Argyrophilic nucleolar organizer region in MIB-1 positive cells in non-small cell lung cancer: clinicopathological signi fi cance and survival
Dmitriy Sergeevich Kobyakov, Ashot Merudzhanovich Avdalyan, Aleksandr Fedorovich Lazarev, Elena Leonidovna Lushnikova, Lev Moiseevich Nepomnyashchikh
1Budget Institution, Kogalym City Hospital, Kogalym 628484, Russia;
2Laboratory of Molecular Diagnostics of Altai Branch of Russian N.N. Blokhin Cancer Research Center, Barnaul 656049, Russia;
3Federal State Budget Institution Research Institute of Regional Pathology and Pathomorphology of Siberian Branch of Russian Academy of Medical Sciences, Novosibirsk 630117, Russia
Argyrophilic nucleolar organizer region in MIB-1 positive cells in non-small cell lung cancer: clinicopathological signi fi cance and survival
Dmitriy Sergeevich Kobyakov1, Ashot Merudzhanovich Avdalyan2, Aleksandr Fedorovich Lazarev2, Elena Leonidovna Lushnikova3, Lev Moiseevich Nepomnyashchikh3
1Budget Institution, Kogalym City Hospital, Kogalym 628484, Russia;
2Laboratory of Molecular Diagnostics of Altai Branch of Russian N.N. Blokhin Cancer Research Center, Barnaul 656049, Russia;
3Federal State Budget Institution Research Institute of Regional Pathology and Pathomorphology of Siberian Branch of Russian Academy of Medical Sciences, Novosibirsk 630117, Russia
Objective:To evaluate the relation between argyrophilic nucleolar organizer region (AgNOR)-associated proteins and clinicopathological parameters and survival in non-small-cell lung cancer (NSCLC).
Argyrophilic nucleolar organizer region (AgNOR); MIB-1; survival; non-small cell lung cancer (NSCLC)
Introduction
Biomarkers related to signi fi cant clinicopathological parameters of non-small cell lung cancer (NSCLC), particularly survival of patients and ability to predict disease course with a high degree of probability, should be investigated1. Proliferation is a basic process that reveals tumor appearance and development; this process is also a factor in the prediction of the biological behavior of tumors. However, reliable evaluation of theproliferative potential of tumors is difficult to achieve because proliferation involves not only a number of proliferative cells (proliferative activity and growth fraction) but also the velocity of cells undergoing phases of cell cycle (duration of cell cycle)2.
Immunohistochemical assay with Ki-67 antigen is among the most common and available methods to estimate tumor proliferative activity. Ki-67 antigen is detected in cells in late G1, S, G2, and M phases; however, the functional role of this nuclear protein in proliferation remains unclear3. Argyrophilic proteins associated with nucleolar organizer regions (AgNOR) are markers of cell cycle velocity. Nucleolar organizer regions are ribosomal DNA sequences on the short arms of human acrocentric chromosomes (13, 14, 15, 21, and 22) and encode ribosomal RNA (rRNA). A peculiar group of acidic and highly argyrophilic proteins are also localized at the same sites asnucleolar organizer regions, thereby allowing these regions to be very clearly and rapidly visualized by silver nitrate staining procedures. Up to 75% staining of AgNOR consists of two major argyrophilic proteins, namely, C23 (nucleolin) and B23 (nucleophosmin). Nucleolin is a 105 kDa phosphoprotein that plays an important role in the transcription of rRNA molecules; nucleophosmin is a 38 to 39 kDa phosphoprotein involved in late stages of pre-ribosomal particle organization2. Nucleolin and nucleophosmin are detected in cell nuclei during the entire cell cycle; these phosphoproteins increase in quantity by 1.5- to 3-fold in S and G2phases4. Notably, the amount of AgNOR in the interphase is related to the speed of cell proliferation. AgNOR is inversely related to cell cycle duration and tumor-doubling time5. The relationship between interphase AgNOR quantity and cell doubling time can be attributed to proliferating cells that produce an adequate ribosomal complement for daughter cells; a short cell cycle indicates high ribosomal biogenesis per time unit.e amount of AgNOR in the interphase is also related to cell-doubling time because the number of AgNOR in the interphase is related to rRNA transcriptional activity. The evaluation of the quantitative distribution of AgNOR represents a unique tool to obtain information regarding the proliferation rate of tumors during diagnosis from histological sections of routinely processed tissue samples2.
