杂草管理的分子生物学途径
Wun S. CHAO
(美国农业部农业科学研究院/生物科学研究实验室,北达科他州法戈市阿尔布雷希特大道1605号,58102-2765美国)
Wun S. CHAO. 杂草管理的分子生物学途径[J]. 杂草科学,2012,30(1):1-10.
杂草管理的分子生物学途径
Wun S. CHAO
(美国农业部农业科学研究院/生物科学研究实验室,北达科他州法戈市阿尔布雷希特大道1605号,58102-2765美国)
全球气候变化有利于外来杂草的入侵与传播,因为外来种通常可以快速适应环境。除草剂和抗除草剂作物的滥用使抗性杂草严重威胁现代农业的发展,这就需要新技术有效缓解当前的和未来的杂草问题。分子生物学是研究DNA、RNA以及蛋白质分子之间相互作用的科学,该技术已在杂草科学中广泛应用,如决定杂草抗药性机制、抗性杂草的起源、杂草基因型和基因流动的传播、杂草特征的生态适应与进化发展等。这些信息有利于建立可持续发展的杂草管理方案。分子生物技术也具有可直接用于防除杂草的潜力,如可用于开发新的除草措施的技术,包括宏基因组学、病毒诱导基因沉默、转基因雌性不育性等。
全球气候变化; 外来杂草; 病毒诱导基因沉默; 宏基因组学; 转基因雌性不育性
Weeds are ‘plants growing in the wrong place’. They are undesirable and disruptive because they displace native species, reduce crop growth and yield, and can contaminate crop seed. Weed control has been an ongoing effort in human history. Today,because of the upsurge in human traffic and climate change,invasive weeds have increased in number and destructive effects[1]; in addition,due to the extensive use of herbicides and the widespread use of herbicide resistant crops,herbicide-resistant weeds have become a severe threat to the sustainability and profitability of cropping systems. To reduce the cost and protect the soil,an economically feasible and environmentally friendly integrated weed management (IWM) program is often applied to control weeds. IPM is the combination of various management strategies including cultural,mechanical,biological and chemical methods to lower weed impact below the economic threshold. Although IPM emphasizes the use of multiple control methods,molecular technologies have not been included as part of the IPM program; nevertheless,molecular techniques have been used immensely as genetic markers to study ecological and evolutionary questions on weedy characteristics and have been used to provide genetic and mechanistic information on herbicide resistance. Giving the major advances in our knowledge of molecular biology for manipulating genomes and living organisms,these modern technologies have great potential in future weed management programs. This paper will discuss various aspects of using molecular technologies for weed control. Topics of discussion include invasive weeds and climate change,herbicide-resistant weeds,and molecular biology in weed science.
1 Invasive weeds and climate change
Invasive species and climate change are two of the most prevalent forms of ecosystem disturbance. Climate change is associated with rising global average temperature,altered precipitation regimes,changing magnitudes and durations of extreme weather events,and increased atmospheric carbon dioxide and nitrogen deposition[2]. The stressful conditions of these changes could cause species extinction and reduction in biological diversity for local endemics[3]. In contrast,invasive weeds,which tend to be generalists,are able to survive these altered climatic conditions better than more specialized and locally adapted species,and thus out-compete the native species. Invasive or noxious species is defined as an alien species whose introduction does or is likely to cause economic or environmental harm or harm to human health[4]. Among invasive pests,weeds lead to the greatest crop losses ($23 billion) and the greatest control costs ($3 000 million) annually[5]. Climate change affects invasive weeds in two general ways. First,the loss of native plant species results in fewer plant species distributed over large areas of the landscape; such ecosystems potentially have additional space and unused resources for the establishment of invasive weeds[6]. Second,invasive weeds are typically adaptable and aggressive,have a high reproductive potential,and may not have as many natural enemies. Their vigor combined with a lack of natural enemies often leads to outbreak populations[2].
