An initial study of insect succession on decomposing rabbit carrions in Harare, Zimbabwe
Nyasha Mabika, Ron Masendu, Gilbert Mawera
1Department of Anatomy, College of Health Sciences, University of Zimbabwe, Zimbabwe
2Abt Associates, Zimbabwe
An initial study of insect succession on decomposing rabbit carrions in Harare, Zimbabwe
Nyasha Mabika1*, Ron Masendu2, Gilbert Mawera1
1Department of Anatomy, College of Health Sciences, University of Zimbabwe, Zimbabwe
2Abt Associates, Zimbabwe
PEER REVIEW
Peer reviewer
Dr. Nazaire Aizoun, Centre de Recherche Entomologique de Cotonou (CREC), 06 BP 2604, Cotonou, Bénin.
Tel: (229) 95317939
E-mail: aizoun.nazaire@yahoo.fr
Comments
In this research work, authors have demonstrated the importance of establishing the relationship between insects associated with a decomposing dead body.
Details on Page 564
Objective:To investigate insects visiting sun exposed and shaded decomposing rabbit carcasses and to establish the relationship between insects and carcasses which may be of forensic importance in Harare.
Insect succession, Forensic entomology, Decomposing rabbit carrion
1. Introduction
Forensic entomology is the study of the insects associated with a dead body in an effort to determine elapsed time since death[1]. As the dead body decomposes, it attracts a different group of sarcosaprophagous arthropods, especially insects. Some are attracted to the remains which are used as a medium for oviposition or feeding, while others are attracted by the aggregation of other arthropods that are used as a food source[2]. Insect species associated with carrion and their times of colonization vary according to many factors, one of the most important being the geographic region or biogeoclimatic zone. The biogeoclimatic zone defines the habitat, vegetation, soil type and meteorological conditions of the area that obviously have a major impact on the types and species of insects present, as well as their seasonal availability[3].
The majority of literature in the field of forensic entomology has addressed the corpse fauna of theUnited States, Europe, Britain and Australia, but Africa has generally been neglected[4]. There is relatively little information available regarding insects associated with animal carrion and human corpses in Africa. Few African countries such as Cameroon[5,6], South Africa[7-10], Egypt[11], Ghana[12] and Nigeria[13-15] have studied insects associated with decomposing dead bodies. In Zimbabwe, there are no published records of insects associated with a decomposing dead body. Consequently, forensic entomology has not been incorporated into death investigations, despite numerous homicide and poaching cases being reported almost every week. Therefore, the major objectives of the study were to identify insects visiting decomposing rabbit carcasses and to establish the relationship between insects and carcasses which may be of forensic importance in Harare. The preliminary results of this study could be very useful for further forensic work in Harare.
2. Materials and methods
2.1. Site description
The study was conducted at two sites, 30 m apart from the University of Zimbabwe. The surrounding area was generally bare with short grass.
2.2. Carcasses
Two rabbits from the Animal House, University of Zimbabwe, weighing 2.3 kg and 2.5 kg were killed by sharp blows with a blunt metal object on the head. They were put in separate plastic bags and transported to two separate sites about 30 m walking distance from the Animal House. At site 1, the carcass was exposed to the sun while at site 2, the carcass was placed under shade. Wire cages measuring 160 mm×100 mm×30 mm were placed over the carcasses to protect them from birds and scavengers. The mesh size of the cage, measuring 60 mm× 10 mm did not add much to the shading.
2.3. Sampling procedure
Samples were collected twice a day (9:00 and 13:00) for the first week and thereafter once a day for up to the end of the 7 weeks. Sampling started on 1 November 2012 and ended on 19 December 2012. Hand netting (net diameter 330 mm, depth 750 mm, mesh size 5 mm×5 mm) and manual sampling were used to collect flying and crawling insects on the carcasses and the surrounding soil. Operation time at each carcass did not exceed 18 min to ensure uniform and limited disturbance at each site[13]. The collected insects were anesthetized with diethyl ether and preserved in 70% ethanol. However, immature fly samples were kept alive for rearing. A thermometer was held in the cage of each carcass 5 cm above the ground to record the daily ambient temperature.
