Phase selection rules for complex multi-component alloys with equiatomic or close-to-equiatomic compositions
GUO Sheng, LIU Chain T.
① Research Fellow; ②Fellow of National Academy of Engineering, Center of Advanced Structural Materials,Department of Mechanical and Biomedical Engineering, College of Science and Engineering, City University of Hong Kong, Kowloon, Hong Kong, China
Phase selection rules for complex multi-component alloys with equiatomic or close-to-equiatomic compositions
GUO Sheng①, LIU Chain T.②
① Research Fellow; ②Fellow of National Academy of Engineering, Center of Advanced Structural Materials,Department of Mechanical and Biomedical Engineering, College of Science and Engineering, City University of Hong Kong, Kowloon, Hong Kong, China
phase selection, multi-component alloys, amorphous phase, solid solution, intermetallic compounds
Alloying greatly expands the amount of available materials beyond the naturally existing ones, and more importantly offers the material scientists opportunities to initiatively control the composition-structure-property relationship in materials. Since commonly used metallic materials are mostly multi-component alloys, the know-how of alloying through compositional control, certainly plays a critical role in designing materials with desired structure and properties. However, alloying in multi-component alloys is an extremely complicated issue, as the alloyed products could be the amorphous phase, various solid solutions and intermetallic compounds containing two or more alloy components. By narrowing down the scope of the multi-component alloys to those with equiatomic or close-to-equiatomic compositions only, and also aiming at framing out the rules that govern the phase selection upon alloying in multi-component alloys in a broad sense, we have identified here a simple and easily executable two-parameter scheme that can effectively predict the formation of the amorphous phase, solid solutions and intermetallic compounds, in multi-component alloys, simply from the given alloy compositions. We believe this scheme reveals a clear physical scenario governing the phase selection in multi-component alloys, helps to simplify the alloy design, and benefits the future development of advanced metallic alloys like bulk metallic glasses and high entropy alloys.
1 Introduction
The English word, alloy, originates from the Latin word,alligare(bind), and later the Old French,aloier(combine). Intuitively, alloying means combining two or more elements. For example, bronze is an alloy coming from the combining of copper and tin, or we can say the alloying of copper and tin can make bronze.Alloying of copper and tin was an accidental discovery in the ancient time, as copper ores mixed with ores of arsenic, zinc and tin in the primitive fires in the caves[1]. Today, we know that this accidental discovery brought the Bronze Age, as people started to use bronze widely after they found that bronze was superior to other materials (stones and pure copper) in terms of the mechanical and physical properties. The discovery of steel, an alloy of iron and carbon, is another milestone in the human history and steel is still the most widely used material nowadays. The purpose of alloying is always to achieve better properties, as pure metals have few uses but their mechanical, physical or chemical properties can be improved by alloying.
Alloys can exist in different forms: solid solution, the amorphous phase, intermetallic compound, or a mixture of them. Solid solution is the most commonly seen form of alloys. A solid solution is a solid-state solution of one or more solutes in a solvent. Such a mixture is regarded as a solution rather than a compound, when the crystal structure of the solvent remains unchanged by adding (alloying) the solutes, and when the mixture remains in a single homogeneous phase. Certainly,depending on the solvent or the principle element,the solid solution can have various crystal structures, like face-centered cubic (fcc), bodycentered cubic (bcc) or hexagonal close packed(hcp) structure. The solute atoms may incorporate into the solvent crystal lattice substitutionally, by replacing the solvent atoms in the lattice, or interstitially, by fitting into the space between solvent atoms. Intermetallic compound essentially is also a solid-state solution of one or more solutes in a solvent, but it differs with the solid solution in which its crystal structure is neither the same as that of the solvent nor that of the solutes. In intermetallic compounds, different elements are ordered into different sites in the structure, with distinct local environments and often a well-defined and fixed stoichiometry. Complex structures with very large unit cells can be formed in intermetallic compounds[2]. Intermetallic compounds are generally hard and brittle, and they have high melting points. They also present desirable magnetic, superconducting and chemical properties,due to their strong internal order and mixed(metallic and covalent/ionic) atomic bonding.Intermetallic compounds have found applications in various novel materials. For example, Ni3Al is the hardening phase in the nickel-base superalloys, and TiAl has been used in recent turbine blade applications; SmCo5is an outstanding permanent magnetic material and LaNi5has good hydrogen storage properties[3]. Both the solid solution and intermetallic compound are crystalline alloys, in which the atoms are arranged in the so-called lattice exhibiting long range order and symmetry. On the contrast, an amorphous alloy has a disordered and non-crystalline atomic-scale structure; as a result, it is also known as the metallic glass. Actually, the English word amorphous comes from the Greekamorphos(shapeless), froma- (without) +morphē(form). The amorphous alloys without a crystalline form have many unique properties that are superior to their crystalline counterparts, including high strength, high elastic strain and good corrosion resistance. More detailed introduction to the amorphous alloys is to be covered in Section 2.