Willis et al.[1]recently demonstrated that non-native plants and invasive species respond to climate change much better than native species. For example,non-natives are significantly better in tracking seasonal temperatures than natives. Invasive species have significantly shifted their flowering time over the last 100 years to be 11 days earlier than native species. Non-native and especially invasive species have significantly increased in abundance since 1900 relative to the native species. A study performed by Bradley et al.[7]also predicted that climate change is likely to enable the existing invasive plants such as kudzu (Puerarialobata),privet (Ligustrumsinense;L.vulgare),and cogongrass (Imperatacylindrica) to greatly expand their current geographical ranges in the southeast United States.
Carbon dioxide (CO2) elevation is one of the causes of climate change. A growing number of studies indicated that noxious weeds,which generally thrive in marginal and disturbed environments, benefit most from increased atmospheric CO2levels. For example,a study comparing six varieties of weedy rice with six varieties of cultivated rice revealed that weedy rice outperformed the cultivated rice substantially in trials with elevated CO2levels; a 55% increase in plant biomass and 62% increase in leaf area was observed 55 d after sowing for weedy rice relative to the cultivated rice at 500 μmol/mol[CO2]. The reason that weedy rice showed such greater performance in higher CO2levels is believed to be the relatively high genetic diversity,resulting in greater plasticity in response to the changing CO2levels[8].
2 Herbicide-resistant weeds
Besides invasive weeds,herbicide-resistant weeds have become a major threat to agriculture globally. Herbicide-resistant weeds have the inherited ability to survive and reproduce after receiving an otherwise lethal dose of herbicide[9]. Due to the extensive use of herbicides,weeds that exhibit resistance to one or more herbicides have increased continuously within the US and around the world. In addition,since molecular manipulation of crops for weed control has been dominated by the development of genetically modified,herbicide-resistant crops,namely glyphosate -,2,4-D -,and dicamba - resistant crops,the massive use of these herbicide-resistant crops has also triggered an outbreak of herbicide-resistant weeds[10]. The impediments resulting from herbicide-resistant weeds are the increase in the cost for weed control,reduction for herbicide options,and yield loss (http://www.wssa.net/LessonModules/herbicide-resistant-weeds/Lesson1/index.html).
There are two primary mechanisms that generate resistant weeds: target-site and non-target-site resistance[11]. Target-site resistance usually involves point-mutations in target genes (monogenic). Alterations of acetolactate synthase (ALS) represent the largest group of target-site based herbicide resistance with over 100 documented cases. Non-target-site herbicide resistance is more complex and may involve many genes that alter metabolic conversion of the herbicide to less effective compounds,sequestration processes,and/or compartmentalization,exclusion or excretion of the herbicide molecules. To date,four gene families including P450s,GSTs,glycosyltransferases and ABC transporters are known to participate in non-target-site herbicide resistance. Repeated use of a single herbicide (or group of related herbicides) and/or the exclusion of other weed control tactics can lead to the development of target-site and non-target-site resistance[11]. Other terms related to herbicide resistance include cross- and multiple-reisistance. Herbicide cross-resistance refers to a plant that has evolved resistance to one herbicide that also allows it to be resistant to other similar herbicides to which this plant has not been previously exposed. For example,a population of yellow starthistleCentaureasolstitialisin Washington State,United States evolved resistant to Picloram,a picolinic acid herbicides; it also became resistant to another picolinic acid herbicide,Clopyralid[9]. Multiple-resistance refers to a plant that has evolved resistance to more than one class of herbicides with very different modes or sites of action. For example,a population of prostrate pigweedAmaranthusblitoidesin Isreal evolved resistant to most ALS-inhibiting inhibitors and atrazine[12].
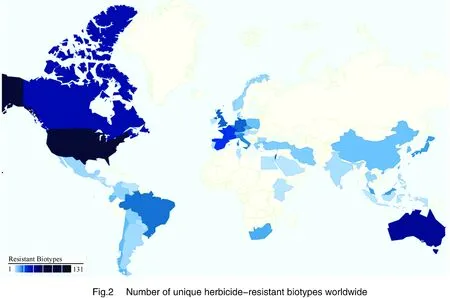
The number of herbicide resistance weeds is increasing at an exponential rate. Figure 1 shows the number of resistant biotypes to different mode of actions (http://www.weedscience.org/summary/MOASummary.asp). As of 2012,the number of ALS inhibitor biotypes is 116. Worldwide,there are now 372 unique herbicide-resistant biotypes (Fig. 2,also see http://www.weedscience.org/Maps/GlobalMap.htm). The United States has the highest number of herbicide-resistant weeds (131). China has 10. Herbicide resistant and invasive weeds have become major problems in today’s agricultural practices. The crisis is expected to worsen rapidly if proper management action is not taken. Many management tactics such as crop rotation,applications of herbicides with different target sites,use of certified seeds etc. can reduce weed loads and selection pressure for resistance evolution. Alternatively,advances in molecular biology have given biotechnology new potential in weed control and management.

