Empowering sonic hedgehog to rescue brain cells after ischemic stroke
Empowering sonic hedgehog to rescue brain cells after ischemic stroke
Ischemic stroke occurs when blood supply to the brain is interrupted. This can cause irreversible injury to the central nervous system (CNS) tissue. Each year in the United States almost 800,000 people experience a new or recurrent stroke. 15% of stroke patients die shortly after insult and only 10% recover completely, leaving the majority of surviving stroke patients with disabilities. Tissue-type plasminogen activator (tPA) is the only available therapy for stroke but its clinical use is limited because of associated danger of intracranial hemorrhage. Therefore, there is an emergent need for stroke therapeutics that are safe and effective when administered at a later time point after insult.
There are two main targets for stroke therapy: 1) neuroprotection, to prevent degeneration of damaged tissue in the early events (within hours and days) and/or 2) regeneration, to promote repair of lost tissue at later events (of days, weeks and months).
Recently, we found that targeting the developmental pathway sonic hedgehog (Shh) with small-molecule Purmorphamine (PUR), administered at 6 hours after middle cerebral artery occlusion (MCAO), an animal model of ischemic stroke, proved benefcial in reducing the infarct area, improving neurological outcome and promoting regeneration (Chechneva et al., 2014).
Shh is a morphogen that plays a fundamental role in CNS development. Binding of Shh to its receptor Patched releases the inhibition of G-protein coupled receptor Smoothened (SMO) to allow the translocation of Gli transcription factors from the cytoplasm into the nucleus. Gli proteins contain zinc-finger DNA binding domains and are key regulators of Shh signaling transduction. Shh can also act through a non-canonical pathway by passing Gli-mediated transcription.
In the healthy adult CNS, Shh of neuronal origin reduces astrocyte reactivity and controls proliferation of neural progenitors. Shh expressed by endothelial cells and astrocytes can infuence the integrity of the BBB (Garcia et al., 2010; Alvarez et al., 2011; Sirko et al., 2013; Chechneva et al., 2014). Thus, in the adult CNS, Shh acts as an intercellular communicator and its cellular origin might be a decisive regulatory factor. In response to CNS injury, Shh signaling is activated (Sims et al., 2009; Chechneva et al., 2014). Within hours after insult, Shh expression in neurons increases (Sims et al., 2009; Chechneva et al., 2014). The degree of Shh activation depends on the severity of tissue damage and increased Shh signaling early after brain injury promotes endogenous neuroprotective mechanisms. We found a transient increase of Shh mRNA levels in the ipsilateral and contralateral cortex at 9 hours after ischemic stroke (Figure 1). An increase of neuronal Shh might impede glial cell activation and the initiation of an inflammatory response. In addition, we observed a reduction of glial fbrillary acidic protein, a marker of astrocyte reactivity, in the ischemic area at the early hours after stroke. This mechanism could prove crucial for cell survival. As a readout of Shh signaling activation, Gli1 was upregulated in the ipsilateral cortex during longer periods of time, from 9 to 24 hours after stroke. Nonetheless, endogenous activation of Shh signaling proved not suffcient to protect CNS tissue after ischemic injury (Huang et al., 2013; Chechneva et al., 2014). Administration of recombinant Shh, however, was shown to reduce oxidative stress and apoptotic cell death, to ameliorate brain edema and to diminish permeability of the BBB, resulting in a decrease of infarct area and an improvement of neurological outcome (Bambakidis et al., 2012; Huang et al., 2013; Xia et al., 2013). Shh signaling activation has been implicated in increased neural progenitor proliferation and induced angiogenesis after treatment with recombinant Shh or a mixture of neurotrophic peptides (Bambakidis et al., 2012; Huang et al., 2013; Xia et al., 2013; Zhang et al., 2013). Hence, an increasing number of evidence demonstrates a benefcial effect of Shh therapy in stroke, indicating that the reiteration of a developmental pathway in the adult brain confers neuroprotection against brain injury associated with cerebral ischemia and promotes regeneration.
