Down-regulated expression of NPM1 in IMS-M2 cell line by (-)-epigallocatechin-3-gallate
Hoang Thanh Chi, Bui Thi Kim Ly, Hoang Anh Vu, Yuko Sato, Phu Chi Dung, Phan Thi Xinh,
1Department of Molecular Cytogenetics, Hematology and Blood Transfusion Hospital in Ho Chi Minh City, Ho Chi Minh, Vietnam
2Department of Medical Genome Sciences, Graduate School of Frontier Sciences, the University of Tokyo, Tokyo, Japan
3Center for Molecular Biomedicine, The University of Medicine and Pharmacy-Ho Chi Minh City, Ho Chi Minh, Vietnam
4Basic nursing science, The Japanese Red Cross College of Nursing Japan, Tokyo, Japan
Down-regulated expression of NPM1 in IMS-M2 cell line by (-)-epigallocatechin-3-gallate
Hoang Thanh Chi1*, Bui Thi Kim Ly2, Hoang Anh Vu3, Yuko Sato4, Phu Chi Dung1, Phan Thi Xinh1,3
1Department of Molecular Cytogenetics, Hematology and Blood Transfusion Hospital in Ho Chi Minh City, Ho Chi Minh, Vietnam
2Department of Medical Genome Sciences, Graduate School of Frontier Sciences, the University of Tokyo, Tokyo, Japan
3Center for Molecular Biomedicine, The University of Medicine and Pharmacy-Ho Chi Minh City, Ho Chi Minh, Vietnam
4Basic nursing science, The Japanese Red Cross College of Nursing Japan, Tokyo, Japan
PEER REVIEW
Peer reviewer
Dr. Narayan D. Chaurasiya, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi, University, MS 38677, USA.
Tel: 662 202 6317 (Cell)
662 915 1364 (Office)
E-mail: narayan.chaurasiya@gmail.com
Comments
This is a good and useful study in which the authors have evaluated the down-regulated expression of NPM1 in IMS-M2 cell line by EGCG. The results obtained in this work clearly suggested that EGCG was good for treatment of AML patients.
Details on Page 574
Objective:To investigate the inhibited effect of epigallocatechin-3-gallate (EGCG) on the expression of NPM1 in IMS-M2 cells harboring the NPM1 mutations.
NPM1, EGCG, IMS-M2, Apoptosis
1. Introduction
Nucleophosmin 1 (NPM1), one of the isoforms of NPM protein (also known as B23, numatrin or NO38), is a protein that shuttles between the nucleus and cytoplasm found at high levels in the granular regions of the nucleolus[1,2]. Many findings have revealed a complex scenario of NPM1 functions and interactions. Some main functions have been ascribed to NPM1: 1) promoting the biogenesis of the ribosome by acting as a chaperone that carries pre-ribosomal particles from the nucleolus to the cytoplasm and by facilitating the processing and maturation of ribosome RNA[1,3]; 2) controlling the duplication of the centrosome during the cell cycle[4]; 3) modulating the function of tumor-suppressor transcription factors, such as interferon regulatory factor 1 (IRF-1) and p53[5]; and regulating the function and stabilityof the p19ARFtumor suppressor[6] and the apoptosis[7].
NPM1 is an essential protein, since the inactivation of the gene encoding for NPM1 in the mouse germ line leads to developmental defects that cause embryonic death in midgestation[8]. In humans, accumulating evidences suggest that NPM1 is directly implicated in the pathogenesis of cancer. NPM1 is over-expressed in solid tumors of diverse histological origin or is involved in tumor progression[9,10]. In several hematologic malignancies, the NPM1 locus is lost or translocated leading to the formation of oncogenic fusion proteins[11]. Moreover, NPM1 is mutated in about one-third of adult patients with acute myeloid leukemia (AML), which makes NPM1 mutations the most frequent genetic lesions so far that identified inde novoAML[12].
