/AlCl3at 373.2 K and 5 mA·cm-2. Generally speaking, the current efficiency decreased to the range of 95.3%-99.6% when the temperature increased from 313.2 to 373.2 K. With the increase of current density, the current efficiency first increased to a maximum at 10-20 mA·cm-2and then slightly decreased in each IL. Moreover, the current efficiency were raised as the molar ratio of[Amim]Cl/AlCl3changed from 1∶1.5 to 1∶2.0.
The current efficiency in electrodeposition of aluminium reflects the yield of deposits under different experimental conditions. To the best of our knowledge, the yield of aluminium is mainly affected by cathodic side reactions and crystal growth process.The cathodic side reactions, including the reduction of[Amim]+cations and oxidation of deposited aluminium are more likely to take place as temperature and current density increases. Meanwhile, part of the rough and poorly adherent coatings which has been derived from nonuniform crystal growth is prone to fall off the substrates. The lower temperature and current density followed by favorable surface morphology of deposits often result in higher yield of aluminium and current efficiency.
3.5 Crystal structures and composition analysis of aluminium deposits
The relationship between crystal structures and surface morphology of aluminium deposits was further explored by XRD. In order to facilitate the following analysis, the peaks resulting from copper substrates have been corrected and removed from the spectra. As expected, the XRD patterns of all the samples match well with the standard values for aluminium in JCPDS cards. It is found that the crystallographic orientations of deposits change along with experimental conditions (see Figs. 11, 12 and 13).These results indicate that the influence of experimental parameters on the crystal growth of aluminium deposits follows the order temperature>current density>molar ratio of [Amim]Cl to AlCl3. The low temperature and high current density usually increases the peak intensity of (200) plane, thus promoting the crystal growth in this direction on copper substrates. Furthermore, the changes in crystal patterns can also reflect the surface morphology of deposits. According to the measurement of SEM micrographs, the bright,smooth and well adherent deposits almost exhibit a preferred crystallographic orientation of (200).
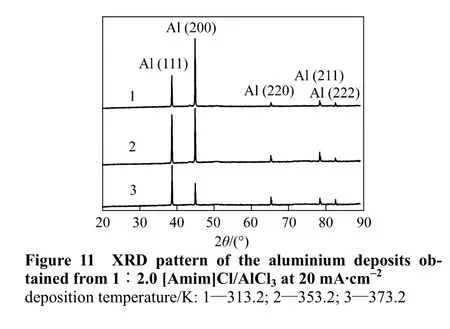

Figure 12 XRD pattern of the aluminium deposits obtained from 1∶2.0 [Amim]Cl/AlCl3 at 313.2 K
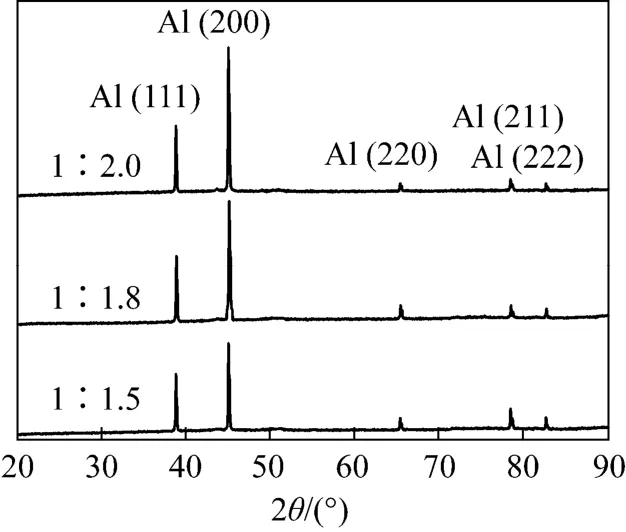
Figure 13 XRD pattern of the aluminium deposits obtained at 313.2 K and 20 mA·cm-2 from 1∶2.0, 1∶1.8, 1∶1.5[Amim]Cl/AlCl3 ILs
The compositions of aluminium deposits prepared at different conditions have been characterized by EDAX. The results show that mass content of aluminium in these samples is more than 98%. As Fig. 14 illustrates, a strong peak of aluminium and an extremely weak peak of oxygen can be seen from the EDAX spectrum of the deposits shown in Fig. 7 (a).The detected oxygen may probably result from the oxidation of aluminium in the atmosphere, and the mass content of aluminium is as high as 99.6%. It is demonstrated that the high-purity deposits generally show a smooth and compact surface, which has effectively prevented the further oxidation of aluminium.