Munakata et al.6proposed the method of double staining involving Ki-67 antigen and AgNOR to evaluate AgNOR (cell cycle duration) in proliferating cells. However, limited research has been conducted regarding the proliferative potential of tumors by double staining of Ki-67 antigen and AgNOR7-13. Studies have also been performed regarding the importance of AgNOR14-19. Despite these studies, the results of double staining of Ki-67 antigen and AgNOR have not yet been evaluated by computer image analysis and in relation to clinicopathological parameters under TNM system and survival in NSCLC. The TNM system is described as follows.e T status re fl ects tumor size and collection of size-independent tumor descriptors, such as visceral pleural invasion, main bronchus involvement, atelectasis, or obstructive pneumonitis. The N status describes regional lymph nodes. The M status shows distant metastasis. Therefore, the current study aimed to explore the AgNOR area in MIB-1-positive cells in relation to clinicopathological parameters and survival in NSCLC.
Materials and methods
We studied 207 surgical specimens of NSCLC resected from 2007 to 2009 in the Altai Krai Oncology Dispensary.e mean age of patients was 59 years (range, 35-75 years); the patients included 177 males (86%) and 30 females (14%). Lobectomy and pneumonectomy were performed in 145 (70%) and 62 (30%) patients, respectively. Preoperative chemotherapy and radiation therapy were not conducted. Postoperative chemotherapy, most frequently with cisplatin and etoposide, was administered to 30 patients (14%). Postoperative radiation therapy, with a total focal dose ranging from 50 to 60 Gr, was conducted in 64 patients (31%). The clinicopathological parameters of NSCLC were determined in accordance with the TNM classi fi cation of seven reviews20. In the current study, no cases were found with M1status under the TNM system, but cases with multiple tumors were detected. The greatest tumor dimension was measured (in cm).is study was examined and approved by the corresponding ethics committee; this study was also performed in accordance with the ethical standards presented in the Declaration of Helsinki. Written informed consent was obtained.
Tissue fragments were fixed for 18 to 24 h in 10% neutral buffered formalin. After standard processing of the surgical material, we prepared histological slices of 4 μm in thickness. Specimens were stained with hematoxylin and eosin to con fi rm the original pathological diagnosis. For differential diagnostic purposes, histochemical (periodic acid-Schiff-alcian blue and according to Kreiberg) and immunohistochemical staining were applied. Immunohistochemical staining was performed using Ventana Discovery XT automated stainer, as described by the manufacturer (Ventana Medical System, Tucson, AZ, USA). Primary antibodies were used for cytokeratins 7 (clone SP52), 20 (clone SP33), high molecular weight (clone 34βE12), and 5/6 (clone D5/16B4). The epidermis and the gastric mucosa were used as stain control.