Mutations of NPM1 in AML disrupt the nucleolarlocalization signal, causing accumulation of NPM1 in the cytoplasm. AML with mutated NPM1 is generally characterized by good response to induction chemotherapy[13] and favorable prognosis (in the absence of a concomitant FLT3-ITD mutation)[14,15]. However, a significant number of cases with NPM1-mutated AML still show poor outcome, especially those associated with FLT3-ITD mutation and elderly patients. Currently, some possible approaches on the development of a targeted therapy for NPM1-mutated AML were attributed, which including: 1) interfering with the aberrant transport of the NPM1 leukemic mutant; 2) inhibiting the capability of the residual wild-type nucleophosmin and other nucleolar components to act as hub proteins for assemblement of the nucleolus; and 3) intervening on minimal residual disease (NPM1 mutant copy transcripts) before overt hematological relapse occurs (so-called preemptive therapy). Evaluating the activity of epigenetic drugs (e.g.5-azacytidine) or agents acting on differentiation and apoptosis in NPM1-mutated AML is also warranted[14].
In this paper, we have demonstrated that epigallocatechin-3-gallate (EGCG) can down-regulate the expression of NPM1 in IMS-M2 cells harboring the NPM1 mutations. Moreover, EGCG also suppressed the cell proliferation and induced apoptosis in IMS-M2 cells. We suggested that EGCG could be considered as a reagent for treatment of AML patients with NPM1 mutations.
2. Materials and methods
2.1. Cell lines and culture conditions
IMS-M2 cells have been described previously[16]. Briefly, IMS-M2 was established from the bone marrow cells taken from a 59-year-old patient with AML (FAB M2), chromosome abnormalities of 48, XX, add (6) (q27), +8, inv(12) (p13q15), add (15) (q25), +add (15) (q25). This cell line harbors the mutation of NPM1 gene and the fusion of ETV6 to neurotrophin-3 receptor TRKC[16]. A leukemic cell lines MOLM13[17] with NPM1 wild-type[18] was used as control.
The cells were grown in RPMI 1640 medium (Sigma-Aldrich, Japan K.K., Tokyo, Japan) supplemented with 10% heatinactivated fetal bovine serum (JRH Biosciences, Lenexa, KS, USA), 100 IU/mL penicillin, and 0.1 mg/mL streptomycin (Nakalai Tesque, Kyoto, Japan) in a humidified incubator of 5% CO2at 37 °C.
2.2. Reagents
A purified powder of EGCG was generously gifted by Dr. Yukihiko Hara (Japan). EGCG was dissolved in dimethylsulfoxide (DMSO) (Wako Pure. Chemical Industries, Osaka, Japan). Controlled cells were cultured with the same concentration of carrier DMSO as used in the highest dose of reagents. The concentration of DMSO was kept under 0.1% throughout all the experiments to avoid its cytotoxicity.
2.3. Cell proliferation assays
Cell proliferation was determined by trypan blue dye exclusion test as described previously[17]. Briefly, cells were seeded in 6-well plates at a density of 1×105cells/mL in the presence of different concentrations of EGCG for 48 h. After the treatment, 10 µL of the cell suspension was mixed with 10 µL of 0.4% trypan blue, and alive cells were counted manually using a hemacytometer. Results were calculated as the percentage of the values measured when cells were grown in the absence of EGCG.
2.4. Morphologic assessment to detect apoptotic cells
For detecting fragmented nuclei and condensed chromatin, cells at a density of 1×105cells/mL were treated with reagents. After indicated durations, cells were harvested and fixed onto slides by using a cytospin (Shandon, Shandon Southern Products Ltd., Cheshire, UK). Cells then were stained with Wright-Giemsa solution. Morphology of cells was observed under an inverted microscope.
2.5. Western blot analysis
Cells were plated onto 10 cm dishes at a density of 1× 105cells/mL in the presence of various concentrations of EGCG. After incubation for indicated durations, cells were collected and washed twice with PBS (-). Cells were then dissolved in a protein lysis buffer containing 5 mmol/L EDTA, 50 mmol/L NaF, 10 mmol/L Na2H2P2O7, 0.01% Triton X-100, 5 mmol/L HEPES, 150 mmol/L NaCl, 1 mmol/L Na3VO4, 1 mmol/ L phenylmethylsulfonyl fluoride, and 75 µg/mL aprotinin on ice for 30 min with brief vortex of 4 times every 10 min. After centrifugation at 13 000 r/min at 4 °C for 10 min, total cell lysates were collected for western blot analysis. Protein samples were electrophoresed through a polyacrylamide gel and transferred to a Hypond-P membrane (Amersham, Buckinghamshire, UK) by electro-blotting. After washing,the membrane was probed with antibodies and antibodybinding was detected using enhanced chemiluminescence ECL (Amersham). The following antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA): total Akt (sc-1618), anti-rabbit IgG-HRP (sc-2317) and anti-mouse IgG-HRP (sc-2031). Anti-actin (A2066) was from Sigma-Aldrich. Phospho-Akt (Ser473), caspase-3 and NPM antibody were from Cell Signaling Technology Japan (Tokyo, Japan). Anti-PARP antibody was from WAKO Chemicals (Osaka, Japan).