4 CONCLUSIONS

Figure 14 EDAX spectrum of the aluminium deposits shown in Fig. 7 (a)
A systematic research on the low-temperature electrodeposition of aluminium from Lewis acidic[Amim]Cl/AlCl3ILs is presented. These electrolytes showed not only easier operation for synthesis, but also more favorable physicochemical properties than traditional chloroaluminate-based ILs. The electrochemical measurements revealed that the electrodeposition of aluminium involved a three-dimensional instantaneous nucleation process with diffusioncontrolled growth. The increase in the molar concentration of Al(III) could enhance the nucleation density and rate of aluminium. By galvanostatic method,smooth, dense and well adherent aluminium deposits were obtained on copper substrates over a wide range of temperature, current density and molar ratio of[Amim]Cl to AlCl3. Most of these deposits exhibited not only finer microstructures, but also improved surface morphology than those prepared from the other classic ILs, such as [Bmim]Cl/AlCl3and [Emim]Cl/AlCl3. The current efficiency in electrodeposition and purity of aluminium has both been achieved with high values. Therefore, it is expected that [Amim]Cl/AlCl3ILs can be promising alternatives to conventional electrolytes in the low-temperature electroplating and electrorefining of aluminium.
1 Wang, D.Q., Shi, Z.Y., “Aluminizing and oxidation treatment of 1Cr18Ni9 stainless steel”,Appl.Surf.Sci., 227, 255-260 (2004).
2 Paredes, R.S.C., Amico, S.C., d’Oliveira, A.S.C.M., “The effect of roughness and pre-heating of the substrate on the morphology of aluminium coatings deposited by thermal spraying”,Surf.Coat.Technol., 200, 3049-3055 (2006).
3 Boogaard, A., van den Broek, J.J., “Crystallisation and electrical resistivity of sputter-deposited aluminium-germanium alloy films”,Thin Solid Films, 401, 1-6 (2001).
4 Yang, D., Jonnalagadda, R., Rogers, B.R., Hillman, J.T., Foster, R.F.,Cale, T.S., “Texture and surface roughness of PRCVD aluminum films”,Thin Solid Films, 332, 312-318 (1998).
5 Gálová, M., “Electrodeposition of aluminium from organic aprotic solvents”,Surf.Technol., 11, 357-369 (1980).
6 Zhao, Y.G., van der Noot, T.J., “Electrodeposition of aluminium from nonaqueous organic electrolytic systems and room temperature molten salts”,Electrochim.Acta, 42, 3-13 (1997).
7 Zhang, M.M., Kamavaram, V., Reddy, R.G., “New electrolytes for aluminum production: ionic liquids”,JOM, 11, 54-57 (2003).
8 Legrand, L., Tranchant, A., Messina, R., “Behaviour of aluminium as anode in dimethylsulfone-based electrolytes”,Electrochim.Acta,39, 1427-1431 (1994).
9 Jiang, T., Chollier Brym, M.J., Dubé, G., Lasia, A., Brisard, G.M.,“Studies on the AlCl3/dimethylsulfone (DMSO2) electrolytes for the aluminum deposition processes”,Surf.Coat.Technol., 201, 6309-6317(2007).
10 Connor, J.H., Brenner, A., “Electrodeposition of metals from organic solutions”,J.Electrochem.Soc., 103, 657-662 (1956).
11 Li, J.C., Nan, S.H., Jiang, Q., “Study of the electrodeposition of Al-Mn amorphous alloys from molten salts”,Surf.Coat.Technol.,106, 135-139 (1998).
12 Jafarian, M., Mahjani, M.G., Gobal, F., Danaee, I., “Electrodeposition of aluminum from molten AlCl3-NaCl-KCl mixture”,J.Appl.Electrochem., 36, 1169-1173 (2006).
