Synthesis,Crystal Structure and Spectroscopic Properties of 2,7-bis(3-cyanophenyl)-9,9-diethyl-fluorene
LIU Zhi-peng,LI Xing,JIANG Jing,ZHAO Ya-yun,ZHAO Xiu-hua,CHEN Hong-bing
(The State Key Laboratory Base of Novel Functional Materials and Preparation Science,Faculty of Materials Science and Chemical Engineering,Ningbo University,Ningbo 315211,China)
1 Introduction
Organic light-emitting diodes(OLEDs)have high potential as the most ideal and potential display technology in the 21st century[1],due to many advantages,e.g.,self-emission,fast response,full solid device,full-color,easy fabrication,high efficiency,wide view-angle,ultrathin thickness.To realize full-colordisplays, high performance red,green,and blue light-emitting materials are required.In contrast to red and green emitters,only a few blue emitters show application potential but much inferior performance in efficiency,stability and color purity.The highly efficient blue light-emitting materials can be used as not only emissive layer in OLEDs,but also host materials for efficient blue and white light source.Among the promising blue emitters,fluorene and its derivatives have drawn much attention from materials chemists and device physicists because of wide energy gap and high luminescent efficiency[2-4].However,a major problem in using these kinds of materials in OLEDs is the undesirable lower energy emission band,which changes the pure blue emission into blue-green color for the form of excimer.In order to improve this problem,covalently incorporating electron-donatingand electron-withdrawing groups onto the polymer backbone is an effective way to enhance the charge injection/transport and suppress aggregation/excimer formation[5-10].
Herein,we report the synthesis(Fig.1)and X-ray crystal structure determination of the title compound.At the same time,the UV-Vis absorption and fluorescence spectra of this compound in CH2Cl2solution as well as in film are also discussed.

Fig.1 Synthesis of the title compound
2 Experiments
2.1 Reagents and Instruments
All reagents were analytically pure grade and were used without further purification.Elemental analyses were obtained on a Perkin-Elmer 240C elemental analyzer.IR spectra were recorded on a Nicolet FT-IR 460 spectrophotometer in KBr pellet.1H NMR spectra were gotten on a Bruker Avance 400 spectrometer with TMS as an internal standard in CDCl3.Crystal structure determination was carried out on a Bruker Smart APEX-2 CCD diffractometer.UV-Vis spectra were taken on a UV-250IPC spectrometer.Fluorescence spectra were recorded on a Hitachi F-4600 fluorescence spectrophotometer.
2.2 Synthesis of The Title Compound
We refer to the relevant literature about the method of synthesis of 2,7-dibromo-9,9-diethyl-fluorene[11].
A mixture of 2,7-dibromo-9,9-diethyl-fluorene(2 mmol,0.76 g),3-cyanophenylboronic acid(5 mmol,0.70 g),K2CO3(10 mmol,1.40 g)and Pd(OAc)2(0.1 mmol,0.024 g)in i-PrOH(50 mL)and H2O(10 mL)was stirred at 85℃under air for 24 h.The reaction mixture was monitored by TLC.After completion of the reaction,the mixture was extracted with CH2Cl2(3 ×20 mL).The organic layers were combined,washed with brine(50 mL),dried over MgSO4,and concentrated under reduced pressure to give the crude product.The crude product was purified by column chromatography on silica(petroleum ether/methylene dichloride=6/1,V/V),which afforded the product as white powder(0.36 g,yield:42%).Anal.Calcd(%)for C31H24N2(Mr=424.52):C,87.74;H,5.66;N,6.60.Found(%):C,87.70;H,5.65;N,6.65.IR(KBr,ν/cm-1):474(w),602(w),692(m),764(w),793(vs),818(s),888(m),117 7(w),125 2(m),141 0(m),143 3(m),146 5(m),157 7(m),159 9(m),222 5(vs),287 1(w),292 0(s),295 9(s),303 3(w),306 9(w).1H NMR(CDCl3,400 MHz)δ:0.384 ~ 0.420(t,J=7.2,7.2 Hz,6H,hydrogen of methyl),2.114 ~2.169(dd,J=7.2,7.2 Hz,4H,hydrogen of methylene),7.537 ~ 7.540(d,J=1.2 Hz,2H,hydrogen of phenyl),7.570 ~ 7.579(t,J=2,1.6 Hz,2H,hydrogen of phenyl),7.590 ~ 7.598(t,J=1.6,1.6 Hz,2H,hydrogen of phenyl),7.637 ~7.663(m,2H,hydrogen of phenyl),7.827 ~7.847(d,J=8 Hz,2H,hydrogen of phenyl),7.891 ~7.918(m,2H,hydrogen of phenyl),7.952 ~7.960(t,J=1.6,1.6 Hz,2H,hydrogen of phenyl).The powder was dissolved in the mixture of methanol and methylene dichloride(methanol/methylene dichloride=1/1,V/V),and slow evaporation of the solvent yielded colorless single crystals after about 7 d.