3 Molecular biology in weed science
3.1Molecularmarkers
Molecular biology is the study of molecular interaction between DNA,RNA and proteins. Molecular biology can be used to study molecular mechanisms of weedy traits and biological questions such as determination of target-site and non-target-site resistance mechanisms[11]. Genomics-based techniques (microarray, quantitative PCR) were applied to identify biological pathways that contribute to a weedy trait,dormancy[13-16]. Molecular biology is also used to study spread of weed genotypes,gene flow,origin of weedy species,and ecological and evolutional reasons of weedy character development[17-19]. Information obtained from all these studies can be used to identify critical genes or physiological and/or developmental processes that can be targeted chemically or culturally to control weeds. Also,information on points of origin for invasive weeds can be used to search for alternative control agents such as insects and pathogens. Among the different molecular tools,DNA sequence-based genetic markers (also called molecular markers) have been most frequently used to detect variation at the DNA level,which in turn is applied to diagnose the evolution of herbicide resistance and/or answer the population,ecological and revolutionary questions mentioned above. Most molecular markers are based on polymerase chain reaction (PCR) amplification and/or sequencing of genomic DNA fragments. A brief description and potential pros and cons of some of the molecular markers are presented below.
Simplesequencerepeats(SSR): SSR are tandem repeats of sequence units usually 2 to 5 base pairs (bp) in length[20]. The recent emergence of next generation sequencing enables rapid SSR discovery at a reasonable cost[21]. Polymorphisms are identified by designing PCR primers for the DNA flanking the SSR region and visualized by agarose or polyacrylamide gel electrophoresis. SSR markers are widely used because they are (1) distributed all over the genome; (2) highly variable,allowing the identification of many alleles at a single locus which is particularly important for species with limited genetic variability; (3) co-dominant and thus provide more information such as the level of heterozygosity; and (4) highly reproducible. SSR markers are also called microsatellites,short tandem repeats (STR),or variable number tandem repeats (VNTR).
Intersimplesequencerepeats(ISSR): ISSR markers are developed from regions between common SSR motifs. Based on the premise that SSR loci are spread all over the genome,a single primer of the repeated region is used with the addition of an anchoring tag (2 to 4 nucleotide residues) on either the 5′ or 3′ end of the primer (e.g.,3′-anchored primers: CACACACACACACACARG; and 5′-anchored primers: BDBCACACACACACACAC-RG; R = A/T,B = T/G/C,D = A/T/G)[22]. The anchoring tag is needed because without it,the inter-repeat section can be amplified but locus-specificity would not be warranted. Since ISSR markers target multiple loci,single-primer amplification will yield multiple polymorphic bands. ISSR markers do not require prior sequence information and they are in general dominant markers[23],and thus not able to distinguish if the amplified DNA segment is from a heterozygous (1 copy) or homozygous (2 copies) locus. Two to 10 times more individuals are needed using dominant markers to achieve the comparable resolving power of co-dominant markers[23]. The amplified fragments are visualized by agarose or polyacrylamide gel electrophoresis.
Amplifiedfragmentlengthpolymorphism(AFLP): AFLP (or AFLP-PCR) is a PCR-based tool,and like ISSR markers,AFLPs target regions of the entire genome and do not require prior sequence information[24]. Genomic DNA is digested with restriction enzymes,and specific oligonucleotide adaptors containing primer sequences are ligated to the cohesive ends of the restriction fragments. A subset of the restriction fragments is then selected and amplified. The amplified fragments labeled with fluorescent or radioactive tags are visualized on denaturing polyacrylamide gels. Although AFLPs are highly reproducible and yield a large number of fragments,they are considered labor-intensive markers. Also,because of the banding pattern complexity and that AFLP markers can be either dominant or co-dominant,it is difficult to tell if any given polymorphic band is from a heterozygous or homozygous locus.