Commonly used metallic alloys are mostly multi-component alloys,i.e., having more than three constituent elements. Alloying in the multicomponent alloys can be an extremely complicated issue, as there exist so many possibilities of the alloyed products depending on the alloy compositions, and this is certainly beyond the capability of phase diagrams, which vividly tell the formed phases at particular compositions but are only valid for limited binary and ternary alloy systems[4]. The phase diagrams are still of help if there are less than three major elements in the alloys, as other minor alloying elements are less likely to alter the phase constitution significantly.Indeed, most conventional alloys are based on one element, like Fe, Al, Mg, Cu, Ti, Ni, or two elements, like FeAl or TiAl. As a result, the Fe-C binary phase diagram can still be used as a guidance to study steels although there exist many more alloying elements in steels apart from Fe and C. The situation changes dramatically in high entropy alloys (HEAs), a recently emerged multicomponent alloys where the alloying elements are mixed in equiatomic ratio or close-to-equiatomic ratio[1,5-6]. In HEAs, there is no well-defined principle element and there is no clue to what the alloyed products can be. In addition, phase diagrams give the phase information in the equilibrium conditions, and although nonequilibrium conditions can now be taken into account, they cannot be used directly to guide the design of amorphous alloys. Admittedly, the eutectic compositions[5]or sometimes intermetallic compound compositions[6]have been suggested to be indicators of good glass formers, and phase diagrams can help in these conditions.
Framing out workable rules to predict the phase selection in multi-component alloys upon alloying is a daunting task, considering the complexity of the possible alloyed products as briefly mentioned above. However, the vision of possessing the capability to predict the phase selection in complex multi-component alloys through simple compositional control, is tremendously attractive as it will greatly simplify the alloy design. This work aims at sheding some light on this important issue, by narrowing down the scope of studied alloy systems and defining the phase selection in a broader sense. Specifically,inspired by HEAs, only multi-component alloys with equiatomic or close-to-equiatomic compositions are considered. In equiatomic alloys the relative composition of each element is not a concern as in conventional multi-component alloys,which on one hand makes the target alloy systems(at least apparently) simple, but on the other hand means none of the alloying elements is playing a negligible role. Since equiatomic multi-component alloys are still a novel metallurgy concept and discussions on the phase selection issue in this new alloy system are few[7-10], it justifies an informative discussion here. By saying to define the phase selection in a broader sense, we mean to differentiate here only the amorphous phase, solid solution and intermetallic compound, and do not further differentiate different types of solid solutions or different intermetallic compounds. To make a fair comparison, all the alloying covered here follows the liquid-solid route, using the copper mould casting or melt spinning methods. The statistical analysis of the phase selection in multicomponent alloys with equiatomic or close-toequiatomic compositions comes after a brief introduction to two intensively studied and both scientifically and technically important multicomponent alloys: bulk metallic glasses (BMGs)and high entropy alloys (HEAs). Understanding the phase selection is of great importance to the future development of the BMGs and HEAs.
2 Bulk metallic glasses (BMGs)
In the 1960s, amorphous alloys, or more commonly referred to as metallic glasses, were discovered by Duwezet al. unintentionally[11]as they initially aimed at testing the solid solubility of two components that normally cannot form a solid solution under the rapid cooling condition. In the early time of the development of metallic glasses,the amorphous phase could only be achieved with high cooling rates of 103~106K·s-1. Although bulk metallic glasses (BMGs), with the smallest dimension over 1 mm were reported at some noble-metal based alloys like Pd-Cu-Si and Pd-Ni-P with low cooling rates of ~10 K·s-1, the high cost of these alloys did not generate a general interest toward them in the materials community[12]. The discovery of multi-component BMGs with low critical cooling rates (the lowest cooling rates to achieve the fully amorphous condition) by Inoue and his colleagues in the late 1980s marked the second milestone in the history of the metallic glasses[13-14]. Noble metals were not a must in these multi-component BMGs. Since then, a large amount of BMGs have been developed in various alloy systems[15]and as seen in Fig. 1, the largest BMGs up to today have a diameter of 80 mm and a length of 85 mm in the Pd-Cu-Ni-P system, made by a water quenching method[16].