In our study, we used an agonist of the SMO receptor, PUR, and found that the neuroprotective effect of PUR administered intravenously at 6 hours after ischemic stroke was associated with a reduction of apoptotic cell death, indicated by a decrease of pro-apoptotic factors caspase-3, Bad and Bax. Surprisingly, PUR did not alter the ischemia-induced level of inflammatory mediators tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β) or interleukin-6 (IL-6), brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF) or vascular endothelial growth factor (VEGF) or Shh signaling molecules (Shh, Ptch1, Smo, Gli2 or Gli1), but increased the expression of tPA in ischemic neurons at 3 hours after treatment. tPA deploys multiple mechanisms with a broad range of effects depending on its concentration and targeted cell type, and has been linked to synaptic plasticity, excitotoxicity, blood brain barrier (BBB) breakdown, and cell fate determination. We found that increased tPA expression in ischemic neurons after treatment with SMO agonist PUR was associated with reduced apoptotic cell death, indicating a previously undescribed connection of tPA with neuronal survival after ischemic injury. Administration of the thrombolytic agent tPA, currently the only FDA-approved treatment available for sudden onset ischemic stroke, is limited to a narrow therapeutic time window due to risk of exacerbated bleeding. Interestingly, treatment with PUR decreased BBB permeability, giving additional credit to the targeting of Shh signaling with PUR as an alternative approach to tPA treatment fordelayed stroke therapy. Treatment with PUR might induce just the right amount of tPA required for neuroprotection or tPA of neuronal origin possesses an effect different to tPA administered exogenously. Further understanding of the detailed mechanism of PUR-induced tPA expression in neurons early after stroke and the role of neuronal tPA in the regulation of BBB integrity by PUR are required. To evaluate the impact of neuronal tPA expression on PUR-induced neuroprotection, the effect of PUR in the presence of tPA inhibitors needs to be evaluated. As a small molecule compound, PUR has advantages in the ease of administration, it is considerably less expensive and has a longer shelf-life compared to Shh protein itself. Thus, PUR indeed could qualify for novel therapeutic use in the treatment of ischemic stroke with a potentially broader therapeutic time window than tPA.
After an initial activation early after stroke, Shh signaling is downregulated but a second increase of its activity is detected around day 7 in association with glial scar formation and neural progenitor proliferation (Figure 1) (Sims et al., 2009; Chechneva et al., 2014). The glial scar isolates damaged area with a high degree of infammation from the healthy CNS environment. But it also represents a physical barrier that restricts axonal outgrowth and cell migration, limiting regeneration of lost tissue. In addition, the infammatory milieu present in the infarct area does not support the survival of newly generated neurons. Reactive astrocytes of the glial scar exhibit a hypertrophic morphology, a high proliferation rate and Shh expression. Shh provides an autocrine regulation to astrocytes by promoting their proliferation, reactivity and stem cell properties (Sirko et al., 2013; Chechneva et al., 2014). It appears that neuronal loss results in the loss in regulatory signals, such as Shh of neuronal origin, and that a compensatory mechanism for this loss manifests in the overexpression of Shh by reactive astrocytes that in turn stimulates their proliferation and further increases Shh expression.
In our study, a second treatment with PUR was administered at 4 days after stroke to examine its effect on regeneration. The evaluation of brain tissue at 14 days after insult revealed a reduction of reactive astrogliosis and infammation in PUR-treated animals. We also observed an increase of newly generated neurons and synaptogenesis in the peri-infarct and infarct area and neovascularization in the infarcted zone. It has been shown that bone marrow stromal cells transplanted intravenously for 7 days daily after MCAO increased tPA expression in neurons and astrocytes present in the ischemic boundary zone at 14 days after insult. The increase of tPA was regulated by Shh signaling and associated with an increase of markers for synaptic plasticity and functional improvement in mice (Ding et al., 2013). Whether treatment with PUR is providing missing regulatory signals to balance the system and how much tPA might be involved in the regulatory mechanism at this time point are questions that require further investigations.
The origin of neuroblasts found in the ischemic area in both vehicle and PUR-treated animals at 14 days after insult remains unclear. In both groups, we observed chains of neuroblasts migrating from the subventricular (SVZ) zone toward the ischemic cortex along blood vessels. However, a recent report showed that reactive astrocytes expressing Shh after invasive injury possess the properties of neural progenitors when isolated and propagatedin vitro(Sirko et al., 2013). Treatment with PUR increased the number of newly generated neurons in the ischemic area. PUR might promote the migration of cells from the SVZ, and/or have a direct effect on astroglial neural progenitor proliferation and differentiation and/or sustain neuroblasts survival by controlling the inflammatory microenvironment. Analysis at multiple time points to address these questions as well as integration of more treatment paradigms are required to further elucidate the role of Shh signaling in CNS regeneration.
Shh activity in the adult brain may lead to tumorigenesis. To address the concern of cancer risk, we did not observe evidence of tumor formation in mice treated with PUR. Moreover, PUR did not signifcantly increase the level of ischemia-induced Gli1, a Shh target gene of tumorigenic potential, giving credit to the compound’s safety profile and translational potential. Whether Gli1 activation by PUR was masked by an endogenously increased activity of Shh signaling after ischemic stroke or PUR acted through non-canonical pathways needs to be further evaluated.
