Modified technique of Wolbachia removal from Malaysian Aedes albopictus
Sylvia Joanne, Indra Vythilingam*, Nava Yugavathy, Jonathan Inbaraj Doss
1Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia
2Julius Centre University of Malaya, Department of Social and Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia
Modified technique of Wolbachia removal from Malaysian Aedes albopictus
Sylvia Joanne1, Indra Vythilingam1*, Nava Yugavathy1, Jonathan Inbaraj Doss2
1Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia
2Julius Centre University of Malaya, Department of Social and Preventive Medicine, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia
PEER REVIEW
Peer reviewer
Prof. Mak Joon Wah, Vice-President (Research), International Medical University. Kuala Lumpur, Malaysia. E-mail: joonwah_mak@imu.edu.my
Comments
The study has provided a viable method to produce Wolbachia free Ae. albopictus which is increasingly implicated as a vector of dengue transmission in many endemic countries. Susceptibility studies with dengue serotypes in Wolbachia positive or negative mosquitoes will be easily available now.
Details on Page 560
Objective:To develop an artificial and modified Wolbachia removal technique using tetracycline from naturally Wolbachia infected Aedes albopictus (Ae. albopictus) so as to be able to produce generations of Wolbachia free offsprings.
Wolbachia, tetracycline, Aedes albopictus
1. Introduction
Wolbachia pipientisis an intracellular bacteria found in most of the arthropods, nematodes and isopods[1,2]. They are vertically transmitted rickettsia endosymbiont bacteria[3]. In order to ensure the parasite being successfully transmitted maternally,Wolbachiatend to alter reproduction properties of their host[4]. Common alteration that have been reported are male killing, feminization, parthenogenesis and cytoplasmic incompatibility (CI)[5,6].Wolbachiamodifies the spermatogenesis causing no viable offspring to be produced when infected male mates with uninfected female or female infected differently from the male[7]. Well understood CI can be used to reduce population of the host.
Aedes albopictus(Ae. albopictus) is an arthropod known to be naturally infected withWolbachia pipientisbacteria. Most strains ofAe. albopictusscreened in Malaysia were superinfected with both twoWolbachiastrains (wAlbA andwAlbB).Aedes aegyptiandAe. albopictusare the major vectors for dengue in Malaysia. They are lethal vectors which transmit many deadly pathogens including dengue fever virus, chikungunya fever virus and West Nile Virus[8]. The combination of dengue blocking activity and rapid spread due to CI has led researchers to suggest thatWolbachiacan be used to develop public health strategies to reduce dengue incidence in human[9,10].
In order to study the CI and effect ofWolbachiaon MalaysianAe. albopictus, it is necessary to have bothWolbachiainfected and uninfected strains.
Ae. albopictusis a species naturally infected withWolbachiatherefore obtaining a natural strain withoutWolbachiawould be very rare[11]. Therefore an artificialWolbachiaremoval technique is needed.
Previous studies have suggested treatment of larvae with tetracycline antibiotic to removeWolbachiafromAe. albopictus[3,11]. However, reduced fecundity and egg viability was observed when the above mentioned method was implemented. Another study proposed treatment of only the adult mosquito with tetracycline antibiotic[11]. This managed to overcome the reduced fecundity and egg viability issue. However, when the method was implemented, the resulting offsprings were found not to be totally free fromWolbachia.
In this study, a modifiedWolbachiaremoval method fromAe. albopictusis reported. It has minimal effect on the mosquito fecundity and egg viability and was able to produce generations ofWolbachiafree offsprings.
2. Materials and methods
2.1. Mosquito strain
A strain ofAe. albopictusobtained from Bukit Lagong, Selayang, Kuala Lumpur, Malaysia in August 2013 was used in this study. Mosquitoes were maintained in cages with 10% sucrose with 100 mg B-Complex solution. They were blood fed and eggs were collected weekly. Mosquito infection status was confirmed using polymerase chain reaction (PCR) amplification and sequencing.