2.6. Statistical analysis
All datas were expressed as the mean±SD. Statistical analyses were done using Student’st-test, in whichP<0.05 was the minimum requirement for a statistically significant difference.
3. Results
3.1. Growth-inhibitory effect of EGCG on IMS-M2 cells
First, to test on growth-inhibitory effect of EGCG, IMSM2 cells were incubated either with the carrier DMSO alone (0 µmol/L EGCG) or with 5, 10, 20, 40 or 80 µmol/L EGCG for 48 h. Cell proliferation was evaluated using the trypan blue exclusion test. The effect of EGCG on cell growth has been demonstrated in various cancers. In IMS-M2 cells, the percent of cell proliferation showed that EGCG suppressed the cell growth of IMS-M2 (Figure 1). The effect of EGCG on IMS-M2 cells is dependent on cell density as coincide with the results observed in GIST cell lines[19]. At a density of 1 ×105cells/mL and 1×104cells/mL, IC50of EGCG was around 20 and 5 µmol/L, respectively (Figure 1). The cell growth inhibition by EGCG diminished dramatically with increasing cell densities, similar to phenomena reported in colorectal carcinoma cells and GIST cell[19].
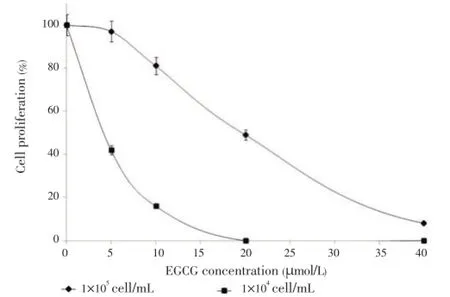
Figure 1. Effect of EGCG on cell proliferation of IMS-M2 cells.IMS-M2 cells at a density of 1×105cells/mL or 1×104cells/mL were treated with 5, 10, 20 or 40 µmol/L EGCG or DMSO alone (0 µmol/L EGCG) as control for 48 h. The number of alive cells was counted after trypan blue exclusion test. Results were calculated as the percentage of the control values.
3.2. Down-regulation of NPM1 in EGCG-treated IMS-M2 cells
Next, we checked whether EGCG can affect on expression status of NPM1 protein. IMS-M2 cells were treated with different concentration of EGCG. After 8 h, the cells were harvested and extracted and then total cell lysates were subjected to western blot analysis. Interestingly, the expression of NPM1 was suppressed in EGCG-treated IMS-M2 cells in dose-dependent manner (Figure 2A). The time course was performed with the EGCG concentration and fixed at 60 µmol/ L for 3 and 8 h. The results shown that EGCG also inhibited the NPM1 expression in a time dependent manner (Figure 2B). Moreover, exposing MOLM13 cells that harboring wild-type NPM1 to different concentration of EGCG revealed that EGCG could not inhibited the expression of NPM1 (Figure 2C).
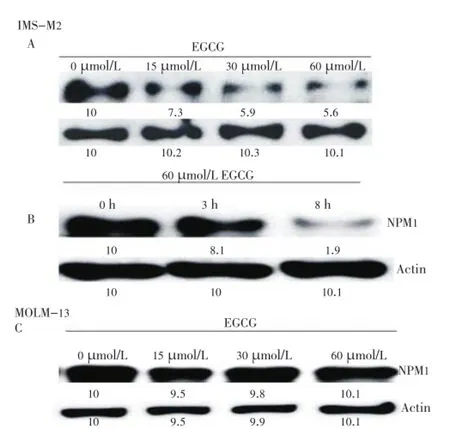
Figure 2. Down-regulation of NPM1 expression in FLT3-mutated cells.Panel A and B showed the results of down-regulation of NPM1 expression by EGCG treatment in IMS-M2 cells as well as MOLM13 control cells shown in Panel C. The cells at a density of 1×105cells/ml were treated with 15, 30, or 60 µmol/L EGCG or DMSO alone (0 µmol/L EGCG) as control for 8 h (A and C) or treated with 60 µmol/L EGCG for an indicated duration (B). Total cell lysates were subjected to western blot analysis with indicated antibodies.