2.4. Maggot rearing
Two rearing containers, one for each carcass, measuring 205 mm long×195 mm high×160 mm wide were prepared. A layer of sandy soil was placed at the base to provide the maggots with a substrate to burrow into during the prepupal stage. A piece of tissue measuring 60 mm×60 mm was dissected from the lateral side of the carcass and placed in the rearing container to feed the maggots. A wire screen with mesh size measuring 2 mm×2 mm for air circulation was put over the rearing container. At Day 8, maggots were collected from each carcass and reared in the separate rearing units. The rearing units were placed under room temperature (27-28 °C) and were checked daily. When the flies emerged, they were killed by placing the rearing containers into a freezer for 15 min. The dead flies were put in labeled vials containing 70% ethanol.
2.5. Sample identification
Adult flies were observed under a dissecting microscope and identified using dichotomous keys[16,17].
3. Results
3.1. Ambient temperature
The minimum recorded temperature on the sun exposed carcass was 27 °C while the maximum recorded was 36 °C. Minimum temperature recorded on the shaded carcass was 26 °C and the maximum was 30 °C.
3.2. Decomposition stages
Three insect orders-Diptera, Coleoptera and Hymenoptera were identified in association with both carcasses during decomposition throughout the study. Four decomposition stages were identified. These were fresh, bloated, decay and post decay stages (Tables 1 and 2). The fresh stage began as soon as the carcasses died up to Day 2. Bloating was observed on Day 3 and both carcasses began to smell and the odour was much more pronounced on the sun exposed carcass. The fifth day marked the decay stage. During this stage, the sunexposed carcass ruptured whilst the shaded carcass was partially ruptured. By Day 9, both carcasses were showing signs of dryness and this marked the post decay stage.
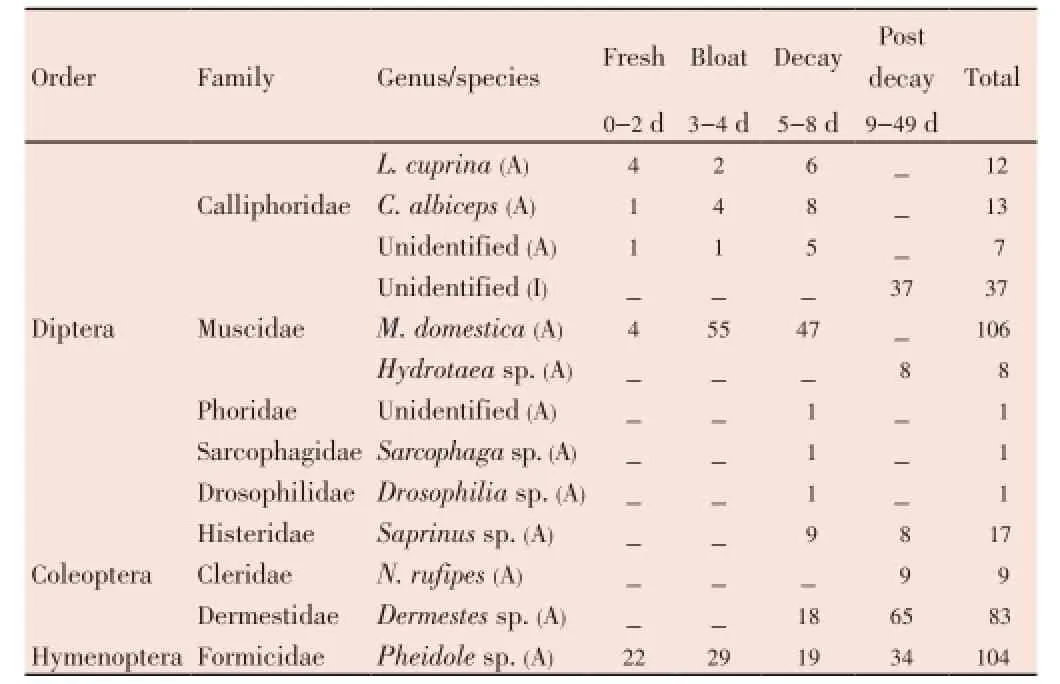
Table 1 Insect succession and abundance from a sun-exposed rabbit carcass during decomposition stage.