2.2. Infection status
A minimum of 30 blood fed mosquitoes were randomly caught for each new generation, blood fed, allowed to lay eggs and then extracted using Dneasy Blood and Tissue Extraction Kit according to the protocol provided by the manufacturer (Qiagen, CA, USA). Extracted DNA were stored at -20 °C until needed. All samples were screened for the presence ofWolbachiausing multiplex PCR with Promega (Promega, Madison, WI) reagents for amplification of the wsp gene with diagnostic primers (Genomics BioSci & Tech, China).
ThewAlbA strain gene was amplified with the wsp 328F and 691R primer pair whereaswAlbB strain gene was amplified with the wsp 183F and 691R primer pair. PCR was conducted in a 20 µL reaction per individual. This consisted of 10 µL ddH2O, 4 µL 5X Green GoTaq® Flexi Buffer, 1.6 µL magnesium chloride, 0.4 µL dNTPs, 0.6 µL of each primer (183F, 328F and 691R), 0.2 µL of GoTaq® Flexi DNA polymerase and 2 µL template. Samples were denatured for 5 min at 94 °C, followed by 35 cycles of 1 minute at 94 °C, 1 min at 55 °C and 1 min at 72 °C. A negative control was run along with each batch of PCR amplification by substituting 2 µL of sample with 2 µL of ddH2O[12].
A total of 8 µL of each sample was run in 1% agarose gel to detect the presence of amplified DNA fragments. One hundred kilobyte ladder (Promega, Madison, WI) was used to confirm presence ofwAlbA (363 bp) andwAlbB (508 bp) genes[12] (Figure 1).
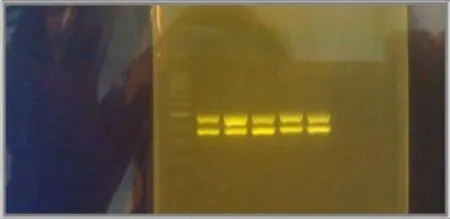
Figure 1. Gel electrophoresis result image. Lane 1 is the 100 kB ladder. Lane 2-6 are my samples.The upper row are wAlbB amplified gene at 508 bp and the lower row bands are wAlbA amplified gene at 363 bp.
2.3. Tetracycline treatment
AllWolbachiaremoval studies were conducted on strains with confirmedwAlbA andwAlbB superinfection. Studies were conducted as stated in Table 1. Treatment 1 was conducted as previously described by Otsuka and Takaoka in 1997[3]. Treatment 5 and 6 were conducted as described previously by Dobson and Rattanadechakul in 2001[11]. Treatments 2, 3, 4 and 7 consisted of a modified technique were conducted by this group. Larvae after the treatment period in treatment 1, 2, 3 and 4 were transferred back into water without tetracycline and reared to adulthood. In each treatment, randomly caught 25 blood fed adult mosquitoes were allowed to lay eggs first and then tested for presence ofWolbachiausing PCR method as mentioned above. If noWolbachiainfection was found in all tested mosquitoes, the eggs obtained were hatched. Larvae after 24 h treatment in treatment 5 was transferred back into water without tetracycline and reared to adulthood.
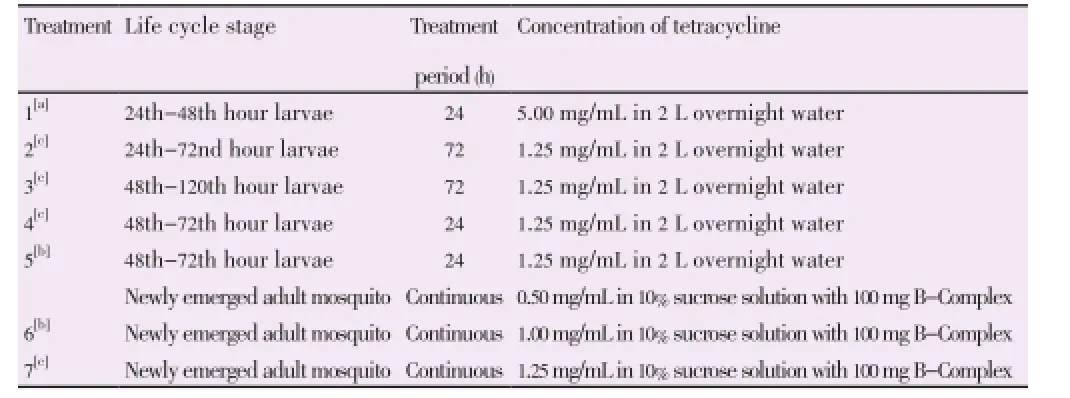
Table 1 Tetracycline treatment design.