Based on the review of corresponding histological specimens, three tissue cores were obtained from each patient by using paraffin blocks with a needle of 1.5 mm in internal diameter.ree tissue microarrays were prepared, each containing 12×18 cores. Histological slices of 4 μm in thickness were obtained from the tissue microarrays. Slices from the tissue microarrays were immunohistochemically stained following the manufacturer’s protocol for DAKO: streptavidin–biotin method with primary antibodies to Ki-67 (clone MIB-1, DAKO) and chromogen as new fuchsin.e slices were autoclaved at 120 ℃ for 20 min in 0.01 M citrate bu ff er (pH=6.0) before staining was performed. The slices were subsequently incubated with chromogen and washed in bidistilled water. The slices were then stained with silver nitrate by one-step method6in a humid chamber at 37 ℃for 19 min. Further staining of nuclei was not performed, and the slices were placed in a water medium (Faramount, DAKO). Only one representative core from each patient was analyzed.Silver-stained specimens were examined under OLYMPUS CX-41 microscope equipped with a Plan C ×100/1.25 oil lens.e images were digitized and transferred directly into the computer with OLYMPUS DP72 digital camera and cellSense v.1.1 soware with resolution of 1,360×1,024 pixels.e AgNOR area (in μm2) in each nucleus of 100 random MIB-1-positive cells (from 10 to 30 digital images) was measured. Semi-automated image processing was performed with ImageJ v.1.42.e images were subjected to conversion into 8-bit image, background, normalization, segmentation, binary processing, and final measurements. To eliminate measurement errors, we excluded granules with a size of <0.1 μm2from analysis. The internal control of stromal lymphoid follicles was attended by MIB-1-positive centroblasts (positive control) and MIB-1-negative small lymphocytes (negative control). The entire AgNOR quanti fi cation was performed by one of the authors.
Statistical analysis
Statistical analysis was performed using STATISTICA 6.0. Data values were expressed as median and interquartile range. Data were processed by Kruskal-Wallis test, and significant differences were assessed by Mann–Whitney U test. Comparisons were performed using χ2test. Probabilities of 5-year overall survival were calculated by Kaplan-Meier method (in %), and between-group comparisons were conducted using log-rank test. Univariate and multivariate Cox proportional hazards model was used for the following factors: age at surgery (<59 vs. ≥59 years), gender (male vs. female), type of surgery (lobectomy vs. pneumonectomy), postoperative chemotherapy (yes vs. no), postoperative radiatio n therap y (yes vs. n o), T status (T1vs. T2to T3), greatest tumor dimension (<3 vs. ≥3 cm), N status (N0vs. N1to N3), TNM stage (I vs. II to III), histology (adenocarcinoma vs. squamous-cell cancer), and di ff erentiation (well vs. moderate to poor). Statistical signi fi cance was set at P<0.05.
Results
Immunohistochemical staining results of slides with primary antibodies for MIB-1 and subsequent staining results with silver nitrate were detected in the form of black round granules (AgNOR) located against a red nucleus (MIB-1-positive cells) or against a brown nucleolus or pale yellow nucleus (MIB-1-negative cells; Figure 1). In the nuclei, isolated black round granules (dots) appeared in the nucleoplasm (usually in MIB-1-negative cells) and/or multiple black round granules (dots) as a cluster (usually in MIB-1-positive cells). The morphometric results of the AgNOR area in MIB-1-positive cells in different clinicopathological parameters of NSCLC are shown in Table 1.
In NSCLC, the median AgNOR area in MIB-1-positive cells was 10.47 μm2(range, 8.57-12.69 μm2), and this value was chosen as cut-o ff point. Cases with AgNOR area ≥10.47 μm2in MIB-1-positive cells were counted as large-area cases (101 cases, 49%) and < 10.47 μm2as small-area cases (106 cases, 51%). The AgNOR area in MIB -1-positive cells was significantly larger in groups T2and T3than in T1. In NSCLC with the greatest tumor dimension of <3 cm, the AgNOR area in MIB-1-positive cells was smaller than in those with tumor size ≥3 cm. A statistically signi fi cant increase in the AgNOR area in MIB-1-positive cells of NSCLC with metastases to regional lymph nodes was o bserved versus n on-metastatic tumo rs (Figure 1A-D).The AgNOR area in MIB-1-positive cells was smaller in TNM stage I than in stages II to III. No difference was observed in the AgNOR area in MIB-1-positive cells of adenocarcinoma and squamous cell cancer.e AgNOR area in MIB-1-positive cells was larger in moderately and poorly di ff erentiated tumors than in well-di ff erentiated tumors (Figure 1E-H).e A gNOR area in MIB-1-positive cells of NSCLC was correlated with T status (P<0.001), greatest tumor dimension (P<0.001), N status (P<0.001), TNM stage (P<0.001), and differentiation (P<0.001).