2.3 Structure Determination
A colorless crystal with dimensions of 0.46 mm ×0.45 mm × 0.10 mm was selected for X-ray analyses.All X-ray data were collected at 293(2)K on a Bruker Smart APEX-2CCD diffractometer equipped with a graphite-monochromatized Mo-Kα radiation(λ =0.071 073 nm)by using a φ-ω scan mode in the range of 2.95°≤ θ≤ 27.53°.A total of 154 71 reflections including 264 2 unique ones(Rint=0.077 7)were collected,of which 262 5 were considered to be observed(I>2σ(I))and used in the succeeding refinement.Absorption correction based in Multi-scan method was applied[12].The structure was solved by direct methods and refined by full-matrix least-squares techniques with SHELXL-97[13].The non-hydrogen atoms were refined anisotropically,and the hydrogen atoms were introduced geometrically.The final refinement converged to R=0.046 9,wR=0.104 2.(w=1/[σ2(Fo2)+(0.052 5 P)2+0.644 1 P],P=(Fo2+2Fc2)/3),(Δ/σ)max=0.000,S=1.013,(Δρ)max=0.284 and(Δρ)min=-173 e/nm3.
3 Results and Discussion
3.1 Optimization of the Reaction Conditions
To find the optimum solvent,the mixture of 2,7-dibromo-9,9-diethyl-fluorene(2 mmol),3-cyanophenylboronic acid(5 mmol),K2CO3(10 mmol)and Pd(OAc)2(0.1 mmol)was carried out in the DMF/H2O,i-PrOH/H2O,EtOH/H2O,Glycol/H2O and CH3OH/H2O at 85℃ under the air.In those reactions,we found that solvents had significant effects on reaction yields.Only 28% yield of the target product was observed when the mixture was stirred under similar conditions in DMF/H2O(Table 1,entry 1).The reactions in i-PrOH/H2O,EtOH/H2O,Glycol/H2O and CH3OH/H2O all gave moderate yields.As shown in Table 1,the results indicated that the mixture solution of i-PrOH/H2O was a more suitable solvent for the reaction system(Table 1,entries 2,3,4,5).The further studies were carried out by optimizing the conditions of the Suzuki coupling reaction in terms of the palladium species.The mixture of 2,7-dibromo-9,9-diethyl-fluorene(2 mmol),3-cyanophenylboronic acid(5 mmol)and K2CO3(10 mmol)was performed in i-PrOH/H2O and EtOH/H2O with PdCl2as the catalyst(Table 1,entries 6,7).