Taken together, our data indicated that EGCG suppressed the cell proliferation of IMS-M2 cells through specific inhibited on NPM1 mutation.
3.3. EGCG suppressed AKT activity in IMS-M2 cells
It has been shown that constitutively active AKT protect cells from apoptosis. To clarify whether AKT were affected by EGCG in IMS-M2 cells, the activity of AKT in IMS-M2 cells treated with or without EGCG was meadured following the indicated duration as shown in Figure 3A. Western blot analysis using antiphosphospecific-Akt antibody showed that EGCG suppressed AKT phosphorylation in a time dependent manner (Figure 3A). That means EGCG could induce cell death in IMS-M2 cells.
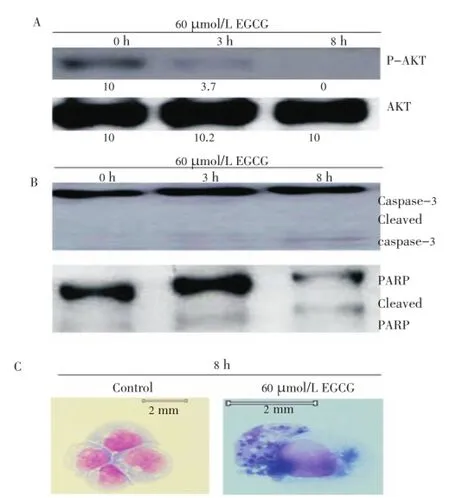
Figure 3. EGCG inhibited AKT activity and induce apoptosis in FLT3-mutated cells. Panel A showed the inhibitory effect of EGCG on AKT activity in IMS-M2 cells. IMS-M2 cells at a density of 1×105cells/ml were treated with 60 µmol/L EGCG or DMSO alone (0 µmol/L EGCG) as control for an indicated duration. Total cell lysates were subjected to western blot analysis with indicated antibodies. Panel B and C showed the evidences of apoptosis induced by EGCG treatment in IMS-M2 cells. The activation forms of caspase-3 and inactivation form of PARP were detected after 8 h treated with 60 µmol/L EGCG (B). The morphology of IMS-M2 cells after treated with or without EGCG were evaluated. After 8 h treated with 60 µmol/L EGCG, cells were fixed onto slides and stained with Wright-Giemsa solution. The arrow indicated that the nuclei of IMS-M2 cells were fragmented by EGCG treatment (C).
3.4. EGCG induced apoptosis in IMS-M2 cells
AKT involved in apoptosis has been clarified. In these cells, AKT phoshorylation was inhibited by EGCG treatment (Figure 3A). To test whether EGCG induced apoptosis in IMS-M2 cells, the cell morphology and the status of some apoptotic markers in IMS-M2 cells were checked after the treatment with EGCG. As a result, after 8 h treatment with 60 µmol/L EGCG, cleaved caspase-3 were detected, then inactivated one enzyme involved in DNA repair, PARP were detected (Figure 3B). Caspase-3 proteolytic cleavage of PARP is a key event in apoptosis. In addition, the observed apoptotic bodies after 60 µmol/L EGCG treatment (Figure 3C) indicated that EGCG caused apoptosis in IMS-M2 cell line harboring NPM1 mutations.
4. Discussion
As mentioned above, one of the potential strategies for treating AML patients with NPM1 mutation is finding any reagents that can enhance the propensity of NPM1-mutated AML cells to die or to be killed[12]. In this study, we demonstrated that EGCG inhibited the cell proliferation and induced apoptosis in IMS-M2 cell line with NPM1 mutation and suggested that EGCG could be a potential reagent for treating AML patients harboring NPM1 mutation.