Figure 1AgNOR in MIB-1-positive and MIB-1-negative cells of NSCLC in squamous cell cancer with absence (A, B) and presence (C, D) of metastases to lymph nodes and in adenocarcinoma with well (E, F) and poor (G, H) di ff erentiation. Double staining for Ki-67 (clone MIB-1) by immunohistochemistry and for AgNOR with silver nitrate, ×1,000. AgNOR, argyrophilic nucleolar organizer region; NSCLC, non-small-cell lung cancer.

Table 1 AgNOR area in MIB-1-positive cells and clinicopathological parameters of NSCLC
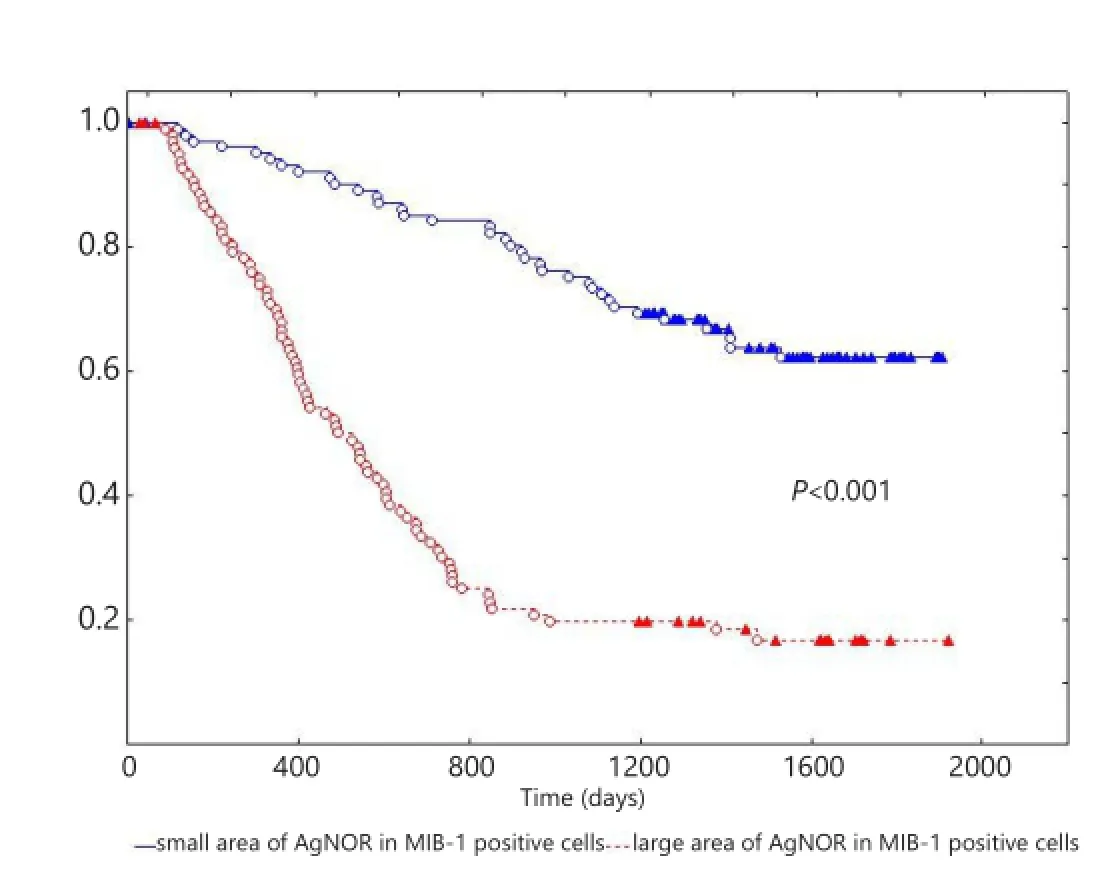
Figure 2Kaplan-Meier curve of patients with NSCLC with small and large AgNOR areas in MIB-1-positive cells. X-axis shows the time of life (in days); Y-axis shows the proportion of surviving patients. NSCLC, non-small-cell lung cancer; AgNOR, argyrophilic nucleolar organizer region.