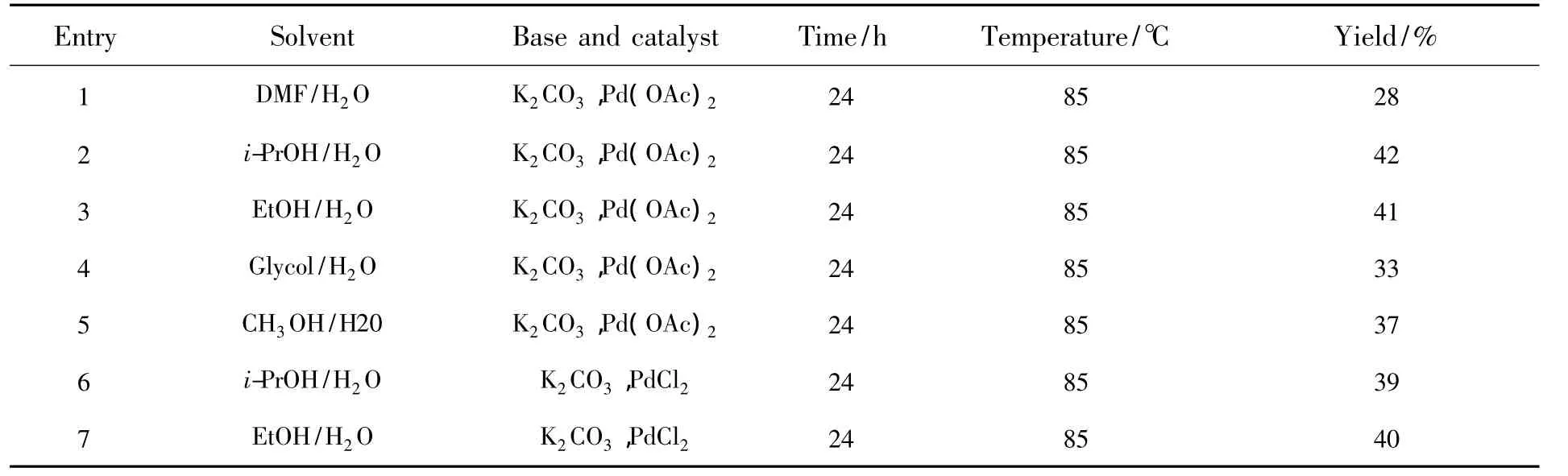
Table 1 Optimization of the reaction conditions
3.2 Structural Description
The selected bond lengths and bond angles of the title compound are listed in Table 2.The molecular structure and crystal packing diagram are shown in Fig.2 and Fig.3,respectively.From Fig.2 we can see that the molecule of title compound is an axis symmetric molecule.There are four planes in the crystal structure.Among them,the angle between plane C1-C2-C3-C4-C5-C6andplaneC12-C13-C14-C16-C17-C18 is 36.080°,while that between plane C1-C2-C3-C4-C5-C6 and plane C1A-C2AC3A-C4A-C5A-C6A is 0.676°.From Fig.3,we can see that the crystal structural unit of the title compound is composed of two molecules.The distance betweenplanes C12-C13-C14-C16-C17-C18ofthosetwo molecules is 0.396 5 nm.The angle between those two molecules is 24.087°.Atypical hydrogen bonds(C13-H13…N1=0.261 86 nm)are existed between those two molecules.The bond length of C15-N1 is 0.114 2(2)nm which is in the normal range.The bond lengths of C7-C8,C7-C10,C8-C9 and C10-C11 are 0.154 1(3),0.155 1(3),0.151 6(4)and 0.152 3(4)nm,respectively.