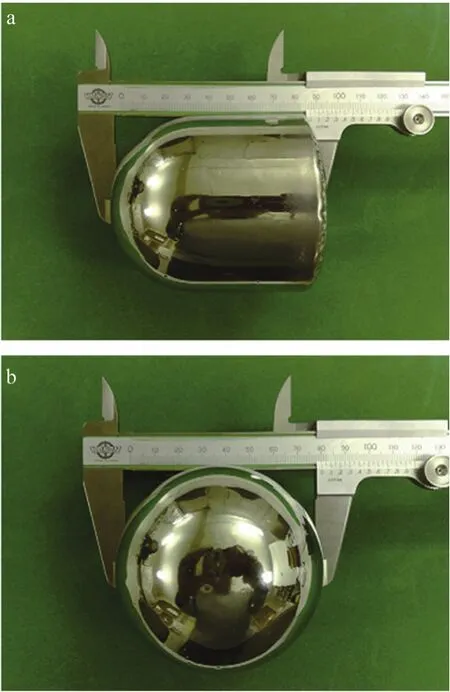
Fig. 1 (a) and (b) side and top views of the world’s largest glassy Pd42.5Cu30Ni7.5P20 alloy, with a cylindrical form of 80 mm in diameter and 85 mm in length[16]
BMGs have been intensively studied over the past two decades, as they present many unique mechanical and functional properties that are absent in crystalline alloys[15,17-21]. BMGs have a high hardness and high fracture strength, and noticeably the Co-based BMGs can have a hardness of 17.1 GPa[22]and compressive fracture strength of 6 GPa;they have a large elastic strain of ~2%, compared with ~0.2% for conventional crystalline alloys[21];their fracture toughness can reach 200 MPa·m1/2[23];they have good soft magnetic properties[24],corrosion resistance[25-26]and wear resistance[17];they have excellent flowability and accurate mould formability in the supercooled liquid region (i.e.,between the glass transition temperature and the crystallization temperature[27]). Although the ductility,especially the tension ductility is still restricting their structural applications, BMGs have found many unique applications such as in kinetic energy penetrators (KEP)[28], biodegradable implants[29],micro fuel cell[30], solar wind collector[18,31], surgical instruments[32]and micro- gear[33-34].
3 High entropy alloys (HEAs)
HEAs emerge as a new type of advanced metallic materials and have received increasing attentions from the materials community. The concept of HEAs was initiated by Yeh and his colleagues in the mid 1990s[1,35], and basically HEAs are defined as multi-component alloys having at least 5 principle elements and all the principle elements are mixed in equiatomic or close-to-equiatomic ratios[36]. HEAs micro-alloyed with some minor elements can still be termed as HEAs[36-37]and this makes no conflict with the atomic percentage requirements for the principle elements.
As mentioned in the Introduction, HEAs differ with conventional multi-component alloys in which there is no dominant principle element in HEAs.This metallurgy concept is a brand new one, as in the past people thought such a high percentage of alloying elements would lead to the formation of many unwanted phases, particularly the hard intermetallic compounds, and hence embrittle the material. Surprisingly, in many HEAs only simple solid solutions with fcc and/or bcc structures(schematically shown in Fig. 2) are obtained and no intermetallic compounds form at all[7,36,38].
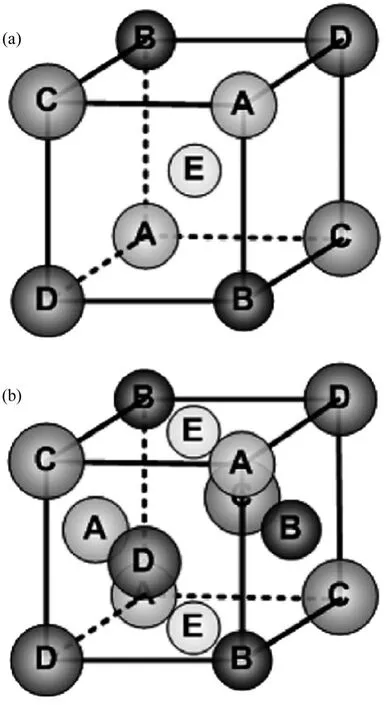
Fig. 2 Schematic illustrations of crystalline structures of (a)bcc and (b) fcc solid solutions composed of five multi-principal elements[60]
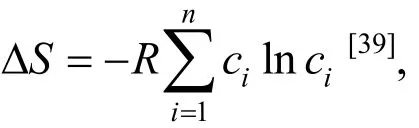
HEAs present some excellent mechanical and physical properties[36]. Their hardness can range from 100 to 1 100 HV; their microstructures can have good thermal stability; they have excellent resistance to temper softening; they can presenthigh-temperature precipitation hardening; they can have excellent corrosion, wear and oxidation resistance; they can have high electrical resistivity with a low or even negative temperature coefficient.To give two specified examples here, a bcc typed AlCoCrFeNiTi0.5(for the expression of the compositions of HEAs, the subscripts indicate its atomic portion and is 1 if not particularly indicated.In this case, Al, Co, Cr, Fe and Ni each accounts for 1/5.5 and Ti accounts for 0.5/5.5 of the constituent elements) alloy has the yield stress, fracture strength, and compressive plastic strain of 2.26 GPa,3.14 GPa, and 23.3%, respectively[40], which are superior to most of the high-strength alloys such as bulk metallic glasses; two refractory high entropy alloys NbMoTaW and VNbMoTaW have a singlephase bcc structure that remains not only stable after exposure to 1 400℃, but also disordered[41],and more interestingly, they still keep a yield strength of 407 MPa and 477 MPa, respectively at 1600℃, much higher than those of the Ni-base superalloys at the same temperature[41]. HEAs have great potentials to be used as high temperature materials, coating materials requiring high hardness and high wear resistance, and corrosion resistant materials with high strength. Some unique applications include the diffusion barrier material between copper interconnects and the Si substrate[42].