Cleavedamplifiedpolymorphicsequence(CAPS): CAPS involves PCR amplification of a target DNA followed by restriction digestion of the PCR products[25]. Differences in restriction fragment lengths due to gain or loss of restriction digestion at the SNP sites in PCR amplicons can be observed on agarose or polyacrylamide gels. They are co-dominant markers,relatively simple and inexpensive,and highly reproducible. However,not all mutations create or disrupt a restriction enzyme recognition site,a modification of CAPS called derived cleaved amplified polymorphic sequence (dCAPS) was developed to specifically introduce a restriction enzyme site at polymorphic locations via modification of PCR primers[26]. Similar to the CAPS technique,dCAPS is simple,relatively inexpensive,and highly reproducible.
Singlenucleotidepolymorphism(SNP): SNPs are single nucleotide variations between individuals at a given position in the genome,which could be in coding and non-coding regions. For a variation to be considered as a SNP,it should occur in at least 1% of the population[27]. SNPs are distributed throughout the genome and occur every 200-500 bp in non-coding DNA[28]. SNPs can be identified by comparing multiple sequences of the same region from expressed sequence (EST) databases and in silico (performed on computer). They are simple to score and represent co-dominant markers. Recent improvements in the speed,cost and accuracy of next generation sequencing (NGS),namely Roche’s (454) GS FLX Genome Analyzer,Illumina’s Solexa 1G sequencer,and most recently Applied Biosystem’s SOLiD system,and other affordable high-throughput genotyping technologies assist the rapid discovery and use of SNPs in non-model species such as weeds by opening the opportunity for large scale sequencing. To further bring down the cost,restriction enzyme-based reduced representation library (RRL) or restriction site-associated DNA (RAD) methods can be used to lower the complexity of the genomes to be sequenced[29-30]. RRL is prepared by first digesting genomic DNA with a restriction enzyme. Fragments are then size selected to 300-700 bp and sequenced after adding sequencing adaptors. Similar to RRL,RAD is also prepared by digesting genomic DNA with a restriction enzyme. Afterwards,fragments are ligated to P1 adaptors (containing primer 1 sequence),randomly sheared,and size selected to 300-700 bp. Fragments with and without P1 adaptors are then ligated to P2 adaptors (containing primer 2 sequence). The resulting fragments are PCR amplified with primer 1 and primer 2 for sequencing[31].
Among much older marker technologies,restriction fragment length polymorphism (RFLP) is based on DNA-DNA hybridization. Although RFLP is a reliable co-dominant marker,it has not been widely used in weed science research because it is labor intensive and requires a large amount of DNA. Random amplified polymorphic DNA (RAPD) is simple,inexpensive and relatively non labor-intensive dominant marker[32]; however,it is notorious for poor reproducibility. Chloroplast DNA (cpDNA) and mitochondrial DNA (mtDNA) are traditional markers useful in phylogeny and in population genetics. Generally,mtDNA has applied more in animal systems,and cpDNA has been a standard tool in plant systems. Since cpDNA is often maternally inherited,cpDNA polymorphism is sensitive to the impact of seed movement on gene flow[33]. Isozymes and allozymes are protein markers; isozymes are enzymes coded by alleles at different loci,and allozymes are enzymes coded by different alleles at the same locus. These protein markers are not commonly used today due to limited number of isozyme loci and high variability among different species and various tissues,namely their enzyme activities are strongly influenced by the environment. Some easily observed phenotypic markers have also been used to study gene flow and to mark linked traits of interest such as herbicide resistance. For example,transgenic plants have been developed that express easily detectable proteins such as GUS (Beta Glucuronidase) or GFP (Green fluorescent protein). These transgenic plants have been helpful in characterizing the possible flow of transgenes such as glyphosate resistance between crop and weed species and within wild populations[34],but the variability in expression levels among different species limits its use[35].