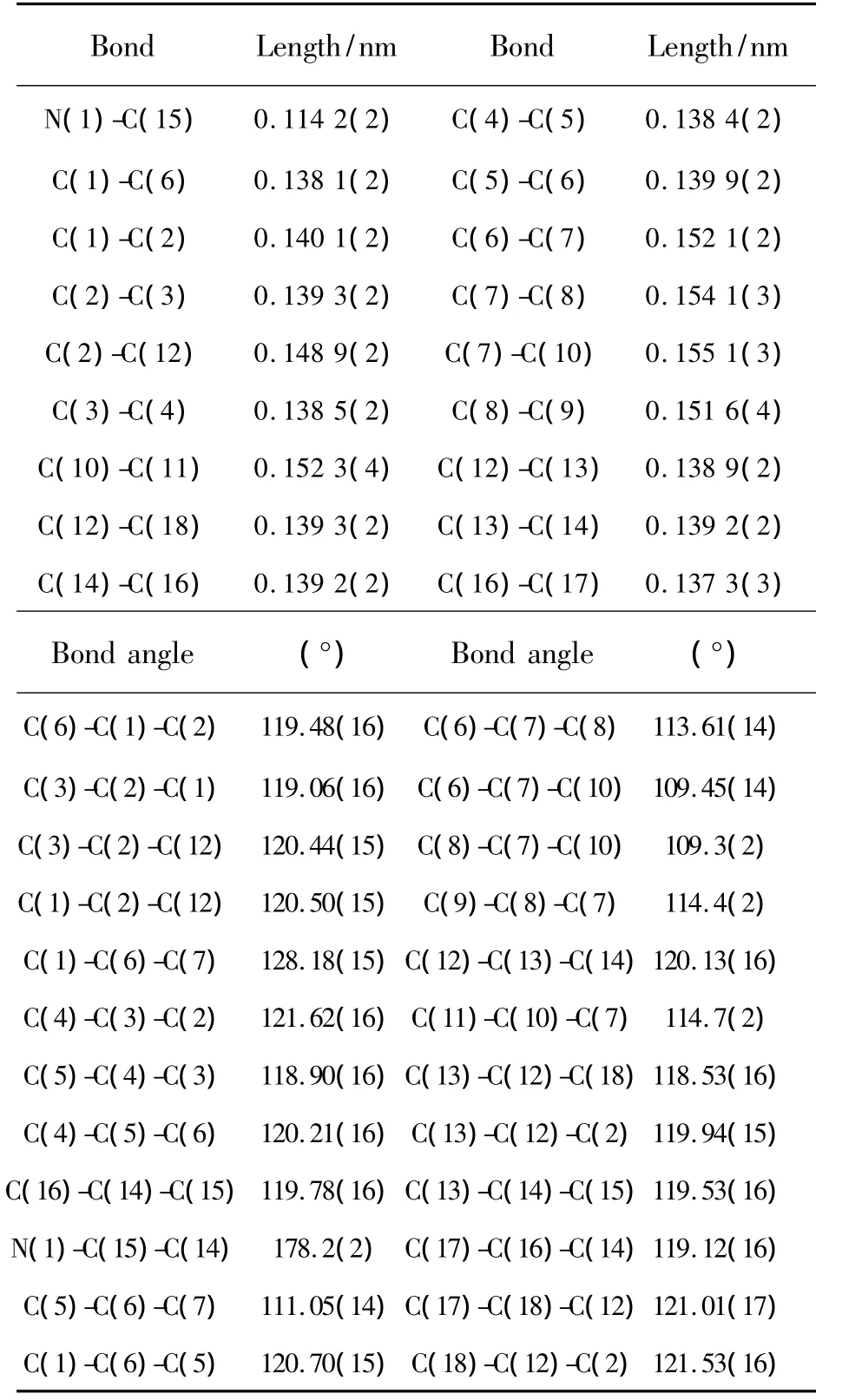
Table 2 Selected Bond Lengths and Bond Angles
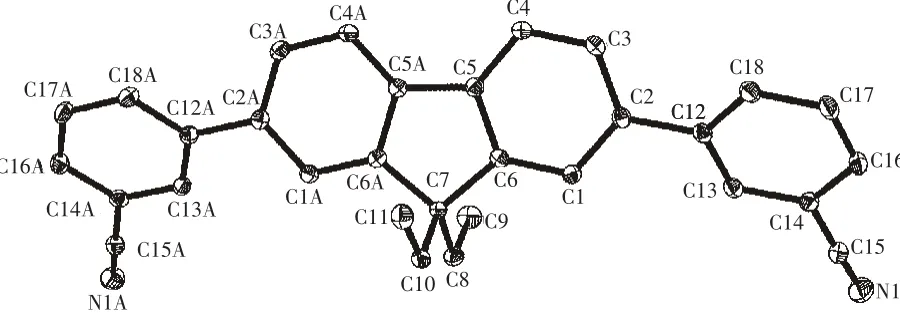
Fig.2 Molecular structure of the title compound

Fig.3 Crystal packing diagram of the title compound
3.3 UV-Vis Absorption Spectra
Fig.4 shows the UV-Vis absorption spectra of the title compound in CH2Cl2solution and in film coated on a quartz substrate.In CH2Cl2solution,the UV-Vis absorption spectra show two peaks at 328 and 240 nm,respectively.The 328 nm low-energy band,which is similar to that observed for other fluorene derivatives[14],is attributed to π-π*electronic transitions in the backbone.The 240 nm high-energy band is attributed to n-σ*transitions of CH2Cl2.However,in film,the peak near 328 nm is observed at 352 nm.Compared to the UV-Vis absorption peak of the compound in CH2Cl2solution,the absorption peak is red-shifted by 24 nm in film,which is attributed to the presence of stronger intermolecular interactions[15].