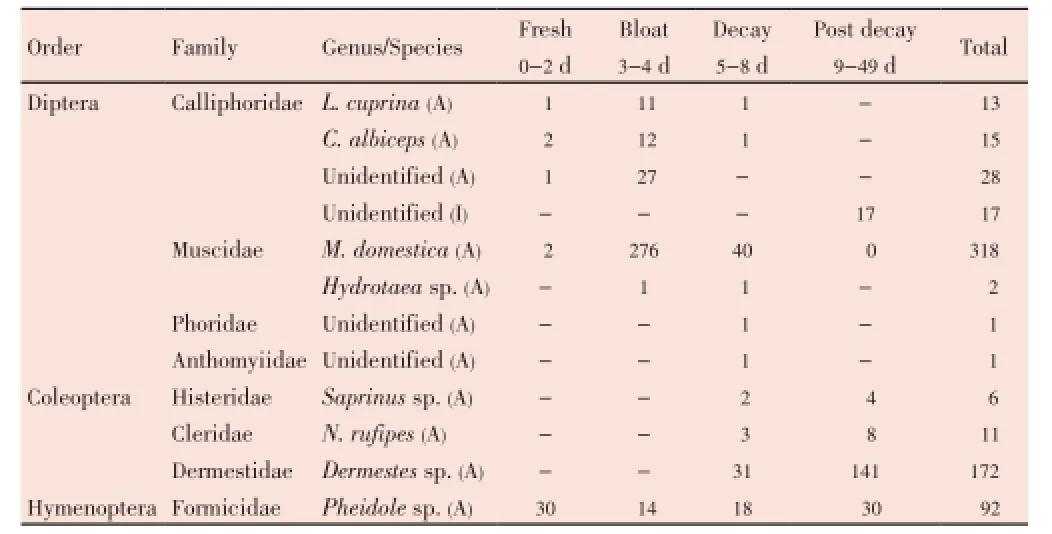
Table 2 Insect succession and abundance from a rabbit carrion placed under a shade during decomposition stage.
3.3. Insect succession
Five dipteran families (Calliphoridae, Muscidae, Sarcophagidae, Phoridae and Drosophilidae) were observed from the sun-exposed carcass (Table 1), whilst four dipetran families (Calliphoridae, Muscidae, Phoridae and Anthomyiidae) were observed from the shaded carcass (Table 2). Early colonizers arriving at both carcasses during the fresh stage were the calliphorids and muscids. The adult calliphorids were observed from the fresh stage up to the decay stage. The species observed wereLucilia cuprina(L. cuprina) andChrysomya albiceps(C. albiceps). AdultMusca domestica(M. domestica) was also observed from the fresh stage up to the decay stage from the sun exposed carcass, whilst from the shaded carcass, it was observed in all the decomposition stages. Maggots were observed on the fourth day from both carcasses.
TheHydrotaeasp. (Muscidae) was observed during the decay stage from the sun exposed carcass, whilst from the shaded carcass it was observed during the bloated and decay stages. Phoridae (unidentified) was observed from both carcasses during the decay stage. AdultSarcophagasp. andDrosophiliasp. were collected from the sunexposed carcass during the decay stage, whilst adult Anthomyiidae (unidentified) was collected from the shaded carcass during the decay stage.M. domesticaprovided the highest number of flies from both the sun-exposed and the shaded carcasses, followed by the calliphorids. On Day 8, maggots were observed moving away from the carcasses. Between Day 12 and Day 15, immature calliphorids were observed on both carcasses.
Three families of beetles (Coleoptera) were observed during the study. These were the Histeridae, Dermestidae and Cleridae. TheSaprinussp. (Histeridae) was the first to arrive at the sun-exposed carcass on Day 6, whilst families Histeridae and Dermestidae were observed from both carcasses during the decay and post decay stages of decomposition. Family Cleridae was collected from the shaded carcass during the decay stage but was not observed during the decay stage from the sun-exposed carcass. TheDermestessp. was the most dominant species from both carcasses.
Ants (Hymenoptera) were observed throughout the decomposition process and only one family, Formicidae (Pheidolesp.) was observed. By Day 21, no new insects were observed and from Day 36 up to Day 49 insects were no longer visiting the carcasses except for the ants.