13 Welton, T., “Room-temperature ionic liquids. Solvents for synthesis and catalysis”,Chem.Rev., 99, 2071-2083 (1999).
14 Binnemans, K., “Ionic liquid crystals”,Chem.Rev., 105, 4148-4204(2005).
15 Galiński, M., Lewandowski, A., Stępniak, I., “Ionic liquids as electrolytes”,Electrochim.Acta, 51, 5567-5580 (2006).
16 Dupont, J., de Souza, R.F., Suarez, P.A.Z., “Ionic liquid (molten salt)phase organometallic catalysis”,Chem.Rev., 102, 3667-3692(2002).
17 Buzzeo, M.C., Evans, R.G., Compton, R.G., “Non-haloaluminate room-temperature ionic liquids in electrochemistry-a review”,ChemPhysChem, 5, 1106-1120 (2004).
18 Abbott, A.P., McKenzie, K.J., “Application of ionic liquids to the electrodeposition of metals”,Phys.Chem.Chem.Phys., 8, 4265-4279(2006).
19 Endres, F., “Ionic liquids: Solvents for the electrodeposition of metals and semiconductors”,ChemPhysChem, 3, 144-154 (2002).
20 Endres, F., MacFarlane, D., Abbott, A.P., Electrodeposition from Ionic Liquids, Wiley-VCH, Weinheim (2006).
21 Hurley, F.H., Wier, T.P., “The electrodeposition of aluminum from nonaqueous solutions at room temperature”,J.Electrochem.Soc., 98,207-212 (1951).
22 Yue, G.K., Zhang, S.J., Zhu, Y.L., Lu, X.M., Li, S.C., Li, Z.X., “A promising method for electrodeposition of aluminium on stainless steel in ionic liquid”,AIChE J., 55, 783-796 (2009).
23 Jiang, T., Chollier Brym, M.J., Dubé, G., Lasia, A., Brisard, G.M.,“Electrodeposition of aluminium from ionic liquids: part I-electrodeposition and surface morphology of aluminium from aluminium chloride (AlCl3)-1-ethyl-3-methylimidazolium chloride([EMIm]Cl) ionic liquids”,Surf.Coat.Technol., 201, 1-9 (2006).
24 Liu, Q.X., Zein El Abedin, S., Endres, F., “Electroplating of mild steel by aluminium in a first generation ionic liquid: A green alternative to commercial Al-plating in organic solvents”,Surf.Coat.Technol., 201, 1352-1356 (2006).
25 Lee, J.J., Bae, I.T., Scherson, D.A., Miller, B., Wheeler, K.A., “Underpotential deposition of aluminum and alloy formation on polycrystalline gold electrodes from AlCl3/EMIC room-temperature molten salts”,J.Electrochem.Soc., 147, 562-566 (2000).
26 Abbott, A.P., Eardley, C.A., Farley, N.R.S., Griffith, G.A., Pratt, A.,“Eletrodeposition of aluminium and aluminium/platinum alloys from AlCl3/benzyltrimethylammonium chloride room temperature ionic liquids”,J.Appl.Electrochem., 31, 1345-1350 (2001).
27 Liu, Q., Liu, K.R., Han, Q., Tu, G.F., “Study on electrodeposition of Al from trimethylphenylammonium chloride (TMPAC)-aluminum chloride (AlCl3) ionic liquids + benzene”,Chin.Surf.Eng., 23,34-39 (2010).
28 Bardi, U., Caporali, S., Craig, M., Giorgetti, A., Perissi, I., Nichlolls,J.R., “Electrodeposition of aluminium film on P90 Li-Al alloy as protective coating against corrosion”,Surf.Coat.Technol., 203,1373-1378 (2009).
29 Abbott, A.P., Qiu, F.L., Abood, H.M.A., Rostom Ali, M., Ryder, K.S.,“Double layer, diluent and anode effects upon the electrodeposition of aluminium from chloroaluminate based ionic liquids”,Phys.Chem.Chem.Phys., 12, 1862-1872 (2010).