Table 1 Comparison of the configuration entropy, ΔS, between HEAs and some conventional multi- component alloys
4 Phase selection in multicomponent alloys with equiatomic or close-to-equiatomic compositions
4.1 High entropy effect
The formation of simple solid solution phases and absence of intermetallic compounds in the seemly complex multi-component alloys with equiatomic or close-to-equiatomic compositions, is somewhat unexpected. The phase selection is essentially determined by the Gibbs energies of the competing phases and intuitively the preferred formation of the solid solution phases can be related to the high configuration entropy of HEAs, as at high temperatures the contribution from the entropy to the Gibbs energy becomes significant. However,such a qualitative understanding does not help to predict the phase selection. In terms of the role of the entropy on the formed phases upon alloying,there is a well-known confusion principle[10]which articulates that the more alloying elements are involved, the lower the chance that the alloy can form well defined crystal structures. The confusion principle works well to explain why BMGs are mostly discovered in multi-component alloys with more than three alloying elements, and it can also be used to explain the formation of disordered solid solution in HEAs. From the perspective of the disorder, disordered solid solutions are chemically disordered and topologically ordered, while the amorphous phases are at least topologically disordered (in the long range). Naturally, the confusion principle raises a question: when the entropy is high, is the formation of the random solid solution or amorphous phase preferred? As a matter of fact, in many HEAs solid solutions tend to form,but in some other HEAs the amorphous phase does form, and even BMGs can form[43-47]. For example,a PdPtCuNiP BMG with a maximum diameter of 10 mm was fabricated by the fluxed water quenching technique[47], as shown in Fig. 3. These BMGs with high entropy are termed as HE-BMGs.In addition, the high entropy does not necessarily lead to the formation of the amorphous phase or disordered solid solutions, as intermetallic compounds or ordered solid solutions have been widely observed in multi-component alloys with equiatomic or close-to-equiatomic compositions[7-9].The available experimental evidences clearly suggest that apart from the entropy effect, there are other factors controlling the phase selection. It is thus the target of this paper to identify those factors playing the decisive roles.

Fig. 3 Pd20Pt20Cu20Ni20P20 alloy cylinders with diameters of(a) 12 mm and (b) 10 mm prepared by water quenching and a B2O3 flux treatment; (c) X-ray diffraction patterns for the two alloys, showing a fully amorphous state for the 10 mm sized one[47]
4.2 A parametric approach
Considering the complexity of the phase selection issue in multi-component alloys, physical metallurgy (semi-empirical) rules rather than strictly robust scientific principles are more likely to act as an effective guidance. Historically, it is also these physical metallurgy rules that lead to the development of many new alloy systems. They are not always right, but they work. In the context of this paper, two well known physical metallurgy rules give some clues to the decisive factors controlling the phase selection in multi-component alloys.
First, the Hume-Rothery rules[48-49]on the formation of binary solid solutions. Hume-Rothery himself listed five factors affecting the stability of alloy phases: electronegativity, atomic size factor,electron concentration, position of the elements in the periodical table, and the orbital type[49-50], and among them the first three factors are more widely recognized. The electron concentration effect is evident when the electronegativity and the size factor are of minor importance[50]. Also, the famous Darken-Garry plot[48]also utilizes only the electronegativity and the size factor to consider the solid solubility issue. Basically, both the Hume-Rothery rules and the Darken-Garry plot claim that, in order to have a large solubility of element A in element B, these two elements shall have a small difference in the electronegativity and atomic size.
