溴代卓酚酮基础的双查尔酮的合成
常明琴,赵勋章,崔 岩,李 阳*
( 1.渤海大学化学化工学院,辽宁锦州121000; 2.锦州市第八中学,辽宁锦州121000)
溴代卓酚酮基础的双查尔酮的合成
常明琴1,赵勋章2,崔岩1,李阳1*
( 1.渤海大学化学化工学院,辽宁锦州121000; 2.锦州市第八中学,辽宁锦州121000)
摘要:卓酚酮基础的双查尔酮3,3'-{ ( 1E,1'E) -1,4-苯撑双[( 1E) -3-羰基并-1-烯-1,3-二基]}双卓酚酮( 1) 与2.1倍或4.2倍物质的量的液溴在乙酸溶剂中直接进行溴化反应.该溴化反应选择性的发生在卓酚酮环上,得到相应的7-溴卓酚酮( 2)和5,7-二溴卓酚酮基础的双查尔酮( 3),收率分别为81%和73%,其结构通过波谱数据和元素分析得以证实.
关键词:溴化;卓酚酮;双查尔酮;液溴;合成
Brominated aromatic compounds are important synthetic intermediates and products in organic chemistry[1-2].They represent very versatile reagents in C-C coupling reactions,as precursors to organometallic species and in nucleophilic substitutions[3-5].In this context,brominated tropolones have attracted much attention because they play an important role in molecular assemblies for a fast and efficient lead generation towards the new drug discovery[6].Impressive results have been obtained by the group of DEVEAU[7],who reported the utilization of 5-bromo-2-methoxy-tropolone as the key synthon in the synthesis of bioactive biaryl colchicinoids.In addition,the introduction of bromo moieties to troponoid nucleus is reported to inhibit the Hepatitis C virus[8].Although a variety of brominated aromatic compounds have been investigated extensively,syntheses of bromo-substituted tropolones are still limited.In this regard,JIN et al[9]previously reported the bromination reaction of 3-isopropenyltropolones with bromine in acetic acid.Our group reported recently the bromination reaction of 3-( ( 2E,4E) -5-phenylpenta-2,4-dienoyl) tropolone[10].
Recently,we reported the nucleophilic cyclization and oxidation cyclization reactions of tropolone-based bischalcone 3,3'-{ 1,4-phenylenebis [( 1E) -3-oxoprop-1-ene-1,3-diyl ]} bistropolone with hydroxyla-mine and aromatic hydrazines[11].As an extension of these works and promoted by importance of bromo-substituted tropolones,we became interested in the bromination reaction of the tropolone-based bischalcone with the aim of synthesizing structurally new brominated tropolone derivatives,which might be useful for further synthetic manipulation,for example,by cross coupling reactions to obtain more complex troponoid compounds.To the best of our knowledge,no analogous reactions have been reported in the literature.The present studies represent a continuation of our interest in the search for novel and interesting troponoid derivatives.
1 Experimental
1.1 Apparatus and Chemicals
The melting points were determined by using a WRS-1B melting point apparatus and themometer was uncorrected.The IR spectra of the compounds in KBr pellets were obtained in the range of 400-4 000 cm-1on a Shimadzu FTIR-8400S spectrophotometer.1H NMR ( 600 MHz) and13C NMR ( 150 MHz) spectra were recorded on a Brucker AVANCE NMR spectrometer using CF3COOD or DMSO-d6as the solvent.The reported chemical shifts (δ values) are given downfield from tetramethylsilane ( TMS) as the internal standard.Mass spectra were determined on a MSD VL ESI1 spectrometer.Elemental analyses were performed for C and H using an Elementar Vario EL-III element analyzer.The progress of reactions was monitored by thin layer chromatography ( TLC) on silica gel GF254 using ethyl acetate/petroleum ether ( 1∶1) as eluent.