30 Atkin, R., Zein El Abedin, S., Hayes, R., Gasparotto, L.H.S., Borisenko,N., Endres, F., “AFM and STM studies on the surface interaction of[BMP]TFSA and [EMIm]TFSA ionic liquids with Au(III)”, J. Phys.Chem. C, 113, 13266-13272 (2009).
31 Armand, M., Endres, F., MacFarlane, D., Ohno, H., Scrosati, B.,“Ionic-liquid materials for the electrochemical challenges of the future”, Nature Mater., 8, 621-629 (2009).
32 Zein El Abedin, S., Moustafa, E.M., Hempelmann, R., Natter, H.,Endres, F., “Additive free electrodeposition of nanocrystalline aluminium in a water and air stable ionic liquid”, Electrochem. Commun., 7, 1111-1116 (2005).
33 Zein El Abedin, S., Moustafa, E.M., Hempelmann, R., Natter, H.,Endres, F., “Electrodeposition of nano- and microcrystalline aluminium in three different air and water stable ionic liquids”, Chem-PhysChem, 7, 1535-1543 (2006).
34 Rodopoulos, T., Smith, L., Horne, M.D., Rüther, T., “Speciation of aluminium in mixtures of the ionic liquids [C3mpip][NTf2] and[C4mpyr][NTf2] with AlCl3: An electrochemical and NMR spectroscopy study”, Chem. Eur. J., 16, 3815-3826 (2010).
35 Zhang, H., Wu, J., Zhang, J., He, J.S., “1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: A new and powerful nonderivatizing solvent for cellulose”, Macromolecules, 38, 8272-8277(2005).
36 Huddleston, J.G., Visser, A.E., Reichert, W.M., Willauer, H.D., Broker,G.A., Rogers, R.D., “Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation”, Green Chem., 3, 156-164 (2001).
37 Bonhôte, P., Dias, A., Papageorgiou, N., Kalyanasundaram, K.,Grätzel, M., “Hydrophobic, highly conductive ambient-temperature molten salts”, Inorg. Chem., 35, 1168-1178 (1996).
38 Zheng, Y., Xuan, X.P., Wang, J.J., Fan, M.H., “The enhanced dissolution of β-cyclodextrin in some hydrophilic ionic liquids”, J. Phys.Chem. A, 114, 3926-3931 (2010).
39 Xuan, X.P., Guo, M., Pei, Y.C., Zheng, Y., “Theoretical study on cation-anion interaction and vibrational spectra of 1-allyl-3-methylimidazolium-based ionic liquids”, Spectrochim. Acta A, 78,1492-1499 (2011).
40 Scharifker, B., Hills, G., “Theoretical and experimental studies of multiple nucleation”, Electrochim. Acta, 28, 879-889 (1983).
41 Lee, J.J., Miller, B., Shi, X., Kalish, R., Wheeler, K.A., “Aluminum deposition and nucleation on nitrogen-incorporated tetrahedral amorphous carbon electrodes in ambient temperature chloroaluminate melts”, J. Electrochem. Soc., 147, 3370-3376 (2000).
42 Tsuda, T., Boyd, L.E., Kuwabata, S., Hussey, C.L., “Electrochemistry of copper(I) oxide in the 66.7-33.3 mol% urea-choline chloride room-temperature eutectic melt”, J. Electrochem. Soc., 157, F96-F103(2010).
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- Retrospect and Perspective of Micro-mixing Studies in Stirred Tanks*
- In-situ Synthesis and Catalytic Properties of ZSM-5/Rectorite Composites as Propylene Boosting Additive in FluidCatalytic Cracking Process*
- Solvent Extraction of Yttrium by Task-specific Ionic Liquids Bearing Carboxylic Group*
- An Experimental Study of Liquid-Liquid Microflow Pattern Maps Accompanied with Mass Transfer*
- Liquid-Liquid-Liquid Three Phase Extraction Apparatus: Operation Strategy and Influences on Mass Transfer Efficiency*
- Micron-sized Magnetic Polymer Microspheres for Adsorption and Separation of Cr(VI) from Aqueous Solution*