3.4. Flies emerging from the rearing units
The families Calliphoridae (L. cuprina, Luciliasp. andC. albiceps), Sarcophagidae (Sarcophagasp.) and Sepsidae (Sepsissp.) emerged from the sun-exposed reared carcass whilst families Calliphoridae (L. cuprina, Luciliasp. andC. albiceps) and Sepsidae (Sepsissp.) emerged from the shaded reared carcass. The Calliphorids emerged after 11 d, whilst the Sarcophagid and Sepsids emerged after 15 d.
4. Discussion
Colonization species of greatest importance in the early stages of decomposition usually are those from the three dipteran families: Calliphoridae, Sarcophagidae and Muscidae[18,19]. This was confirmed in the study as the Calliphorids and Muscids were the first to arrive at both carcasses when the carcasses were soft and producing exudes for the flies to feed on. According to a study by Shiet al.[20], sarcophgids are the primary colonizers in warmer temperatures and tropical areas. However, in this study, a representative of the Sarcophagidae (Sarcophagasp.)was observed as a secondary colonizer arriving during the decay stage. However, it was a bit unusual in this study to collect one sample throughout the study compared to studies elsewhere.
Generally, the sequence and duration of insect succession in the sun and shaded sites followed the same general pattern. However, the same study by Okiweluet al. confirmed that the sun exposed carcass decomposed faster than the shaded carcass[13]. Maggots were observed on the fourth day, whilst a study by Dupontet al. observed maggots from rats’ carcasses on the second day of decomposition[5]. This difference could be attributed to the size of the carcasses[21]. Maggot migration from the carcasses was observed on day 8 and this observation was also confirmed in piglets by Castroet al[22]. Immature calliphorids were observed between Day 12 and Day 15 on both the sun-exposed and the shaded carcasses. These immature calliphorids were probably emerging from the pupae whose larvae had burrowed into the soil from the carcasses.
During the post decay stage of decomposition, the carcasses were showing signs of dryness. Hence, the number of flies visiting the carcasses began to decrease. Beetles (Coleoptera) are considered the most common during this stage.Dermestessp. was the dominant species being collected from the decay stage from the sunexposed carcass. However, other studies have collected this species as early as the bloat stage[23]. According to VanLaerhoven and Anderson[24], the presence of these beetles so early in the decay process might be a function of peak seasonal appearance rather than the decompositional state of the carcass.
Hymenoptera (Formicidae) were observed throughout the decomposition process, and appeared to have no impact on the decomposition process. This is contrary to the observations made by Morretiet al. where ants fed on carcasses and maggots[25]. As a result, they categorized them as an important component of the Sarcophagidae community. By Day 21, no new insects were observed, implying that succession was over. From Day 36 to Day 49, no insects were collected, but ants were observed up to Day 49.
At Day 8, maggots were removed from each carcass and reared in separate rearing units. The families Calliphoridae, Sarcaphagidae and Sepsidae emerged from the sun-exposed carcass whilst families Calliphoridae and Sepsidae emerged from the shaded carcass. Immature calliphorids and sarcophagids emerged after 11 d, whilst Sepsids emerged after 15 d. Of note was that Sepsids, which were not observed from both carcasses during the decomposition, were observed from the rearing units. This family may have been missed during the decomposition of the carcasses possibly because of their size since they are small.
From the study, adultM. domesticawas the most abundant species. However, they were only collected as adults from the decomposing carcasses and were also not observed from the rearing units. This probably indicates that they visited the carcasses to feed and not to breed. Of the dipteran species collected during the study,L. cuprinaandC. albicepscould be important for further forensic studies since they were collected from the carcasses and also observed from the rearing units.
In conclusion, since this was a preliminary study, there is need to repeat and replicate it at different times of the year so as to provide multiple sets of baseline succession data for Harare, encompassing all seasons. However, the information obtained during this study could be useful for providing initial database information as no succession data were previously available in Harare. Furthermore, these results could also possibly stimulate other entomologists in Zimbabwe and initiate future studies.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
We thank Professor M Villet for his advice during the preparation of this manuscript. Special thanks also go to the Chief Technician of Animal House, University of Zimbabwe, Mr. T. Magwiroto and his technical staff for their technical assistance. This research was funded by the Department of Anatomy, College of Health Sciences, University of Zimbabwe (Grant No. Jato 35).