3.2Moleculartechnologiesinweedmanagement
Molecular tools have been applied to study weeds for preventive measures since they can determine the molecular,biochemical,ecological,and evolutionary reasons of how weeds adapt to their environment. When the causes of how weeds thrive and survive are identified,farmers can change management practices to slow the adaptation process and limit economic damage. The question remains as to whether molecular technologies can be used to control weeds directly. The incentive of using modern technologies is to reduce dependence on herbicides and to control troublesome (invasive and herbicide resistant) weeds. At present,a large amount of effort has been invested in modifying crops to increase their competitiveness such as generating herbicide,disease,and drought resistant crops. Much less effort has been devoted to directly weaken the capacity of weeds to thrive/survive using molecular technologies,since the tasks are considered high risk and the results are usually unpredictable. Nonetheless,in theory,molecular technologies can be used to develop new (alternative) chemicals,to improve/generate biocontrol agents,and to reduce weed competitiveness. The following examples illustrate these concepts.
3.2.1 Develop new (alternative) control measures - metagenomics Metagenomics is the study of many genomes simultaneously from a group of microorganisms (bacteria and archaea,as well as eukarytoic microorganisms: fungi,algae,and protozoa) and identifies mixed assemblages of microorganisms directly from their environment without the need for culturing them first in the laboratory[36]. Thus it is also described as “cultivation-independent” techniques. Metagenomics was developed in the mid-1990s and is now utilized to explore the reservoir (>99%) of soil microorganisms producing metabolites for use in agriculture,biomedicine,and industry. Weed-suppressive metabolites produced by these microorganisms may be isolated through functional screening assays and used to develop new herbicides for weed control. The general procedure for this work includes the following steps: 1) microorganisms are extracted from the environmental sample (e.g. soil,sediment,seawater etc.); 2) total DNA (the metagenome) is extracted,purified,and fractionated; 3) DNA fragments of the appropriate size (40 kb to 100 kb) are ligated into a cloning vector and then transformed into host cells; 4) each cell carries a DNA fragment from the metagenome,and all cells together constitute the metagenomic library; 5) novel weed-suppressive metabolites are isolated using functional screening assays by testing their suppressive effects on seed germination,plant growth,and the growth of herbicide-resistant weeds. The challenges of this technology are: (1) metagenomic library is not total inclusive,(2) some secondary metabolites cannot be synthesized in the bacteria host and/or are difficult to purify,(3) the compound may be not safe for the environment,and (4) the actual use of these secondary metabolites for weed control may not be cost-effective.
3.2.2 Generate biocontrol agents - virus-induced gene silencing (VIGS) Rector[37]has suggested using molecular biology approaches to improve biological weed control agents such as to increase lethality,specificity,and plasticity of these agents,and thus will not be restated here. The idea of using the concept of VIGS to generate biocontrol agents was proposed a few years ago[38-39],which modifies viral genomes by incorporating fragments of host genes that are targeted to be silenced.
VIGS employs modified viruses to specifically reduce endogenous gene activity based on post-transcriptional gene silencing (PTGS). Its RNA degradation mechanism is very similar to the pathways of RNA interference (RNAi). The mechanism of VIGS is not fully understood; however,many key findings over the years have established a clear picture of how VIGS functions[40-42]. VIGS is initiated when dsRNA is recognized by Dicer-like ribonucleases and converted to 21 to 24 nucleotides small interfering RNA (siRNA). These siRNAs are then incorporated into RNA-induced Silencing Complex (RISC) where strand separation occurs,namely passenger and guide strand. The passenger strand is degraded by small RNA degrading nucleases,and the guide strand facilitates the sequence-specific cleavage of a target RNA. Argonaute,the catalytic component of the RISC complex,searches for a complementary sequence of mRNA and induces cleavage and/or translational inhibition of the cognate RNAs. The virus-derived silencing signal can be amplified and spreads systemically throughout the plant. For long-range transport,a RNA-dependent RNA polymerase may be required to amplify the silencing signal[43].