Table 2 Five-year overall survival stratified according to cut-off point of AgNOR area in MIB-1-positive cells and clinicopathological parameters of NSCLC
The 5-year overall survival of patients with NSCLC was (39.3±3.8)%. The overall survival of patients with NSCLC exhibited statistically significant difference (P<0.001) based on the AgNOR area in MIB-1-positive cells at (61.2±5.4)% in small area vs. (16.2±4.2)% in large area (Figure 2).e overall survival of patients with NSCLC was also strati fi ed based on the AgNOR area in MIB-1-positive cells, and the clinicopathological parameters showed statistically signi fi cant di ff erence (Table 2).In univariate analysis, the following factors affected the survival of patients: AgNOR area in MIB-1-positive cells (χ2=59.9, P<0.001), N status (χ2=52.2, P <0.001), TNM stage (χ2=44.2, P<0.001), differentiation (χ2=21.7, P<0.001), greatest tumor dimension (χ2=21.3, P<0.001), type of surgery (χ2=8.7, P=0.002), T status (χ2=6.9, P=0.01), and histological characteristics (χ2=5.2, P=0.02). Age, gender, postoperative chemotherapy, and radiation therapy did not a ff ect the survival of patients with NSCLC in univariate analysis. In multivariate analysis (χ2=120.2), the survival of patients was a ff ected by the AgNOR area in MIB-1-positive cells (β=1.05, standard error =0.23, P<0.001), greatest tumor dimension (β=0.94, standard error =0.35, P=0.007), metastases to regional lymph nodes (β=0.79, standard error =0.34, P=0.02), histology (β=0.23, standard error =0.11, P=0.03), and differentiation (β =0.66, standard error =0.29, P=0.02). Likewise, age, gender, type of surgery, postoperative chemotherapy, radiation therapy, T status, and TNM stage did not influence the survival of patients with NSCLC in multivariate analysis.
Discussion
Our study found the relationship between the AgNOR area in MIB-1-positive cells of NSCLC and the following clinicopathological parameters under the TNM system: T status, greatest tumor dimension, N status, TNM stage, and differentiation. Other studies have also shown the relation of individual clinicopathological parameters under the TNM system with AgNOR in MIB-1-positive cells. Yamaguchi13found that AgNOR increases in MIB-1-positive cells of NSCLC in T4vs. T1to T3and in N2to N3vs. N0to N1. Kidogawa et al.10also found that AgNOR increases in MIB-1-positive cells of breast cancer with the largest size of >2 cm vs. tumor size <2 cm. Tomobe et al.12found a sequential increase in AgNOR in MIB-1-positive cells of bladder cancer in T1, T2, and T3. In contrast to our current results obtained from objective analysis (computer image analysis and measurement of AgNOR area), these previous results were obtained from subjective analysis (visual counting of AgNOR number). Other studies have shown the relationship between clinicopathological parameters under TNM system and AgNOR14-18.us, our study showed that the AgNOR area in MIB-1-positive cells was correlated with clinicopathological parameters, thereby re fl ecting the relationship among molecular, biological, and clinicopathological parameters of NSCLC.
The survival of patients with NSCLC with small AgNOR area in MIB-1-positive cells is better than that of those with greater AgNOR area, as well as in homogeneous groups of clinicopathological parameters. In univariate and multivariate regression analyses, the AgNOR area in MIB-1-positive cells independently affected the survival of patients with NSCLC. Our results correlate with those of studies on NSCLC9, breast cancer7,8,10,11, and bladder cancer12. Studies on AgNOR in malignant tumors have also revealed that the content of AgNOR is an independent prognostic factor16-19. The prognostic value of A gNOR in MIB-1-positive cells is related to di ff erent rates of NSCLC proliferation. A large AgNOR area in MIB-1-positive cells indicates short cell cycle of proliferating cells and high proliferation speed. By contrast, small area implies long cell cycle of proliferating cells and low proliferation speed.us, our study showed that the AgNOR area in MIB-1-positive cells was correlated with the survival of patients with NSCLC; this result revealed the relationship of molecular and biological parameters, as well as biological behaviors of tumors. The current results provide further insights into an accurate assessment of actual survival curves and prognosis of patients with NSCLC.