Comments
Background
The knowledge regarding insect succession on decomposing rabbit carrions is useful for forensic studies. Thus, the current study was conducted as a preliminary study in order to have initial database information in Harare, Zimbabwe, where no study in this field was available.
Research frontiers
The present research work about insect succession on decomposing rabbits in Harare, Zimbabwe was conducted in order to identify insects visiting decomposing rabbit carcasses and to establish insects which may be of forensic importance in Harare.
Related reports
It was known that many insect species colonized animal carrions such as rabbit carcass. The presence of bothL. cuprina,C. albicepsreported in the current study would help to develop forensic entomology in Harare.
Innovations and breakthroughs
Studies on insects associated with a decomposing dead body have already conducted in many African countries. Authors have demonstrated the need to establish this correlation in Zimbabwe, where no study demonstrated this relationship yet.
Applications
From the literature survey, certain arthropods follow a predictable succession sequence. This scientific study supports and suggests the need to carry out further studies in order to define some strategies which allow to easily analyse the arthropod fauna in the time estimating since death.
Peer review
In this research work, authors have demonstrated the importance of establishing the relationship between insects associated with a decomposing dead body.
[1] Bhat MA, Shrivastav AB, Qurereshi SR, Quadri SA. Forensic exploitation of veterinary entomology. Int J Agro Vet Med Sci 2011; 5(4): 429-437.
[2] Payne JA. A summer carrion study of the baby pig Sus sacrofa Linnaeus. Ecology 1965; 46: 592-602.
[3] Anderson GS. Factors that influence insect succession on carrion. In: Byrd JH, Castner JL, editors. Forensic entomology: the utility of arthropods in legal investigations. 2nd ed. Boca Raton, FL: CRC Press; 2010, p. 201-250.
[4] Harvey MI, Mansell MW, Villet MH, Dadour IR. Molecular identification of some forensically important blowflies of Southern Africa and Australia. Med Vet Entomol 2003; 17(4): 363-369.
[5] Dupont FY, Champlain DL, Cyrille AA, Felix BB. A preliminary study of arthropod associated with carrion in Yaounde, Cameroon: a first step in forensic entomology in Central Africa. J Ecol Nat Environ 2011; 3(6): 215-220.
[6] Dupont FY, Felix BB, Daniel C, Champlain DL. Biodiversity study of the arthropods collected on rat carrion in Yaounde, Cameroon: first study of forensic entomology in Central Africa. Int J Biosci 2012; 2(1): 1-8.
[7] Kelly JA, Van De Linde TC, Anderson GS. Influence of clothing a wrapping on carcass decomposition and arthropod succession: a winter study in Central South Africa. Can Soc Forensic Sci 2008; 41(3): 135-147.
[8] Richards CS, Villet MH. Data quality in thermal summation development models for forensically important blowflies. Med Vet Entomol 2009; 23(3): 269-276.
[9] Villet MH. African carrion ecosystems and their insect communities in relation to forensic entomology. Pest Technol 2011; 5: 1-15.
[10] Midgel JM, Collett IJ, Villet MH. The distribution, habitat, diet and forensic significance of the scarab Frankenbergerius forcipatus (Harold, 1881) (Coleoptera: Scarabaeidae). Afr Invertebr 2012; 53(2): 745-749.
[11] Galal LA, Abd-El-hameed SY, Attia RA, Uonis DA. An initial study on arthropod succession on exposed human tissues in Assiut, Egypt. Mansoura J Forensic Med Clin Toxicol 2009; 17(1): 55-74.
[12] Kyerematen RA, Boateng BA, Twumasi E. Insect diversity and succession pattern on different carrion types. J Res Biol 2012; 2(7): 1-8.
[13] Okiwelu SN, Ikpamii T, Umeozor OC. Arthropods associated with mammalian carcasses in Rivers State, Nigeria. Afr J Biomed Res 2008; 11: 339-342.
[14] Ekanem MS, Dike MC. Arthropod succession on pig carcasses in South Eastern Nigeria. Pap Avulsos Zool (Sao Paulo) 2010; 50(35): 561-570.