1.2Procedure for the preparation 3,3'-( ( 1E,1' E) -1,4-phenylenebis( 3-oxoprop-1-ene-3,1-diyl) ) bis( 7-bromotropolone) ( 2)
A solution of bromine ( 0.33 g,2.1 mmol) in acetic acid ( 5 mL) was added drop-wise to a stirred solution of tropolone-based chalcone 1 ( 0.426 g,1 mmol) and sodium acetate ( 0.25 g,3 mmol) in acetic acid ( 60 mL) at laboratory temperature.After stirring for 4 h,water ( 100 mL) was added to the reaction mixture.The precipitate was collected and recrystallised from trifluoroacetic acid ( TFA) to give mono-brominated product 2 as brown crystals in 81% yield,mp: 295-297℃.IR ( KBr,ν,cm-1) : 3 184 ( OH),1 669 ( C =O),1 614 ( C=O),1 586,1 532,1 474,1 384, 1 342,1 250,1 142;1H NMR ( CF3COOD,600 MHz)δ: 7.25 ( dd,2H,J = 11.0,11.0 Hz,tropolone H-5),7.38 ( d,J = 15.6 Hz,2H,=CH),7.51 ( d,J = 7.2 Hz,2H,Ph-H),7.61 ( d,J = 15.6 Hz,2H,=CH),7.78 ( d,J = 7.2 Hz,2H,Ph-H),7.82 ( d,J = 11.0 Hz,2H,tropolone H-6),8.15 ( d,J = 11.0 Hz,2H,tropolone H-4),9.65 ( s,2H,OH) ;13C NMR ( DMSO-d6,150 MHz)δ: 123.13,127.85,129.27,132.05,133.49,139.58,142.70,143.34,145.37,154.92,171.39,182.18.MS ( ESI,m/z) : 583.1,584.9,587.1 [M + H]+.Anal.Calcd.for C26H16Br2O6( %) : C 53.45,H 2.76; Found ( %) : C 53.64,H 2.62.
1.3Procedure for the preparation 3,3'-( ( 1E,1' E) -1,4-phenylenebis( 3-oxoprop-1-ene-3,1-diyl) ) bis( 5,7-dibromotropolone) ( 3)
A solution of bromine ( 0.672 g,4.2 mmol) in acetic acid ( 10 mL) was added drop-wise to a stirred solution of tropolone-based chalcone 1 ( 0.426 g,1 mmol) and sodium acetate ( 0.5 g,6 mmol) in acetic acid ( 60 mL) at laboratory temperature.After stirring for 4 h,water ( 100 mL) was added to the reaction mixture.The precipitate was collected and recrystallised from trifluoroacetic acid ( TFA) to give the dibrominated product 3 as brown crystals in 73% yield,mp >300℃.IR ( KBr,ν,cm-1) : 3 185 ( OH),1 663 ( C = O),1 617 ( C = O),1 578,1 552,1 491,1 457,1 361,1 339,1 246,1 156;1H NMR ( CF3COOD,600 MHz)δ: 7.43 ( d,J =15.6 Hz,2H,CH =),7.55 ( d,J = 7.2 Hz,2H,Ph-H),7.69 ( d,J =15.6 Hz,2H,= CH),7.79 ( d,J = 7.2 Hz,2H,Ph-H),7.87 ( d,J = 2.0 Hz,2H,tropolone H-6),8.17 ( d,J =2.0 Hz,2H,tropolone H-4),9.78 ( s,2H,-OH) ;13C NMR ( DMSO-d6,150 MHz)δ: 125.27,129.45,130.52,134.14,137.28,143.82,144.09,145.70,148.32,158.67,174.73,183.98.MS ( ESI,m/z) : 741.1,742.8,744.9 [M + H]+.Anal.Calcd.for C26H14Br4O6( %) : C 42.09,H 1.90; Found ( %) : C 41.96,H 2.05.