AML with mutant NPM1 accounts for approximately onethird of all AMLs. Because of its distinctive molecular, pathologic, immunophenotypic and clinical characteristics [13,20], NPM1-mutated AML has been included as a provisional entity in the 2008 World Health Organization classification of lympho-hemopoietic neoplasms. Despite the advantages of understanding about the role of NPM1 in leukegenesis, the development of a targeted therapy for NPM1-mutated AML has still been a problem. For a long time, the predominant abnormal accumulation of NPM1 mutant in cytoplasm attracted the scientists’ concern in finding drugs that can redirect NPM1 from cytoplasm to nucleus. However, it is very difficult to intervene on the abnormal traffic of the NPM1 mutant[20]. Leptomycin B is such a typical example, it can redirect NPM1 mutant to nucleoplasm but cannot direct to nucleolus (the physiological site of NPM1) [12]. Currently, Balusuet al. suggested another direction, that is, to interfere on the level or the oligomerization status of NPM1 that influence its capability to properly build up the nucleolus in NPM1-mutated AML cells[21]. Agreement with Balusuet al., we suggested that finding any reagents that can reduce or even completely inhibit the expression of NPM1 in AML with NPM1 mutation leading to instability of nucleolus could be consider as potential strategies for treating AML with NPM1 mutation.
EGCG, the major polyphenol of green tea, has been used as a beverage for over 5 000 years. EGCG offers several potential clinical advantages compared to other traditional cancer drugs. Most modern medicines currently available for treating cancers are very expensive, while EGCG is globally available as tea, inexpensive to isolate and can be administered orally[22]. In addition, traditional cancer drugs that often destroy some healthy cells along with cancerous cells, while EGCG was noticed as an apoptosis inducer agent that is non-toxic to healthy cells[17,19,22,23]. Moreover, EGCG appears to target biochemical and genetic functions unique to cancer cells[17,22]. In this report we have shown that EGCG specifically targeted on NPM1 expression in IMS-M2 cells, but not in MOLM13 cells that carrying NPM1 wild-type. Some of the anti-carcinogenic agents currently in use have toxic adverse effects. However, data from clinical trials reported to date suggests that EGCG has a very acceptable safety profile[24]. It is noted that green tea is now developing as a cancer preventive drug in the USA and Europe[25]. Currently, there are 83 ongoing clinical trials studying the effects of EGCG on different pathologies[26]. These benefits support further development of EGCG as a potentially useful anti-carcinogenic agent.
AML patients harboring mutant NPM1 often carry FLT3 mutations, particularly the ITD-type mutations and poor prognosis[14]. In another paper, we have also demonstrated that EGCG could down-regulate the expression of FLT3 in FLT3 mutated cell lines (not yet published data) suggesting that EGCG can be a potential reagent for treating AML patients harboring NPM1 /FLT3 mutation.
Conflict of interest statement
We declare no conflict of interest.
Acknowledgements
This work was supported by the Japan Foundation for Promotion of International Medical Research Co-operation (JF-PIMRC); the Global COE Program ‘‘Center of Education and Research for the Advanced Genome-Based Medicine-For personalized medicine and the control of worldwide infectious diseases-’’, MEXT, Japan; the Honjo international scholarship Foundation and the Hematology and Blood Transfusion Hospital in Ho Chi Minh City.
Comments
Background
This is a good work and very informative that the author evaluated the down-regulated expression of NPM1 in IMSM2 cell line by EGCG. Moreover, EGCG also suppressed the cell proliferation and induced apoptosis in IMS-M2 cells. In this study, the authors have suggested that EGCG could be considered as a reagent for treatment of AML patients withNPM1mutations.
Research frontiers
Studies are being performed in order to determine the effect of EGCG on Down-regulated expression of NPM1 in IMS-M2 Cell line.
Related reports
There are very limited reports on related to this study. But the authors have developed a good technique for a treatment of AML using EGCG.
Innovations and breakthroughs
In this study, the authors have evaluated the effect of EGCG
for treatment of AML.
Applications
This study is very useful for treatment of AML patients.
Peer review
This is a good and useful study in which the authors have evaluated the down-regulated expression of NPM1 in IMS-M2 cell line by EGCG. The results obtained in this work clearly suggested that EGCG was good for the treatment of AML patients.
[1] Lindstrom MS. NPM1/B23: a multifunctional chaperone in ribosome biogenesis and chromatin remodeling. Biochem Res Int 2011; 2011: 195209.