[15] Arimoro FO. Colonization and invertebrate succession on mammalian carcasses in Ethiope River, Nigeria Delta, Nigeria. Appl Sci Res J 2013; 1(1): 7-21.
[16] Smith KG. A manual of forensic entomology. London: British Museum of Natural History; 1986.
[17] Whitworth T. Keys to the genera and species of blow flies (Diptera: Calliphoridae) of the West Indies and description of a new species Lucilia Robineau-Desvoidy. Zootaxa 2010; 2663: 1-35.
[18] Grassberger M, Frank C. Initial study of arthropod succession on pig carrion in a central European urban habitat. J Med Entomol 2004; 41: 511-523.
[19] Goddard J, Fleming DJ, Seltzer JL, Anderson S, Chesnut C, Cook M, et al. Insect succession on pig carrion in North-Central Mississippi. Midsouth Entomologist 2012; 5: 39-53.
[20] Shi YW, Liu XS, Wang HY, Zhang RJ. Seasonality of insect succession on exposed rabbit carrion in Guangzhou, China. Insect Sci 2009; 16(5): 425-439.
[21] Greenberg B. Flies as forensic indicators. J Med Entomol 1991; 28: 565-577.
[22] Castro CP, Serrano A, Martins Da Silva P, Garcia MD. Carrion flies of forensic interest: a study of seasonal community composition and succession in Libson, Portugal. Med Vet Entomol 2012; 26(4): 417-431.
[23] Early M, Goff ML. Arthropod succession patterns in exposed carrion on the island of O’ahu, Hawaiian Islands, USA. J Med Entomol 1986; 23: 520-531.
[24] VanLaerhoven SL, Anderson GS. Insect succession on buried carrion in two biogeoclimatic zones of British Columbia. J Forensic Sci 1999; 44: 32-43.
[25] Morreti T, Solis DR, Godoy WA. Ants (Hymenoptera: Formicidae) collected with carrion-baited traps in Southeast Brazil. Open Forensic Sci J 2013; 7: 1-5.
10.12980/APJTB.4.2014C1031
*Corresponding author: Nyasha Mabika, Department of Anatomy, College of Health Sciences, University of Zimbabwe, Zimbabwe.
Tel: +263 0773440191
E-mail: mabikan2@yahoo.co.uk
Foundation Project: Funded by the Department of Anatomy, College of Health Sciences, University of Zimbabwe (Grant No. Jato 35).
Article history:
Received 25 Apr 2014
Received in revised form 10 May, 2nd revised form 18 May, 3rd revised form 24 May 2014
Accepted 12 Jun 2014
Available online 28 Jul 2014
Methods:Two rabbits weighing 2.3 kg and 2.5 kg were killed by sharp blows on the head. One was exposed to the sun while the other was placed under shade. The carcasses were allowed to decompose and insects were collected twice a day for the first week and thereafter once a day up to the end of the 7 weeks. Maggots were also collected from the decomposing carcasses and reared.
Results:Five dipteran families (Calliphoridae, Muscidae, Sarcophagidae, Phoridae and Drosophilidae) were identified from the sun-exposed carcass. Species collected included Lucilia cuprina (L. cuprina), Chrysomya albiceps (C. albiceps), Musca domestica, Sarcophaga sp. and Drosophila sp. Four families (Calliphoridae, Muscidae, Phoridae, Anthomyiidae) were identified from the shaded carcass. Representatives of these families included L. cuprina, C. albiceps, Musca domestica, and Hydrotaea sp. Three Coleopteran families (Histeridae, Cleridae and Dermestidae) were identified from both carcasses. The observed species were Saprinus sp., Necrobia rufipes and Dermestes sp. Formicidae (Hymenoptera) was represented by only one species (Pheidole sp.). Flies which emerged from the rearing units were L. cuprina, Lucilia sp., C. albiceps, Sarcophaga sp. and Sepsis sp.).
Conclusions:Of the dipteran species collected during the study, L. cuprina and C. albiceps could be important for further forensic studies since they were collected from the carcasses and also observed from the rearing units.
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Antioxidant potential of Rumex vesicarius L.: in vitro approach
- Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