A viral vector carrying host gene fragments can prevent the expression of homologous,chromosomal genes of the host. Thus,introducing a vital gene into a host weed species via a virus could also inhibit the expression of that gene and prevent its ability to grow (Fig. 3). Viral RNAs can be prepared in vitro. Plants are then inoculated with a mixture of viral RNA and other components. After inoculation,viral RNA and proteins are produced in the cytoplasm,and viruses are produced and move systemically to other parts of the plant. The endogenous host gene is then suppressed. This approach is very specific because only plants carrying highly similar genes,such as those of a modified virus,would be affected. When weeds develop resistance,the solution could be as simple as the replacement of a new lethal host gene sequence. In addition,viruses can propagate themselves and systemically spread from tissue to tissue,perhaps avoiding the need for repeated applications. The challenges are that virus genome modifications can be problematic and plant defense mechanisms may thwart the spread of the genetically-modified virus.
3.2.3 Reduce weed competitiveness - transgenic

female-sterility Direct modification of weed genomes to control their own growth and spreading is a challenging task and has not been applied to date. However,ideas of direct genome modification for weed control have been proposed[37,44]. One idea is termed transgenic female-sterility,which is to introduce genes conferring female sterility into the genome of a problematic target weed and to reduce competitiveness of that weed[37]. It relies on transformation of the target weed with a construct designed to spread through a weed population by way of a plant transposon vector,which is based on a formerly proposed insect model using Transposons with Armed Cassettes-Targeted Insect Control Strategy (TAC-TICS) system[45-46]. The premise for insect model is that (1) the active TAC constructs increase in copy number within the genome of each individual through a replicative transposition process,and (2) normal chromosome segregation during gamete formation allows the TAC constructs to be distributed to each offspring.
Transgenic female sterility targets female flower parts and would use plant transposons,such as Ac/Ds,in a gene construct that cause female-sterility in the weed. The details of generating female-sterility were stated by Rector[37]and thus are briefly summarized here. Female sterility can be generated by destroying female reproductive organs (e.g. the stigma,style,or ovule) using organ-specific promoters to express a destructive gene such as RNase. This trait could spread to successive generations by pollen of female-sterile plants to wild-type target plants,which serve as the female parents. Transposon vector would increase penetration of the female-sterility gene construct by the target population,leading to increasing proportions of female-sterile seed in the seed bank. The challenges for this strategy are (1) the weeds have to be amenable to transformation,(2) transposon construct is able to spread the destructive genes throughout the populations of the recipient species,(3) identifying organ-specific promoters with absolute specificity may be problematic,and (4) there is always a possibility of hybridization between the target and other closely related species.
It is apparent that there is no single perfect solution for weed control. Combating ‘weeds’ will be an ongoing struggle and may never be completely solved. It is thus possible that none of current control methods will work,and farm land abandonment may become inevitable. However,the advantage of molecular biology is that it kindles creative approaches in solving these problems. The aforementioned molecular technologies can assist in improving the biological constituents of a weed management program,such as increasing crop competitiveness,developing alternative chemicals,improving/generating biocontrol agents,or reducing weed competitiveness. Although some of these approaches are challenging and considered high risk,they may be the only solution when all other methods have failed.
[1]Willis C G,Ruhfel B R,Primack R B,et al. Favorable climate change response explains non-native species’ success in Thoreau’s woods[J]. PLoS ONE,2010,5:e8878.
[2]Bradley B A,Blumenthal D M,Wilcove D S,et al. Predicting plant invasions in an era of global change[J]. Trends in Ecology & Evolution,2010a,25:310-318.
[3]Thomas C D,Cameron A,Green R E,et al. Extinction risk from climate change[J]. Nature,2004,427:145-148.
[4]National Invasive Species Council (NISC) (2006) Invasive species definition,clarification,and guidance white paper[EB/OL]. http://www.invasivespeciesinfo.gov/docs/council/isacdef.pdf. Accessed 19 January 2012.
[5]Ziska L H,Blumenthal D M,Runion G B,et al. Invasive species and climate change: an agronomic perspective[J]. Climatic Change,2011,105:13-42.