[2] Mitrea DM, Grace CR, Buljan M, Yun MK, Pytel NJ, Satumba J, et al. Structural polymorphism in the N-terminal oligomerization domain of NPM1. Proc Natl Acad Sci U S A 2014; 111(12): 4466-4471.
[3] Rees-Unwin KS, Faragher R, Unwin RD, Adams J, Brown PJ, Buckle AM, et al. Ribosome-associated nucleophosmin 1: increased expression and shuttling activity distinguishes prognostic subtypes in chronic lymphocytic leukaemia. Br J Haematol 2010; 148(4): 534-543.
[4] Yu Y, Maggi LB Jr, Brady SN, Apicelli AJ, Dai MS, Lu H, et al. Nucleophosmin is essential for ribosomal protein L5 nuclear export. Mol Cell Biol 2006; 26(10): 3798-3809.
[5] Jian Y, Gao Z, Sun J, Shen Q, Feng F, Jing Y, et al. RNA aptamers interfering with nucleophosmin oligomerization induce apoptosis of cancer cells. Oncogene 2009; 28(47): 4201-4211.
[6] Saporita AJ, Chang HC, Winkeler CL, Apicelli AJ, Kladney RD, Wang J, et al. RNA helicase DDX5 is a p53-independent target of ARF that participates in ribosome biogenesis. Cancer Res 2011; 71(21): 6708-6717.
[7] Li Z, Hann SR. The Myc-nucleophosmin-ARF network: a complex web unveiled. Cell Cycle 2009; 8(17): 2703-2707.
[8] Grisendi S, Bernardi R, Rossi M, Cheng K, Khandker L, Manova K, et al. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 2005; 437(7055): 147-153.
[9] Gimenez M, Souza VC, Izumi C, Barbieri MR, Chammas R, Oba-Shinjo SM, et al. Proteomic analysis of low-to high-grade astrocytomas reveals an alteration of the expression level of raf kinase inhibitor protein and nucleophosmin. Proteomics 2010; 10(15): 2812-2821.
[10] Leal MF, Mazzotti TK, Calcagno DQ, Cirilo PD, Martinez MC, Demachki S, et al. Deregulated expression of nucleophosmin 1 in gastric cancer and its clinicopathological implications. BMC Gastroenterol 2014; 14: 9.
[11] Qian Z, Joslin JM, Tennant TR, Reshmi SC, Young DJ, Stoddart A, et al. Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chem Biol Interact 2010; 184(1-2): 50-57.
[12] Falini B, Gionfriddo I, Cecchetti F, Ballanti S, Pettirossi V, Martelli MP. Acute myeloid leukemia with mutated nucleophosmin (NPM1): any hope for a targeted therapy? Blood Rev 2011; 25(6): 247-254.
[13] Liu YR, Zhu HH, Ruan GR, Qin YZ, Shi HX, Lai YY, et al. NPM1-mutated acute myeloid leukemia of monocytic or myeloid origin exhibit distinct immunophenotypes. Leuk Res 2013; 37(7): 737-741.
[14] Falini B, Martelli MP, Bolli N, Sportoletti P, Liso A, Tiacci E, et al. Acute myeloid leukemia with mutated nucleophosmin (NPM1): is it a distinct entity? Blood 2011; 117(4): 1109-1120.
[15] Daver N, Liu Dumlao T, Ravandi F, Pierce S, Borthakur G, Pemmaraju N, et al. Effect of NPM1 and FLT3 mutations on the outcomes of elderly patients with acute myeloid leukemia receiving standard chemotherapy. Clin Lymphoma Myeloma Leuk 2013; 13(4): 435-440.
[16] Chi HT, Ly BT, Kano Y, Tojo A, Watanabe T, Sato Y. ETV6-NTRK3 as a therapeutic target of small molecule inhibitor PKC412. Biochem Biophys Res Commun 2012; 429(1-2): 87-92.
[17] Ly BT, Chi HT, Yamagishi M, Kano Y, Hara Y, Nakano k, et al. Inhibition of FLT3 expression by green tea catechins in FLT3 mutated-AML cells. PLoS One 2013; 8(6): e66378.
[18] Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A, Macleod RA, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia 2005; 19(10): 1760-1767.