Second, Inoue’s three empirical rules to form the bulk metallic glasses[13]. As mentioned in Section 2, it is the feasibility of fabricating BMGs using cost effective elements that actually ignited the frenzy of massive research activities towards BMGs. According to Inoue, to form BMGs the alloy compositions shall have more than three elements, the atomic size difference among the three main elements (A, B, C) needs to be large enough (>12%), and the mixing enthalpies between the main elements (A-B, A-C, B-C) have to be very negative. A similar principle has also been proposed by Johnson[51].
Hume-Rothery rules and Inoue’s rules seem to suggest that three factors are controlling the phase selection: (1) topology, mainly the atomic size; (2)chemistry, the electronegativity, electron concentration or the mixing enthalpy; and (3)complexity, simply put the genre and amount of the constitution elements. We then attempt to use three parameters characteristic of these three controlling factors to search for the phase selection rules, based on a statistical analysis of the available experimental evidences in multi-component alloys with equiatomic or close-to-equiatomic compositions.
The atomic size difference is a simple and effective parameter to reflect the topological factor,and it appears in both the Hume-Rothery rules and Inoue’s rules. For the multi-component alloys, the atomic size polydispersity,δ, can be a good parameter to represent the atomic size difference among the constituent elements[7-8,52]. It is defined as



It is emphasized here again that the configuration entropy defined here assumes the alloys being at the fully random state and ignores the actual atomic site occupancy in the specified phase (solid solution, the amorphous phase or intermetallic compound). As the target of this work is multi-component alloys with equiatomic or close-to-equiatomic compositions, differences inmixSΔamong the analyzed alloys (all with highmixSΔ) are small[7]and hencemixSΔis not considered here. However,the highmixSΔ should be the necessary though not the sufficient condition, for the solid solution to be the preferred phase in some alloy systems[36,55].In addition, it has been shown thatmixSΔ has a close correlation to the glass forming ability (how easy to form the BMGs) and a highermixSΔ generally favors the easy glass formation[7]. This is in line with Inoue’s rules on the complexity of the alloying elements to form BMGs. We then evaluate howδandmixHΔ can act as controlling factors for the phase selection in multi-component alloys with equiatomic or close-to-equiatomic compositions. The selection of these two parameters is also based on a statistical analysis[7],from which we identified the key factors that control the phase selection in multi-component alloys with equiatomic or close-to-equiatomic compositions.
4.3 Statistical analysis
Figure 4 shows the result from the statistical analysis of the available experimental evidences on the phase selection in multi-component alloys with equiatomic or close-to-equiatomic compositions,using the two parameters, a topology naturedδand a chemistry naturedmixHΔ (source data given in Ref. [56]). It is surprising to see that the phase selection can be very reasonably delineated by these two parameters alone. Basically, solid solutions can form whenδis small (δ<~0.066),andmixHΔis either slightly positive or insignificantly negative (~ −11.6 kJ·mol-1<mixHΔ<3.2 kJ·mol-1); exactly contrary to these requirements, the amorphous phase can form whenδis large (δ>0.064), andmixHΔ is noticeably negative (mixHΔ< −12.2 kJ·mol-1); interestingly,intermetallic compounds can form in the intermediate conditions in terms ofδandmixHΔ.It is noted here that the intermetallic compound forming region does not indicate that intermetallic compounds are the sole alloyed products, but rather refer to the condition where the amount of intermetallic compounds is sufficient to be detected by the X-ray diffraction.

Fig. 4 A δ -ΔHmix plot delineating the formation conditions for solid solutions, the amorphous phase and intermetallic compounds. The shadowed blocks indicate the crossover values on δ and ΔHmix, where the formation of both the solid solution and amorphous phase is possible. The olive dash-dotted ellipse indicates the intermetallic compound forming region defined by δ and ΔHmix
The atomic size difference,δ, apparently plays a critical role determining the phase selection. Solid solutions forming at smallδin the multicomponent alloys can be seen as an extension of the Hume-Rothery rules on the solid solubility of binary solid solutions. The necessity of a largeδto form the amorphous shall originate from the requirement on the sufficient atomic-level stress to destabilize the solid solution phase[57-58]. According to Egami[57], the amorphous phase is stabilized partly because the solid solution phase of the corresponding composition is topologically unstable, which explains why the formation of the solid solution phase and amorphous phase has an opposite requirement from the topology perspective.Previous studies also suggest that a largeδis required to form BMGs, for non-equiatomic multi-component alloys[7-9]. In other words,δis related not only to the glass formation, but also to the glass forming ability.