Adult mosquitoes in treatment 5, 6 and 7 were blood fedafter two weeks and one month for egg collection. Twenty five mosquitoes from which eggs were collected were tested for presence ofWolbachiausing PCR method as mentioned above. Eggs collected from the treatment 5, 6 and 7 were allowed to hatch in 2 L overnight water. Egg hatching rate were calculated to determine egg viability for each treatment. Once the F1generation mosquitoes were obtained, 25 blood fed adult mosquitoes were randomly caught from each colony, allowed to lay egg first and then tested for presence ofWolbachiausing PCR method as mentioned above. AverageWolbachiainfectivity of F1for treatment 6 and 7 was obtained by calculating the mean infected mosquito numbers for three replicates ofWolbachiatesting. The same calculation was done for averageWolbachiainfectivity of F2for treatment 7.
3. Results
The strain ofAe. albopictusused in this study had 100% bothwAlbA andwAlbB infection. Figure 1 shows the result of PCR amplification when bothwAlbA andwAlbB is present. The F4 eggs were used in thisWolbachiaremoval study.
Percentage of eggs hatched that survived to pupation, percentage of adult mosquitoes emerged, percentage ofWolbachiainfection status of the F0and percentage of F1eggs hatched were calculated for all treatments 1-7. Results are shown in Table 2.
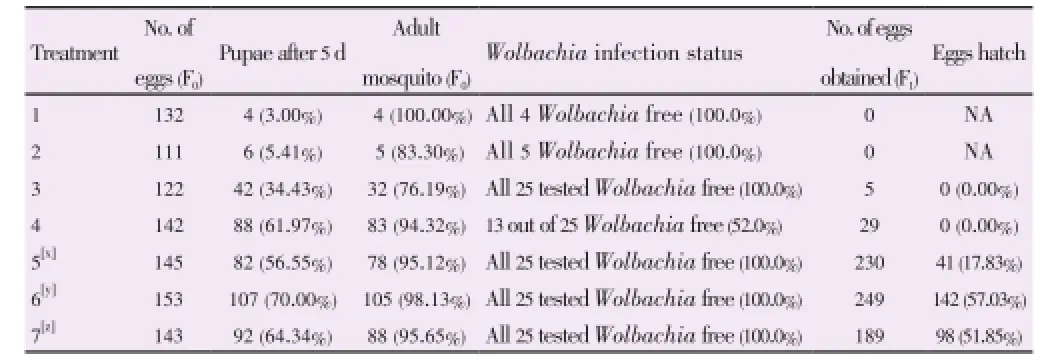
Table 2 Percentage eggs hatch in F0and F1tetracycline treated strains.
Treatment 5, 6 and 7 had F1eggs therefore studies were continued to obtain the percentage of F1adult mosquitoes,Wolbachiainfectivity status of F1colony andWolbachiainfectivity status of F2colony (only treatment 7). Results for this continuation studies are shown in Table 3.
4. Discussion
Tetracycline is a group of broad-spectrum antibiotics. Its overall usage has been reduced with the increasing bacterial resistance[13]. SinceWolbachiais an endosymbiotic bacteria, tetracycline at the right concentration and delivery method should be able to remove them from their respective hosts. This concurs with previous studies conducted[11].
Treatment 1 which was conducted based on Otsuka method was not effective in this study as it caused low egg viability, high larval mortality and sterile adult mosquitoes[3]. Same issue have been reported by Dobson and Rattanadechakul in 2001[11]. This may have been due to the high concentration of the tetracycline used to treat the larvae.
Similar larval treatments were carried out with reduced concentration to 1.25 mg/mL in treatment 2, 3 and 4 at different exposure periods.