Conclusion
In NSCLC, clinicopathological parameters under the TNM system are related to molecular and biological parameters, including AgNOR area in MIB-1-positive cells. The survival of patients with NSCLC with a small AgNOR area in MIB-1-positive cells is better than that of patients with a large AgNOR area. Molecular, biological (AgNOR area in MIB-1-positive cells), and clinicopathological (greatest tumor dimension, metastases to regional lymph nodes, histology, and di ff erentiation) parameters are independent prognostic factors of NSCLC.
Acknowledgements
We gratefully acknowledge Boris Borisovitch Salnikov for his help in the English translation of our article.
Con fl ict of interest statement
No potential con fl icts of interest are disclosed.
1. Larsen JE, Minna JD. Molecular biology of lung cancer: clinical implications. Clin Chest Med 2011;32:703-740.
3. Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol2006;206:624-635.
4. Sirri V, Roussel P, Hernandez-Verdun D.e AgNOR proteins: qualitative and quantitative changes during the cell cycle. Micron 2000;31:121-126.
5. Canet V, Montmasson MP, Usson Y, Giroud F, Brugal G. Correlation between silver-stained nucleolar organizer region area and cell cycle time. Cytometry 2001;43:110-116.
6. Munakata S, Hendricks JB. A multilabeling technique for simultaneous demonstration and quantitation of Ki-67 and nucleolar organizer regions (AgNORs) in para ffi n-embedded tissue. J Histochem Cytochem 1994;42:789-793.
7. Abboud P, Lorenzato M, Joly D, Quereux C, Birembaut P, Ploton D. Prognostic value of a proliferation index including MIB1 and argyrophilic nucleolar organizer regions proteins in node-negative breast cancer. Am J Obstet Gynecol 2008;199:146,e1-146,e7.
8. Biesterfeld S, Farokhzad F, Klüppel D, Schneider S, Hufnagl P. Improvement of breast cancer prognostication using cell kineticbased silver-stainable nucleolar organizer region quanti fi cation of the MIB-1 positive tumor cell compartment. Virchows Arch 2001;438:478-484.
9. Bigras G, Marcelpoil R, Brambilla E, Brugal G. Interest of targeting AgNORs measurement in cycling cells: in vivo cell kinetic evaluation of non-small cell lung cancer. Anal Cell Pathol 1996;11:183-198.
10. Kidogawa H, Nanashima A, Yano H, Matsumoto M, Yasutake T, Nagayasu T. Clinical signi fi cance of double staining of MIB-1 and AgNORs in primary breast carcinoma. Anticancer Res 2005;25:3957-3962.
11. Lorenzato M, Abboud P, Lechki C, Browarnyj F, O’Donohue MF, Ploton D, et al. Proliferation assessment in breast cancer: a d ouble-staining technique for AgNOR quanti fi cation in MIB-1 positive cells especially adapted for image cytometry. Micron 2000;31:151-159.
12. Tomobe M, Shimazui T, Uchida K, Hinotsu S, Akaza H. Argyrophilic nucleolar organizer region in proliferating cell has a predictive value for local recurrence in super fi cial bladder tumor. J Urol 1999;162:63-68.
13. Yamaguchi S. Relationship between the responses to simultaneous double staining for Ki-67 and AgNOR and the clinicopathological features of non-small cell pulmonary carcinoma. Acta Med Nagasaki 1994;39:147-152.