Fig.5 Fluorescence spectra of the title compound in CH2Cl2solution(a)and in film(b)
3.4 Fluorescence Spectra
Fig.5 shows the fluorescence spectra of the title compound in CH2Cl2solution and in film coated on a quartz substrate.In CH2Cl2solution,the compound exhibits deep-blue fluorescence with shoulder emission peaks of 382 and 366 nm upon excitation at 330 nm,as is the characteristic of most conjugated fluorene derivatives[16-18].The emission spectra of the compound show a red-shift(λmax=408 nm)in film as compared to the spectra in CH2Cl2solution,likely because of a self-absorption effect.The Stokes shift between the UV-Vis absorption and fluorescence maxima is 54 nm for the CH2Cl2solution and 56 nm for the film.The relatively large Stokes shift of the film is favorable for preventing the self-absorption of the emitted light in OLED devices[19].This indicates that the compound may be suitable as a candidate for a promising blue-emitting material.

Fig.4 UV-Vis absorption spectra of the title compound in CH2Cl2solution(a)and in film(b)
3.5 Fluorescence Quantum Yield
Fluorescence quantum yield(Φ)is the ratio of photons absorbed to photons emitted through fluorescence.The reliable method for recording Φ is the comparative method which involves the use of well characterized standard samples with known Φ values[20].The relative fluorescence quantum yield was obtained using quinine sulfate as a standard,assuming a quantum yield of 0.55 in 0.05 mol/L aqueous sulphuric acid[21].The fluorescence quantum yield(Φ)is calculated using the standard sample,according to the following equation:

where the subscripts u and s denote the test and standard,respectively,A is the absorbance of the solution,F is the integral area of the fluorescence spectra.The fluorescence quantum yield of the title compound in CH2Cl2solution is 0.64.This result indicates that the title compound has a higher fluorescence quantum yield.
4 Conclusion
In summary,A novel fluorene derivative,2,7-bis(3-cyanophenyl)-9,9-diethyl-fluorene has been synthesized by Suzuki coupling reaction.The compound exhibits deep-blue fluorescence emissions under UV excitation at 330 nm with high fluorescence quantum yield(0.64)in CH2Cl2solution and a large Stokes shift(56 nm)in the film,so it may be a promising blue-emitting material for OLED application.
[1]Scherf U,List E J W.Semiconducting polyfluorenes:Towards reliable structure-property relationships[J].Adv.Mater.,2002,14(7):477-487.
[2]Grisorio R,Mastrorilli P,Nobile C F,et al.New spiro-functionalized polyfluorenes:Synthesis and properties[J].Macromol.Chem.Phys.,2005,206(4):448-455.
[3]Anuragudom P,Newaz S S,Phanichphant S,et al.Facile horner-emmons synthesis of defect-free poly(9,9-dialkylfluore-nyl-2,7-vinylene)[J].Macromolecules,2006,39(10):3494-3499.