2 Results and Discussion
As presented in Fig.1,tropolone 1 was first treated with 2.1 equiv.bromine in acetic acid in the presence of sodium acetate at laboratory temperature.The bromination reaction took place exclusively at the 7-po-sition of tropolone ring to give the corresponding products identified as monobromo derivative 2 in 81 % yields.In its1H NMR spectrum,the signals for the four vinyl protons and the four benzene ring protons were observed and their spectral pattern is very similar to that of the starting compound 1,indicating that the bromination reaction occurred selectively on the tropolone ring.Further,the signals from two C-6 and C-4 hydrogens of the tropolone ring appeared as two doublet signals at 7.82 ( d,2H,J = 11.0 Hz) and 8.15 ( d,2H,J = 11.0 Hz),which arose a result of coupling with the C-5 hydrogen.The signal corresponding to the C-5 hydrogen appears as a doublet of doublets at 7.25 ( dd,2H,J = 11.0,10.8 Hz),which arose from coupling with both the C4 and C6 hydrogen atoms.These observations clearly indicated the formation of a 7-bromo-substituted product 2.
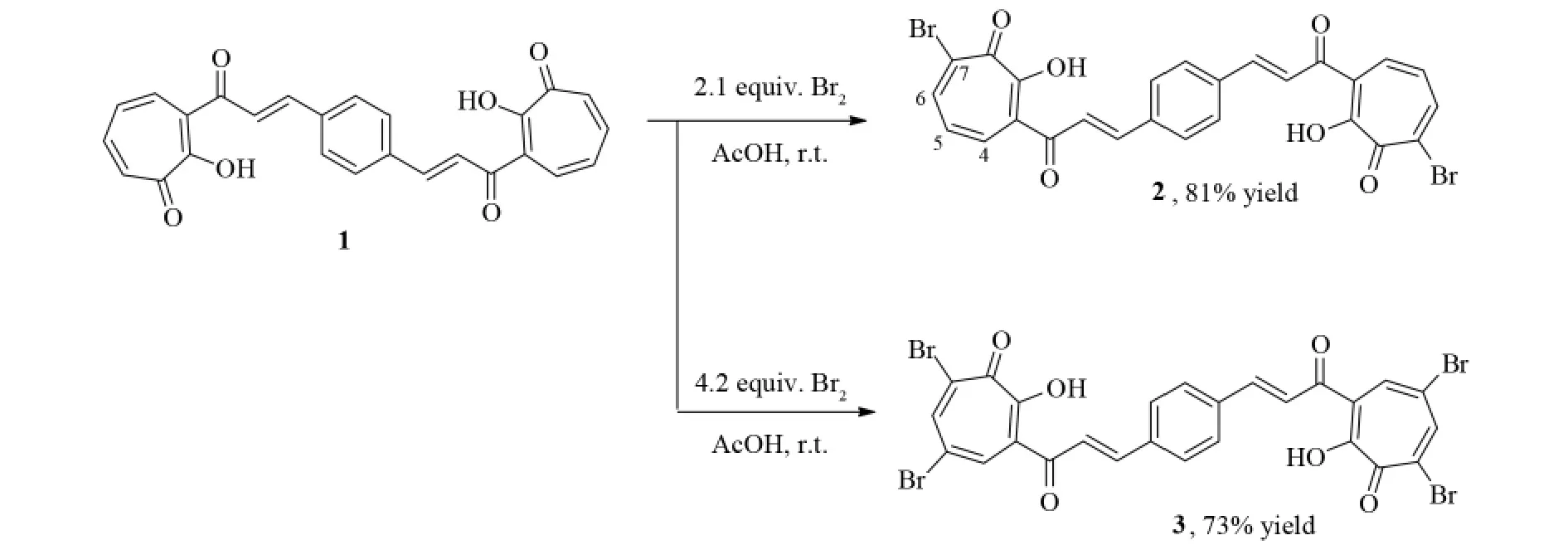
Fig.1 Synthesis of brominated tropolone-based bischalcones 2 and 3
Under the same reaction conditions,the bromination reaction with 4.2 equivalents of bromine gave the corresponding 5,7-dibromotropolone 3 with 73% yield.The structure of 3 was established based on its spectral data.In its1H NMR spectrum,the main feature of the C-6 and C-4 hydrogens of the tropolone ring shows two doublet signals at 7.87 and 8.17 with a coupling constant J = 2.0 Hz,which is a typical value ( 1-3 Hz) for meta-protons coupling in tropolone ring.The observation indicated that the two bromine atoms were substituted at the 5-and 7-positions of the tropolone ring.