High larval mortality was observed when larvae were treated for more than 24 h. However, improved larval mortality was observed when the 48 h larvae were treated instead of the 24 h larvae. This may be because 24 h larvae are too young to withstand the tetracycline treatment.
Treatment 4 was designed to expose 48 h larvae for 24 h which gave lower larval mortality and a higher percentage of adults.
Although low larval mortality was observed, the treatment failed to removeWolbachiacompletely from all surviving adults. Therefore it can be concluded that perhaps the period of treatment or tetracycline concentration was not sufficient.
Treatment 5 was conducted based on Dobson report in 2001 which subjects both larvae and adult mosquitoes tetracycline[11]. This method had low larval mortality and was able to completely removeWolbachiafrom all surviving F0adults. A good number of F1eggs were obtained but the hatching rate of the F1eggs was very low compared to untreated strains.
Treatment 6 was conducted based on the final method from Dobson paper in 2001 which treats only the adult with 1.0 mg/mL[11]. No alternative food source was provided for the mosquitoes. F0Adult mosquitoes were tested forWolbachiaafter 2 weeks exposure to tetracycline sucrose treatment. Mosquitoes were not found to be completely free ofWolbachia. F0adult mosquitoes were again tested forWolbachiaafter 1 month tetracycline treatment and all wereWolbachiafree. Eggs were collected and F1mosquitoes were obtained. Although the experiment was repeated three times, we failed to obtain entirelyWolbachiafree F1adult mosquitoes. Therefore treatment 6 as proposed by Dobson was not effective in this study.
Treatment 7 was designed exactly as treatment 6 with aslight increment of the concentration of tetracycline in the sucrose solution. CompleteWolbachiaremoval from the F0adult mosquitoes was observed in two weeks tetracycline treated mosquitoes. This was confirmed with two replicates. Egg hatching rate was slightly lower than treatment 6 and 93.88% became F1adults. In contrast to treatment 6, F1adults were 100%Wolbachiafree. Average was obtained from three replicates. All F2adults was also found to beWolbachiafree.

Table 3 Wolbachia infectivity status of F1and F2tetracycline treated strains.
Tetracycline treatment of only adult mosquitoes simplifies the process, improves the egg hatchability, reduces larval mortality and increases adult fecundity. The best concentration for the adult treatment is concluded to be 1.25 mg/mL in sucrose solution with no alternative food source. This method is able to remove bothwAlbA andwAlbB completely in just two weeks and gives subsequent generations free ofWolbachia.
This self-sustainingWolbachiafreeAe. albopictuscolony developed can be used to study the effect ofWolbachiaon MalaysianAe. albopictus. Future research may be conducted to develop a singly infectedAe. albopictusstrain with a modified antibiotic treatment as none has been established so far.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This project have been financially supported by University Malaya Research Grant (UMRG) with grant NO.: RG372/11HTM.
Comments
Background
There is increasing interest onWolbachiaendosymbionts inAedesvectors as they are related to fecundity and to dengue transmission. There is a need to obtain strains ofAe. albopictusfree from endosymbionts but existing methods through treatment of larvae with tetracycline have not been satisfactory.
Research frontiers
An improved method to obtainedWolbachiafree and viableAe. albopictusmosquitoes for further studies.
Related reports
Although the use of tetracycline to obtainWolbachiafreeAedeshas been previously studied, the dosage and methods used did not produce satisfactory results.
Innovations and breakthroughs
An improved method of using tetracycline in obtaining subsequent generations ofWolbachiafreeAe. albopictus.
Applications
This study is important for research onWolbachiaand dengue susceptibility.
Peer review
The study has provided a viable method to produceWolbachiafreeAe. albopictuswhich is increasingly implicated as a vector of dengue transmission in many endemic countries. Susceptibility studies with dengue serotypes inWolbachiapositive or negative mosquitoes will be easily available now.
[1] Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 2008; 6: 741-751.
[2] Cordaux R, Pichon S, Hatira HB, Doublet V, Grève P, Marcadé I, et al. Widespread Wolbachia infection in terrestrial isopods and other crustaceans. Zookeys 2012; 176: 123-131.