[6]Manley P. Biodiversity and Climate Change[R/OL]. (2008-05-20). U.S. Department of Agriculture,Forest Service,Climate Change Resource Center. http://www.fs.fed.us/ccrc/topics/biodiversity.shtml.
[7]Bradley B A,Wilcove D S,Oppenheimer M. Climate change increases risk of plant invasion in the Eastern United States[J]. Biological Invasions,2010b,12:1855-1872.
[8]Ziska L H,McClung A M. Differential response of cultivated and weedy (red) rice to recent and projected increases in atmospheric carbon dioxide[J]. Agronomy Journal,2008,100:1259-1263.
[9]Prather T S,Ditomaso J M,Holt J S. Herbicide resistance:definition and management strategies. Publication of division of agriculture and natural resources[M]. UC Davis USA. Publication 8012,2000.
[10]Mortensen D A,Egan J F,Maxwell B D,et al. Navigating a critical Juncture for sustainable weed management[J]. BioScience,2012,62:75-84.
[11]Yuan J S,Tranel P J,Stewart C N. Non-target-site herbicide resistance: a family business[J]. Trends in Plant Science,2007,12:613.
[12]Sibony M,Rubin B. Molecular basis for multiple resistance to acetolactate synthase-inhibiting herbicides and atrazine inAmaranthusblitoides(prostrate pigweed) [J]. Planta,2003,216:1022-1027.
[13]Horvath D P,Chao W S,Suttle J C,et al. Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (EuphorbiaesulaL.) [J]. BMC Genomics,2008,9:e536.
[15]Foley M E,Anderson J V,Chao W S,et al. Initial changes in the transcriptome of Euphorbia esula seeds induced to germinate with a combination of constant and diurnal alternating temperatures[J]. Plant Molecular Biology,2010,73:131-142.
[17]Baker J,Hidayat I,Preston C. Molecular tools for understanding distribution and spread of weed genotypes[J]. Crop Protection,2007,26:198-205.
[18]Slotta T A B. What we know about weeds: insights from genetic markers[J]. Weed Science,2008,56:322-326.
[19]Ward S M,Jasieniuk M. Review: sampling weedy and invasive plant populations for genetic diversity analysis[J]. Weed Science,2009,57:593-602.
[20]Bruford M W,Wayne R K. Microsatellites and their application to population genetic studies[J]. Current Opinion in Genetics and Development,1993,3:939‐943.
[21]Castoe T A,Poole A W,Gu W J,et al. Rapid identification of thousands of copperhead snake (Agkistrodoncontortrix) microsatellite loci from modest amounts of 454 shotgun genome sequence[J]. Molecular Ecology Resources,2010,10:341-347.
[22]Zietkiewicz E,Rafalski A,Labuda D. Genome fingerprinting by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification[J]. Genomics,1994,20:176-183.
[23]Jasieniuk M,Maxwell B D. Plant diversity: new insights from molecular biology and genomics techniques[J]. Weed Science,2001,49:257-262.
[24]Vos P R,Hogers M,Bleeker M,et al. AFLP: a new technique for DNA fingerprinting[J]. Nucleic Acids Research,1995,23:4407-4414.
[25]Konieczny A,Ausubel F M. A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific markers[J]. The Plant Journal,1993,4:403-410.
[26]Neff M M,Neff J D,Chory J,et al. dCAPS,a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications inArabidopsisthalianagenetics[J]. The Plant Journal,1998,14:387-392.
[27]White P S,Kwok P Y,Oefner P,et al. 3rd international meeting on single nucleotide polymorphism and complex genome analysis: SNPs: “some notable progress” [J]. European Journal of Human Genetics,2001,9:316-318.
[28]Brumfield R T,Beerli P,Nickerson D A,et al. The utility of single nucleotide polymorphisms in inferences of population history[J]. Trends in Ecology and Evolution,2003,18:249-256.
[29]Wiedmann R T,Smith T P,Nonneman D J. SNP discovery in swine by reduced representation and high throughput pyrosequencing[J]. BMC Genetics,2008,9:e81.