[4]Li T X,Yamamoto T,Lan H L,et al.Synthesis and electroluminescence properties of fluorene containing arylamine oligomer[J].Polym.Adv.Technol.,2004,15(5):266-269.
[5]Kim Y H,Shin D C,Kim S H,et al.Novel blue emitting material with high color purity[J].Adc.Mater.,2001,13(22):1690-1693.
[6]Ren X F,Ren A M,Zou L Y,et al.Fluorene-based oligomers as red light-emitting materials:A density functional theory study[J].Theor.Chem.Acc.,2010,126(5):305-314.
[7]Lee S H,Nakamura T,Tsutsui T.Synthesis and characterization of oligo(9,9-dihexyl-2,7-fluorene ethynylene)s:For application as blue light-emitting diode[J].Org.Lett.,2001,3(13):2005-2007.
[8]Kreger K,Neuber C,Strohriegl P,et al.Combinatorial development of blue OLEDs based on star shaped molecules[J].Adv.Funct.Mater.,2007,17(17):3456-3461.
[9]Wang J,Wan W,Jiang H Z,et al.C-9 Fluorenyl substituted anthracenes:A promising new family of blue luminescent materials[J].Org.Lett.,2010,12(17):3874-3877.
[10]Kulkarni A P,Zhu Y,Jenekhe S A.Quinoxaline-containing polyfluorenes:Synthesis,photophysics,and stable blue electroluminescence[J].Macromolecules.,2005,38(5):1553-1563.
[11]Kannan R,He G S,Yuan L X,et al.Diphenylaminofluorene-based two-photon-absorbing chromophores with various πelectron acceptors[J].Chem.Mater.,2001,13(5):1896-1904.
[12]Sheldrick G M.Software for Empirical Absorption Correction[M].Germany:University of Göttingen,1996.
[13]Sheldrick G M.Program for the Solution and Refinement of Crystal Structure[M].Germany:University of Göttingen,1997.
[14]Geng Y H,Trajkovska A,Katsis D,et al.Origin of strong chiroptical activities in films of nonafluorenes with a varying extent of pendant chirality[J].J.Am.Chem.Soc.,2003,125(46):14032-14038.
[15]Zhang L,Zhang Q Y,Gao Y Y,et al.Novel quinoxaline-based poly(phenylenevinylene)s copolymers:Synthesis and optical properties[J].Acta Chimica Sinica(化学学报),2009,67(21):2475-2480(in Chinese).
[16]Zeng G,Yu W L,Huang W,et al.Spectral and thermal spectral stability study for fluorene-based conjugated polymers[J].Macromolecules,2002,35(18):6907-6914.
[17]Hou Q,Zhou Q M,Zhang Y,et al.Synthesis and electroluminescent properties of high-efficiency saturated red emitter based on copolymers from Fluorene and 4,7-Di(4-hexylthien-2-yl)-2,1,3-benzothiadiazole[J].Macromolecules,2004,37(17):6299-6305.
[18]Teetsov J,Fox M A.Photophysical characterization of dilute solutions and ordered thin films of alkyl-substituted polyfluorenes[J].J.Mater.Chem.,1999,9(9):2117-2122.
[19]Lai K Y,Chu T M,Elangovan A,et al.Excimer emission from a novel ethyne-based fluorescent dye in organic lightemitting devices[J].Surf.Coat.Technol.,2006,200(10):3283-3288.
[20]Parker C A,Rees W T.Correction of fluorescence spectra and measurement of fluorescence quantum efficiency[J].Analyst,1960,85(1013):587-600.
[21]Hirano J,Hamase K,Miyata H,et al.6,7-Difluoro-1,4-dihydro-1-methyl-4-oxo-3-quinolinecarboxylic acid,a newly designed fluorescence enhancement-type derivatizing reagent for amino compounds[J].J.Fluoresc,2010,20(2):615-624.