[19] Chi HT, Vu HA, Iwasaki R, Thao le B, Hara Y, Taguchi T, et al. Green tea (-)-epigalocatechin-3-gallate inhibits KIT activity and causes caspase-dependent cell death in gastrointestinal stromal tumor including imatinib-resistant cells. Cancer Biol Ther 2009; 8(20): 1934-1939.
[20] Falini B, Bolli N, Liso A, Martelli MP, Mannucci R, Pileri S, et al. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: molecular basis and clinical implications. Leukemia 2009; 23(10): 1731-1743.
[21] Balusu R, Fiskus W, Rao R, Chong DG, Nalluri S, Mudunuru U, et al. Targeting levels or oligomerization of nucleophosmin 1 induces differentiation and loss of survival of human AML cells with mutant NPM1. Blood 2011; 118(11): 3096-3106.
[22] Jin P, Wu H, Xu G, Zheng L, Zhao J. Epigallocatechin-3-gallate (EGCG) as a pro-osteogenic agent to enhance osteogenic differentiation of mesenchymal stem cells from human bone marrow: an in vitro study. Cell Tissue Res 2014; doi: 10.1007/s00441-014-1797-9.
[23] Iwasaki R, Ito K, Ishida T, Hamanoue M, Adachi S, Watanabe T, et al. Catechin, green tea component, causes caspase-independent necrosis-like cell death in chronic myelogenous leukemia. Cancer Sci 2009; 100(2): 349-356.
[24] Mah YJ, Song JS, Kim SO, Lee JH, Jeon M, Jung UW, et al. The effect of epigallocatechin-3-gallate (EGCG) on human alveolar bone cells both in vitro and in vivo. Arch Oral Biol 2014; 59(5): 539-549.
[25] Tsao AS, Liu D, Martin J, Tang XM, Lee JJ, El-Naggar AK, et al. Phase II randomized, placebo-controlled trial of green tea extract in patients with high-risk oral premalignant lesions. Cancer Prev Res (Phila) 2009; 2(11): 931-941.
[26] U.S. National Institutes of Health. Search for studies, ClinicalTrials. gov. Bethesda, Maryland: U.S. National Institutes of Health; 2014. [Online] Available from: http://www.clinicaltrials.gov/ct2/home. [Accessed on 27 January, 2014].
10.12980/APJTB.4.2014APJTB-2014-0177
*Corresponding author: Hoang Thanh Chi, Department of Molecular Cytogenetics, Hematology and Blood Transfusion Hospital in Ho Chi Minh City, 118 Hong Bang Street, District 5, Ho Chi Minh City, Vietnam.
Tel: 84-932-728115
E-mail: hoangchidc1985@yahoo.com
Foundation Project: Supported by the Japan Foundation for Promotion of International Medical Research Co-operation (JF-PIMRC).
Article history:
Received 15 May 2014
Received in revised form 22 May, 2nd revised form 30 May, 3rd revised form 9 Jun 2014
Accepted 23 Jul 2014
Available online 28 Jul 2014
Methods:Cell proliferation assay was performed to test the effects of EGCG on cell growth of IMS-M2 cells harboring the NPM1 mutations. Western blot analysis were performed to test the protein expression of NPM1, AKT, those associated with apoptosis.
Results:EGCG can down-regulate the expression of NPM1 in IMS-M2 cells harboring the NPM1 mutations. Moreover, EGCG also suppressed the cell proliferation and induced apoptosis in IMSM2 cells.
Conclusions:The results suggested that EGCG could be considered as a reagent for treatment of AML patients with NPM1 mutations.
 Asian Pacific Journal of Tropical Biomedicine2014年7期
Asian Pacific Journal of Tropical Biomedicine2014年7期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Antimicrobial activity against periodontopathogenic bacteria, antioxidant and cytotoxic effects of various extracts from endemic Thermopsis turcica
- Proteomics analysis of antimalarial targets of Garcinia mangostana Linn.
- The presence of eucalyptol in Artemisia australis validates its use in traditional Hawaiian medicine
- Jeju seaweeds suppress lipopolysaccharide-stimulated proinflammatory response in RAW 264.7 murine macrophages
- Antioxidant potential of Rumex vesicarius L.: in vitro approach
- Cytotoxicity screening of Melastoma malabathricum extracts on human breast cancer cell lines in vitro