[3] Yasushi O, Hiroyuki T. Elimination of Wolbachia pipientis from Aedes albopictus. Med Entomol Zool 1997; 48: 257-260.
[4] Mains JW, Brelsfoard CL, Crain PR, Huang Y, Dobson SL. Population impacts of Wolbachia on Aedes albopictus. Ecol Appl 2013; 23: 493-501.
[5] Klasson L, Kambris Z, Cook PE, Walker T, Sinkins SP. Horizontal gene transfer between Wolbachia and the mosquito Aedes aegypti. BMC Genomics 2009; 10: 33.
[6] Ingram KK, Hoadley AP, Iandoli M, Kahler J, Marion S, Peteru S, et al. Sporadic infection of Wolbachia in a recently established population of Formica fusca. Psyche 2012; doi:10.1155/2012/432151.
[7] Zabalou S, Apostolaki A, Pattas S, Veneti Z, Paraskevopoulos C, Livadaras I, et al. Multiple rescue factors within a Wolbachia strain. Genetics 2008; 178: 2145-2160.
[8] Hochedez P, Jaureguiberry S, Debruyne M, Bossi P, Hausfater P, Brucker G, et al. Chikungunya infection in travelers. Emerg Infect Dis 2006; 12: 1565-1567.
[9] Mousson L, Zouache K, Arias-Goeta C, Raquin V, Mavingui P, Failloux AB. The native Wolbachia symbionts limit transmission of dengue virus in Aedes albopictus. PLoS Negl Trop Dis 2012; 6: e1989.
[10] Blagrove MS, Arias-Goeta C, Failloux AB, Sinkins SP. Wolbachia strain wMel induces cytoplasmic incompatibility and blocks dengue transmission in Aedes albopictus. Proc Natl Acad Sci U S A 2012; 109: 255-260.
[11] Dobson SL, Rattanadechakul W. A novel technique for removing Wolbachia infections from Aedes albopictus (Diptera: Culicidae). J Med Entomol 2001; 38: 844-849.
[12] Armbruster P, Damsky WE Jr, Giordano R, Birungi J, Munstermann LE, Conn JE. Infection of New-and Old-World Aedes albopictus (Diptera: Culicidae) by the intracellular parasite Wolbachia: implications for host mitochondrial DNA evolution. J Med Entomol 2003; 40: 356-360.
[13] Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001; 65: 232-260.
10.12980/APJTB.4.2014APJTB-2014-0020
*Corresponding author: Indra Vythilingam, Department of Parasitology, Faculty of Medicine, University of Malaya, Kuala Lumpur, 50603, Malaysia.
Tel: +60379677893
E-mail: indrav@um.edu.my
Foundation Project: Supported by University Malaya Research Grant (UMRG) with grant NO.: RG372/11HTM.
Article history:
Received 1 May 2014
Received in revised form 12 May, 2nd revised form 15 May, 3rd revised form 29 May 2014
Accepted 23 Jun 2014
Available online 28 Jul 2014
Methods:In this study, seven different tetracycline treatment methods were conducted to obtain the best removal method. Four methods focused on larvae tetracycline treatment, one method on both larvae and adult tetracycline treatment and the last two methods on adult mosquito sucrose treatment.
Results:All larval tetracycline treatments resulted in either high larvae mortality, sterile F0adult mosquitoes or unsuccessful Wolbachia removal. Treatment of both larvae and adults resulted in reduced larvae mortality, successful Wolbachia removal but slow mosquito fecundity. As for the adult treatment, 1.0 mg/mL as previously published was not able to completely remove Wolbachia in F1generation whereas 1.25 mg/mL successfully removed Wolbachia from F1and F2mosquitoes in 2 weeks.
Conclusions:This method is different from the previously published methods as it provides an improved Wolbachia removal technique from Ae. albopictus with high egg hatchability, low larvae mortality, increased fecundity and better Wolbachia removal rate.
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Antioxidant potential of Rumex vesicarius L.: in vitro approach
- Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