The requirement onmixHΔ to form the solid solution phase is also contrary to that of the amorphous phase. A smallmixHΔ favoring the solid solution is still in line with the Hume-Rothery rules in that elements with similar chemistry natured properties have larger solid solubility.However, a largemixHΔ favoring the amorphous phase rather than the intermetallic compound, is surprising at the first sight. On the one hand, it is noted here that the mixing enthalpy defined in this work is the weight averaged mixing enthalpies of different pairs of alloying elements, and the large mixing enthalpy of an individual pair is hence not sensitively reflected by this averaged parameter. A large mixing enthalpy of an individual pair still promotes the intermetallic compound formation,and this happens to explain the competition between the amorphous phase and intermetallic compounds in the intermediate conditions in terms ofmixHΔ. Our experimental results (see Ref. [56])have proved that, in cases intermetallic compounds inhibit the fully amorphorization, the intermetallic compounds are formed between those elements with very negativemixHΔ, and further the fully amorphorization can be achieved by avoiding the formation of these unwanted intermetallic compounds through the compositional adjustment.For example, the melt-spun CoCrCuFeNiZr was mainly amorphous, but intermetallic compounds ZrX5(X=Cu, Co or Ni) also formed[56], since Zr-Cu,Zr-Co and Zr-Ni have the mixing enthalpy of −23,−41 and −49 kJ·mol-1, respectively, much more negative compared to the mixing enthalpy of other combinations of elements[54]. Simply reducing the Zr concentration in this alloy and making it to a composition of CoCrCuFeNiZr0.6, the fully amorphous phase was obtained[56]. On the other hand, the formation of intermetallic compounds or the amorphous phase is determined simultaneously by ΔSmix(actually T·ΔSmix) and ΔHmix. In the case of forming intermetallic compounds, the more negative ΔHmixdominates as ΔSmixis negligible (note that ΔSmixhere apparently cannot assume a fully random state of the atomic distribution); ΔSmixplays an important role when forming the amorphous phase and in cases when ΔSmixis high and at the same time ΔHmixis very negative, the stabilization of the amorphous phase is possible. The very negative ΔHmixmight also favor the formation of atomic clusters that stabilize the amorphous phase[59]. The revealing of intermediate conditions where the amorphous phase and intermetallic compounds can compete, and furthermore the proposing of convenient solutions to avoid the formation of unwanted intermetallic compounds, are thus of significant importance and provide new perspectives to design amorphous alloys.
5 Conclusions
(1) The phase selection in the seemingly complex multi-component alloys with equiatomic or close-to-equiatomic compositions, can reasonably predicted using a parametric approach based on two parameters: the atomic size polydispersity parameter,δ, and the mixing enthalpy, ΔHmix.
(2) When the mixing entropies are comparable,solid solutions can form whenδis small and ΔHmixis either slightly positive or insignificantly negative; in the contrary, the amorphous phase is stable whenδis large and ΔHmixis significantly negative.
(3) Intermetallic compounds can be formed in the intermediate condition in terms ofδand ΔHmix. The formation of intermetallic compounds or the amorphous phase is dependent on the competition betweenmixSΔ andmixHΔ.Intermetallic compounds are formed whenmixHΔ dominates, while the amorphous phase can be stabilized whenmixSΔ is high and at the same timemixHΔ is very negative. In cases when intermetallic compounds compete with the amorphous phase, the full amorphorization can be achieved via the compositional adjustment to avoid the formation of intermetallic compounds.
(4) The phase selection rules proposed in this work reveals a clear physical scenario that is not seen previously, and it will help to simplify the alloy design of multi-component alloys, and benefit the future development of advanced metallic alloys,such as BMGs, HEAs, BMG composites, and intermetallic compound strengthened high temperature alloys.
Acknowledgements This work is financially supported by the Research Grant Council (RGC), the Hong Kong Government, through the General Research Fund (GRF) under the project number CityU/521411, with City University of Hong Kong.
(2013年1月16日收稿)
[1]RANGANATHAN S. Alloyed pleasures: multimetallic cocktails [J].Current Science, 2003, 85: 1404.
[2]WESTBROOK J H, FLEISCHER R L. Crystal structures of intermetallic compounds [M]. Chichester: John Wiley & Sons, 2000.
[3]BUSCHOW K H J. Intermetallic compounds of rare-earth and 3d transition metals [J]. Reports on Progress in Physics, 1977, 40: 1179.
[4]BAKER H, OKAMOTO H. ASM handbook volume 3: alloy phase diagrams [M]. Ohio: American Society for Metals, 1992.
[5]COHEN M H, TURNBULL D. Composition requirements for glass formation in metallic and ionic systems [J]. Nature, 1961, 189: 131.
[6]WU W F, LI Y. Bulk metallic glass formation near intermetallic composition through liquid quenching [J]. Applied Physics Letters,2009, 95: 011906.
[7]GUO S, LIU C T. Phase stability in high entropy alloys: formation of solid-solution phase or amorphous phase [J]. Progress in Natural Science: Materials International, 2011, 21:433.