In both cases,the phenyl moiety was not involved in the bromination reaction.These observations were consistent with our previous reports[10].In addition,it is worth noting that in our earlier report the reaction of 3-cinnamoyltropolone with excessive bromine afforded cyclized 6,8-dibromo-2-phenyl-4,9-dihydrocyclohepta[b ]pyran-4,9-dione[12].However,in the present case,when 1 was treated with excessive bromine,no similar oxidative cyclisation reaction was observed.
3 Conclusion
In conclusion,the first synthesis of structurally novel brominated tropolone-based bischalcones has been achieved by the direct bromination reaction using elemental bromine.From the perspective of structural diversification and the ease of subsequent derivatization,the newly-synthesized brominated tropolones 2and 3 should be useful for the synthesis of highly substituted troponoid,for example,by modern cross-coupling reactions.We anticipate that this work will promote more interest in the chemistry of troponoids.Further examinations of this transformation are currently in progress,to be reported in due course.
References:
[1]TAYLOR R.Electrophilic aromatic substitution [M ].New York: Wiley,1990: 72-73.
[2]BUTLER A,WALKER J V.Marine haloperoxidases[J].Chem Rev,1993,93( 5) : 1937-1944.
[3 ]DIEDERICH F,STANG P J ( Eds.).Metal-catalyzed cross-coupling reactions [M].Weinheim: Wiley-VCH,1998: 65-66.
[4]BACH T,HEUSER S.Synthesis of 2'-substituted 4-bromo-2,4'-bithiazoles by regioselective cross-coupling reactions [J].J Org Chem,2002,67( 16) : 5789-5795.
[5]高丰琴,何汉江,郭强,等.Suzuki偶联反应合成4'-乙基-2,3-二氟-1,1'-联苯[J].化学研究与应用,2013,25( 1) : 86-90.
[6]MORI A,KAWAKAMI H,KATO N,et al.Photochemical reactions of 2-bromotropone and 2,7-dibromotropone with 9,10-dicyanoanthracene [J ].Org Biomol Chem,2003,1( 10) : 1730-1736.
[7]DEVEAU,A M,MACDONALD T L.Practical synthesis of biaryl colchicinoids containing 3',4'-catechol etherbased A-rings via Suzuki cross-coupling with ligandless palladium in water [J ].Tetrahedron Lett,2004,45 ( 4) : 803-807.
[8 ]BOGUSZEWSKA-CHACHULSKA A M,KRAWCZYK M,NAJDA A,et al.Searching for a new anti-HCV therapy: synthesis and properties of tropolone derivatives [J].Biochem Biophys Res Commun,2006,341( 2) : 641-647.
[9]JIN R H,YIN B Z,JIN Z T,et al.Reactions of 3-isopropenyltropolones with bromine and N-bromosuccinimide: Formation of 8H-cyclohepta[b]furan-8-one derivatives [J].J Heterocycl Chem,1990,27( 3) : 583-586.
[10]GAO W T,YAN Y,LI Y.Synthesis of conjugated ( E,E) -1,3-diene-containing troponoid-based compounds [J].Chem Pap,2012,66( 7) : 691-698.
[11]LI Y,LI F,GAO W T.Novel synthesis of 1,4-phenylene bridged bis-heterocyclic tropone compounds [J ].Heterocycles,2012,85( 4) : 911-917.
[12]GAO W T,JIN,Z T,YIN B Z,et al.Synthesis and reactions of halo-,nitro-,and arylazo-substituted 3-cinnamoyltropolones.Formation of styryl-substituted heterocycle-fused troponoid compounds[J].J Heterocycl Chem,1989,26( 2) : 371-375.
[责任编辑:任铁钢]