14. Eröz R, Unluhizarci K, Cucer N, Ozturk F. Value of argyrophilic nucleolar organizing region protein determinations in nondiagnostic fi ne needle aspiration samples (due to insu ffi cient cell groups) of thyroid nodules. Anal Quant Cytopathol Histpathol 2013;35:226-231.
15. Eroz R, Cucer N, Unluhizarci K, Ozturk F. Detection and comparison of cut-o ff values for total AgNOR area/nuclear area and AgNOR number/nucleus in benign thyroid nodules and normal thyroid tissue. Cell Biol Int 2013;37:257-261.
16. Winzer KJ, Bellach J, Hufnagl P. Long-term analysis to objectify the tumour grading by means of automated microscopic image analysis of the nucleolar organizer regions (AgNORs) in the case of breast carcinoma. Diagn Pathol 2013;8:56.
17. Avdalyan A, Bobrov I, Klimachev V, Lazarev A. Prognostic Value of Microvessel Density in Tumor and Peritumoral Area as Evaluated by CD31 Protein Expression and Argyrophilic Nucleolar Organizer Region Count in Endothelial Cells in Uterine Leiomyosarcoma. Sarcoma 2012;2012:594512.
18. Treré D, Ceccarelli C, Migaldi M, Santini D, Ta ff urelli M, Tosti E, et al. Cell proliferation in breast cancer is a major determinant of clinical outcome in node-positive but not in node-negative patients. Appl Immunohistochem Mol Morphol 2006;14:314-323.
19. Pich A, Chiusa L, Margaria E. Prognostic relevance of AgNORs in tumor pathology. Micron 2000;31:133-141.
20. Sobin LH, Gospodarowicz MK, Wiekind C. eds. TNM classi fi cation of malignant tumours. 7th edition. Oxford: Wiley-Blackwell, 2009.
Cite this article as:Kobyakov DS, Avdalyan AM, Lazarev AF, Lushnikova EL, Nepomnyashchikh LM. Argyrophilic nucleolar organizer region in MIB-1 positive cells in non-small cell lung cancer: clinicopathological signi fi cance and survival. Cancer Biol Med 2014;11:264-269. doi: 10.7497/ j.issn.2095-3941.2014.04.005
Dmitriy Sergeevich Kobyakov
E-mail: dskob@yandex.ru
Received August 29, 2014; accepted December 11, 2014. Available at www.cancerbiomed.org
Copyright © 2014 by Cancer Biology & Medicine
Methods:A total of 207 surgical specimens diagnosed as NSCLC were included in this study. Double-staining procedures were performed using antigen Ki-67 (clone MIB-1) and silver nitrate by immunohistochemical and AgNOR-staining methods.
Results:The AgNOR area in MIB-1-positive cells of NSCLC is related to clinicopathological parameters under the TNM (tumor, node, and metastasis) system. The survival of patients with small AgNOR area in MIB-1-positive cells is better than that of patients with large AgNOR area. Molecular, biological (AgNOR area in MIB-1-positive cells), and clinicopathological (greatest tumor dimension, metastases to regional lymph nodes, histology, and differentiation) parameters are independent prognostic factors of NSCLC.
Conclusion:e AgNOR area in MIB-1-positive cells is related to clinicopathological parameters and survival in NSCLC.
 Cancer Biology & Medicine2014年4期
Cancer Biology & Medicine2014年4期
- Cancer Biology & Medicine的其它文章
- Minimally invasive local therapies for liver cancer
- Combining targeted therapy and immune checkpoint inhibitors in the treatment of metastatic melanoma
- Recent advances in lymphatic targeted drug delivery system for tumor metastasis
- Emerging function of mTORC2 as a core regulator in glioblastoma: metabolic reprogramming and drug resistance
- E ff ect of EGFR-TKI retreatment following chemotherapy for advanced non-small cell lung cancer patients who underwent EGFR-TKI
- Sequential maximum androgen blockade (MAB) in minimally symptomatic prostate cancer progressing after initial MAB: two case reports