[8]ZHANG Y, ZHOU Y J, LIN J P, et al. Solid-solution phase formation rules for multi-component alloys [J]. Advanced Engineering Materials, 2008, 10: 534.
[9]YANG X, ZHANG Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys [J]. Materials Chemistry and Physics, 2012, 132: 233.
[10]GREER A L. Confusion by design [J]. Nature, 1993, 366: 303.
[11]KLEMENT W, WILLENS R H, DUWEZ P. Non-crystalline structure in solidified gold-silicon alloys [J]. Nature, 1960, 187: 869.
[12]CHEN M W. A brief overview of bulk metallic glasses [J]. NPG Asia Materials, 2011, 3: 82.
[13]INOUE A. Stabilization of metallic supercooled liquid and bulk amorphous alloys [J]. Acta Materialia, 2000, 48: 279.
[14]INOUE A, ZHANG T, MASUMOTO T. Zr-Al-Ni amorphous alloys with high glass transition temperature and significant supercooled liquid region [J]. Materials Transactions, 1990, 31: 177.
[15]INOUE A, TAKEUCHI A. Recent development and application products of bulk glassy alloys [J]. Acta Materialia, 2011, 59: 2243.
[16]NISHIYAMA N, TAKENAKA K, MIURA H, et al. The world’s biggest glassy alloy ever made [J]. Intermetallics, 2012, 30: 19.
[17]TREXLER M M, THADHANI N N. Mechanical properties of bulk metallic glasses [J]. Progress in Materials Science, 2010, 55: 759.
[18]AXINTE E. Metallic glasses from “alchemy” to pure science: present and future of design, processing and applications of glassy metals [J].Materials & Design, 2012, 35: 518.
[19]CHEN M W. Mechanical behavior of metallic glasses: microscopic understanding of strength and ductility [J]. Annual Review of Materials Research, 2008, 38: 445.
[20]ASHBY M F, GREER A L. Metallic glasses as structural materials[J]. Scripta Materialia, 2006, 54: 321.
[21]YAVARI A R, LEWANDOWSKI J J, ECKERT J. Mechanical properties of bulk metallic glasses [J]. MRS Bulletin, 2007, 32: 635.
[22]WANG J F, LI R, XIAO R J, et al. Compressibility and hardness of Co-based bulk metallic glass: a combined experimental and density functional theory study [J]. Applied Physics Letters, 2011, 99:151911.
[23]DEMETRIOU M D, LAUNEY M E, GARRETT G, et al. A damage-tolerant glass [J]. Nature Materials, 2011, 10: 123.
[24]SHEN T D, SCHWARZ R B. Bulk ferromagnetic glasses prepared by flux melting and water quenching [J]. Applied Physics Letters, 1999,75:49.
[25]PANG S J, ZHANG T, ASAMI K, et al. Synthesis of Fe-Cr-Mo-C-B-P bulk metallic glasses with high corrosion resistance[J]. Acta Materialia, 2002, 50: 489.
[26]SCULLY J R, GEBERT A, PAYER J H. Corrosion and related mechanical properties of bulk metallic glasses [J]. Journal of Materials Research, 2007, 22: 302.
[27]SCHROERS J. Processing of bulk metallic glass [J]. Advanced Materials, 2010, 22: 1566.
[28]AXINTE E M, CHIRILEANU M P I. Recent progress in the industrialization of metallic glasses [J]. Recent Patents on Materials Science, 2012, 5: 213.
[29]ZBERG B, UGGOWITZER P J, LOFFLER J F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants [J]. Nature Materials, 2009, 8: 887.
[30]SEKOL R C, KUMAR G, CARMO M, et al. Bulk metallic glass micro fuel cell [J]. Small, 2012. doi: 10.1002/smll.201201647.
[31]GRIMBERG A, BAUR H, BOCHSLER P, et al. Solar wind neon from genesis: implications for the lunar noble gas record [J]. Science,2006, 314: 1133.
[32]HUANG J C, CHU J P, JANG J S C. Recent progress in metallic glasses in Taiwan [J]. Intermetallics, 2009, 17:973.
[33]NGUYEN V B, PARK K Y, NA Y S, et al. Micro-forming characteristics of a Zr-based amorphous alloy with a gear-like mold[J]. Metals and Materials International, 2007, 13: 433.
[34]NISHIYAMA N, AMIYA K, INOUE A. Bulk metallic glasses for industrial products [J]. Materials Transactions, 2003, 45: 1245.
[35]YEH J W, CHEN S K, LIN S J, et al. Nanostructured high-entropy alloys with multiple principal elements: novel alloy design concepts and outcomes [J]. Advanced Engineering Materials, 2004, 6: 299.