[30]Baird N A,Etter P D,Atwood T S,et al. Rapid SNP discovery and genetic mapping using sequenced RAD markers[J]. PLoS ONE,2008,3:e3376.
[31]Davey J W,Hohenlohe P A,Etter P D,et al. Genome-wide genetic marker discovery and genotyping using next-generation sequencing[J]. Nature Reviews Genetics,2011,12:499-510.
[32]Williams J G K,Kubelik A R,Livak K J,et al. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers[J]. Nucleic Acids Research,1990,18:6531-6535.
[33]McCauley D E. The use of chloroplast DNA polymorphism in studies of gene flow in plants[J]. Trends in Ecology & Evolution,1995,10:198-202.
[34]Stewart C N. Monitoring transgenic plants using in vivo markers[J]. Nature Biotechnology,1996,14:682.
[35]Hanson M R,Köhler R H. GFP imaging: methodology and application to investigate cellular compartmentation in plants[J]. Journal of Experimental Botany,2001,52:529-539.
[36]Handelsman J. Metagenomics: Application of genomics to uncultured microorganisms[J]. Microbiology and Molecular Biology Reviews,2004,68:669-685.
[37]Rector B G. Molecular biology approaches to control of intractable weeds: New strategies and complements to existing biological practices[J]. Plant Science,2008,175:437-448.
[38]Anderson J V,Chao W S,Horvath D P. A current review on the regulation of dormancy in vegetative buds[J]. Weed Science,2001,49:581-589.
[39]Chao W S. Contemporary methods to investigate seed and bud dormancy[J]. Weed Science,2002,50:215-226.
[40]Becker A,Lange M. VIGS genomics goes functional[J]. Trends in Plant Science,2010,15:1-4.
[41]Llave C. Virus-derived small interfering RNAs at the core of plant-virus interactions[J]. Trends in Plant Science,2010,15:701-707.
[42]Senthil-Kumar M,Mysore K S. New dimensions for VIGS in plant functional genomics[J]. Trends in Plant Science,2011,16:656-665.
[43]Kalantidis K,Schumacher H T,Alexiadis T,et al. RNA silencing movement in plants[J]. Biology of the Cell,2008,100:13-26.
[44]Gressel J. Molecular biology of weed control[M]. London: Taylor & Francis,2002.
[45]Grigliatti T A,Meister G,Pfeifer T A. TAC-TICS: Transposon-based biological pest management systems[M]//Vurro M,Gressel J. Novel biotechnologies for biocontrol agent enhancement and management. Dordrecht,Netherlands:Springer,2007: 327-351.
[46]Pfeifer T A,Grigliatti T A. Future perspectives on insect pest management: Engineering the pest[J]. Journal of Invertebrate Pathology,1996,67:109-119.
MolecularBiologyApproachestoWeedManagement
Wun S. CHAO
(USDA-Agricultural Research Service,Biosciences Research Lab, 1605 Albrecht Boulevard,Fargo,ND 58102-2765 USA)
Global climate change appears to be favorable for invasive weed development and spread because invasive species in general are proficient at succeeding in new environments. To worsen matters,herbicide-resistant weeds have become a severe threat in modern agricultural systems due to the extensive use of herbicides and the widespread use of herbicide resistant crops. Therefore,novel techniques are needed to effectively alleviate current and future weed problems. Molecular biology is the study of molecular interactions between DNA,RNA,and proteins. Molecular tools have been used in weed science to determine mechanisms of how weeds become resistant,origins of resistance,spread of weed genotypes and gene flow,ecological and evolutionary development of weedy characteristics,etc. Information obtained from these studies will contribute in proactive planning and applying environmentally sustainable solutions to weed management. Molecular tools also have the potential to be applied directly for weed control. Several technologies such as metagenomics,virus-induced gene silencing,and transgenic female-sterility are some good examples of how these techniques may be used to develop novel control measures.
global climate change; invasive weed; virus-induced gene silencing; metagenomics; transgenic female-sterility
S451
A
1003-935X(2012)01-0001-10
2012-03-08
Wun S. CHAO(1960—),男,博士,美国农业部农业科学研究院(USDA)研究员。E-mail:wun.chao@ars.usda.gov。