Given the novel protective effect of PUR on ischemic brain injury, it is imperative to rapidly move this exciting research to the clinic. An important issue to investigate is the long-term regenerative beneft of PUR, especially the effect on oligodendrocytes and white matter. It would be interesting to evaluate the benefit of PUR on oligodendrocytes and whether this can presage a long-term defcit in myelin at the injured site. Additionally, more time points post-injury are needed to define optimal timing of PUR administration to achieve its regenerative effect. Another important issue is the need to more comprehensively evaluate PUR’s safety profle. For example, we have not studied the effect of acute activation of the Shh pathway on heart physiology, and we have not investigated thein vivometabolism of PUR. Although the liver can potentially remove a large fraction of it, an important concern is whether PUR induces arrhythmias. We have not recorded electrocardiograms on animals injected with PUR, but we have not observed any unexplained behaviors after administration of PUR.
Taken together, our results represent an important conceptual advance. By revealing a previously unrecognized role of the Shh developmental signaling pathway in neuroprotection and regenerationviaa novel tPA-mediated mechanism that leads to functional recovery in the adult brain after cerebral ischemia, the present study provides new insights into mechanisms and treatments of targeting Shh signaling in neuroprotection and regenerationafter stroke. Our study thus identifes a novel pharmacological approach for post-ischemic stroke treatment with a potentially effcacious and safe new small-molecule Shh agonist.
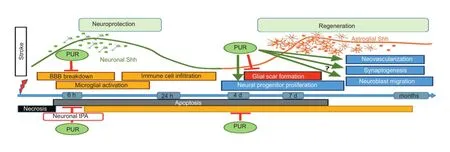
Figure 1 Neuroprotective and regenerative effects of PUR after ischemic stroke.
This work was in part supported by grants from the National Institutes of Health [R01NS061983, R01ES015988] and Shriners Hospitals for Children to W.D.
Olga V. Chechneva, Wenbin Deng*
Department of Biochemistry and Molecular Medicine, University of California, Davis, CA 95616, USA (Chechneva OV, Deng W)
Institute for Pediatric Regenerative Medicine, Shriners Hospitals for Children, 2425 Stockton Blvd, Sacramento, CA 95817, USA (Deng W)
*Correspondence to: Wenbin Deng, Ph.D., wbdeng@ucdavis.edu.
Accepted:2015-01-30
Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M, van Horssen J, de Vries HE, Charron F, Prat A (2011) The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334:1727-1731.
Bambakidis NC, Petrullis M, Kui X, Rothstein B, Karampelas I, Kuang Y, Selman WR, Lamanna JC, Miller RH (2012) Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of Sonic hedgehog. J Neurosurg 116:1114-1120.
Chechneva OV, Mayrhofer F, Daugherty DJ, Krishnamurty RG, Bannerman P, Pleasure DE, Deng W (2014) A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis 5:e1481.
Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M (2013) The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab 33:1015-1024.
Garcia AD, Petrova R, Eng L, Joyner AL (2010) Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci 30:13597-13608.
Huang SS, Cheng H, Tang CM, Nien MW, Huang YS, Lee IH, Yin JH, Kuo TB, Yang CC, Tsai SK, Yang DI (2013) Anti-oxidative, anti-apoptotic, and pro-angiogenic effects mediate functional improvement by sonic hedgehog against focal cerebral ischemia in rats. Exp Neurol 247:680-688.
Sims JR, Lee SW, Topalkara K, Qiu J, Xu J, Zhou Z, Moskowitz MA (2009) Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke 40:3618-3626.
Sirko S, Behrendt G, Johansson PA, Tripathi P, Costa M, Bek S, Heinrich C, Tiedt S, Colak D, Dichgans M, Fischer IR, Plesnila N, Staufenbiec M, Haass C, Snapyan M, Saghatelyan S, Tsai LH, Fischer A, Grobe K, Dimou L, et al. (2013) Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog glia. Cell Stem Cell 12:426-439.
Xia YP, He QW, Li YN, Chen SC, Huang M, Wang Y, Gao Y, Huang Y, Wang MD, Mao L, Hu B (2013) Recombinant human sonic hedgehog protein regulates the expression of ZO-1 and occludin by activating angiopoietin-1 in stroke damage. PLoS One 8:e68891.
Zhang L, Chopp M, Meier DH, Winter S, Wang L, Szalad A, Lu M, Wei M, Cui Y, Zhang ZG (2013) Sonic hedgehog signaling pathway mediates cerebrolysin-improved neurological function after stroke. Stroke 44:1965-1972.
10.4103/1673-5374.153677 http∶//www.nrronline.org/
Chechneva OV, Deng W (2015) Empowering sonic hedgehog to rescue brain cells after ischemic stroke. Neural Regen Res 10(3)∶360-362.
- 中国神经再生研究(英文版)的其它文章
- RAFting the rapids of axon regeneration signaling
- TAM receptors: two pathways to regulate adult neurogenesis
- Synapsing with NG2 cells (polydendrocytes), unappreciated barrier to axon regeneration?
- Targeting the body to protect the brain: inducing neuroprotection with remotely-applied near infrared light
- Novel advancements in threedimensional neural tissue engineering and regenerative medicine
- Functional regeneration of the brain: white matter matters