[36]YEH J W, CHEN Y L, LIN S J, et al. High-entropy alloys - a new era of exploitation [J]. Materials Science Forum, 2007, 560: 1.
[37]LI C, LI J C, ZHAO M, et al. Microstructure and properties of AlTiNiMnBxhigh entropy alloys [J]. Materials Science and Technology, 2008, 24: 376.
[38]GUO S, NG C, LU J, et al. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys [J]. Journal of Applied Physics, 2011, 109: 103505.
[39]SWALIN R A. Thermodynamics of solids [M]. 2nd ed. New York:Wiley, 1991.
[40]ZHOU Y J, ZHANG Y, WANG Y L, et al. Solid solution alloys of AlCoCrFeNiTixwith excellent room-temperature mechanical properties [J]. Applied Physics Letters, 2007, 90: 181904.
[41]SENKOV O N, WILKS G B, SCOTT J M, et al. Mechanical properties of Nb25Mo25Ta25W25and V20Nb20Mo20Ta20W20refractory high entropy alloys [J]. Intermetallics, 2011, 19: 698.
[42]CHANG S Y, CHEN D S. 10-nm-thick quinary (AlCrTaTiZr)N film as effective diffusion barrier for Cu interconnects at 900℃ [J].Applied Physics Letters, 2009, 94: 231909.
[43]GAO X Q, ZHAO K, KE H B, et al. High mixing entropy bulk metallic glasses [J]. Journal of Non-Crystalline Solids, 2011, 357:3557.
[44]MA L Q, WANG L M, ZHANG T, et al. Bulk glass formation of Ti-Zr-Hf-Cu-M (M=Fe, Co, Ni) alloys [J]. Materials Transactions,2002, 43: 277.
[45]CUNLIFFE A, PLUMMER J D, FIGUEROA I, et al. Glass formation in a high entropy alloy system by design [J]. Intermetallics,2012, 23: 204.
[46]ZHAO K, XIA X X, BAI H Y, et al. Room temperature homogeneous flow in a bulk metallic glass with low glass transition temperature [J].Applied Physics Letters, 2011, 98: 141913.
[47]TAKEUCHI A, CHEN N, WADA T, et al. Pd20Pt20Cu20Ni20P20high-entropy alloy as a bulk metallic glass in the centimeter [J].Intermetallics, 2011, 19: 1546.
[48]CAHN R W, HASSEN P. Physical metallurgy [M]. 4th ed. vol.1.Amsterdam: North Holland, 1996.
[49]RUDMAN P S, STRINGER J, JAFFEE R I. Phase stability in metals and alloys [M]. New York: McGraw Hill, 1967.
[50]MIZUTANI U. Hume-Rothery rules for structurally complex alloy phases [M]. Boca Raton: CRC, 2011.
[51]JOHNSON W L. Bulk glass-forming metallic alloys: science and technology [J]. MRS Bulletin, 1999, 24: 42.
[52]MORIGUCHI I, KAWASAKI K, KAWAKATSU T. The effects of size polydispersity in nearly hard-sphere colloids [J]. Journal De Physique II, 1993, 3: 1179.
[53]BOER F R, BOOM R, MATTENS W C M, et al. Cohesion in metals:transition metal alloys [M]. Amsterdam: North-Holland, 1988.
[54]TAKEUCHI A, INOUE A. Calculations of mixing enthalpy and mismatch entropy for ternary amorphous alloys [J]. Materials Transactions, 2000, 41: 1372.
[55]NG C, GUO S, LUAN J H, et al. Entropy-driven phase stability and slow diffusion kinetics in Al0.5CoCrCuFeNi high entropy alloy [J].Intermetallics, 2012, 31: 165.
[56]GUO S, HU Q, NG C, et al. More than entropy in high-entropy alloys: forming solid solutions or amorphous phase [J]. submitted.
[57]EGAMI T, WASEDA Y. Atomic size effect on the formability of metallic glasses [J]. Journal of Non-Crystalline Solids, 1984, 64: 113.
[58]EGAMI T, LEVASHOV V, AGA R, et al. Geometrical frustration and glass formation [J]. Metallurgical and Materials Transactions A, 2008,39: 1786.
[59]SHENG H W, LUO W K, ALAMGIR F M, et al. Atomic packing and short-to-medium-range order in metallic glasses [J]. Nature, 2006,439: 419.
[60]YEH J W, CHEN S K, GAN J Y, et al. Formation of simple crystal structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V alloys with multiprincipal metallic elements [J]. Metallurgical and Materials Transactions A,2004, 35: 2533.
10.3969/j.issn.0253-9608.2013.02.002
(编辑:方守狮)
——以“办部书现象”为例》收稿

