A randomized, controlled trial of Shutangluo fang in treating painful diabetic peripheral neuropathy
Di Hongjie(狄红杰), Chu Xiaoqiu(褚晓秋), Liu Kemian(刘克冕), and Liu Chao(刘超)
Endocrine and Diabetes Center,Jiangsu Province Hospital on Integration of Chinese and Western Medicine,Nanjing University of Traditional Chinese Medicine,Nanjing 210028, China
A randomized, controlled trial of Shutangluo fang in treating painful diabetic peripheral neuropathy
Di Hongjie(狄红杰), Chu Xiaoqiu(褚晓秋), Liu Kemian(刘克冕), and Liu Chao(刘超)*
Endocrine and Diabetes Center,Jiangsu Province Hospital on Integration of Chinese and Western Medicine,Nanjing University of Traditional Chinese Medicine,Nanjing 210028, China
OBJECTIVE:To assess efficacy of Shutangluo fang on painful diabetic peripheral neuropathy(PDPN).
Painful diabetic peripheral neuropathy; Shutangluo fang; Epalrestat
Painful diabetic peripheral neuropathy (PDPN) is one of the common chronic complications of diabetes,which are often associated with considerable morbidity and mortality. It requires medical attention because of its adverse effects on quality of life1. There are many treatment options available include anticonvulsants, antidepressants, opioids and others2. But there are problems of side-effects and contraindications for all these drugs3. Thus, the development of new drugs to manage PDPN remains a high priority.
Shutangluo fang, which has been long used as an anti-diabetes medicine in our department, proved to be effective on diabetic peripheral neuropathy (DPN). This study evaluated the efficacy of Shutangluo fang in relieving PDPN.
METHODS
Overview
This was a randomized, placebo-controlled, parallel-group study. Eighty-two patients with PDPN were invited to participate in this clinical trial starting from July 2013 to May 2016 at endocrine and diabetes center of Jiangsu Province Hospital on Integration of Chinese and Western Medicine. The patients were composed of 46 men and 34 women, with the mean age of 56.54±7.82 years. Each patient read and signed informed consent before initiating any study procedures.
Selection criteria
All the patients enrolled in the study should meet the diagnostic criteria for PDNP issued by Chinese Diabetes Society (CDS)4. The criteria mainly include:① Diabetes for at least 3 months on stable dosage of oral hypoglycemic agent or insulin.②Daily neuropathic pain of at least moderate severity for more than 3 months.③Written consent to participate in the study.
Exclusion criteria
Patients with hemoglobin A1c levels of more than 11% were excluded, as were patients with clinically significant or unstable liver, hematologic, or respiratory illnesses, vitamin def i ciency, symptomatic peripheral vascular disease, or unstable cardiovascular disease.
Study design
All patients were divided into the control group (CG) and Shutangluo fang treatment group (STG) randomly. Both groups recieved conventional therapy (diet treatment, hypoglycemic agents, insulin, and hypotensive agents) and held constant throughout the study. Adequate glucose control was maintained to ensure patient safety. Epalrestat (50 mg) was orally administered three times daily before each meal (150 mg/day) for each patient. The patients in treatment group received Shutangluo fang, which composed of euonymus alatus, trumpetcreeper, rehmannia,rhizoma coptidis and radix puerariae lobatae. The course of treatment lasts for 3 months.
Laboratory studies
All patients were subjected to thorough interview, clinical examination and relevant laboratory investigations. All of them were asked to fill in (by themselves or with assistance) visual analogue score (VAS) first initially, then after 3 months. Biochemical examinations, which included fasting blood glucose (FBG), HbA1c, lipid prof i le was done at the beginning of the study and after 3 months. Electrophysiological studies, in the form of sensory nerve conduction studies (SNCV), were performed at the beginning of the study and after 3 months in both groups.
Efficacy measures
The primary efficacy measure for this study was the reduction in mean of the 24-hour average pain scores (computed from diary scores between two site visits), as measured by an 11-point (0 = no pain, 10 = worst possible pain) Likert scale that was completed daily by the patients in a diary. We considered a reduction difference more than 30% a significant effect, from 15% to 30% in an insignif i cant ef f ect, and less than 15% in no ef f ect5.
Statistical analysis
All statistical testing were two-sided and performed using SAS procedures. All values (e.g. HbA1c) were expressed as means ± SD. Statistical methods used includedx2tests for nominal scale, two-sample tests for comparison of mean values between groups. Values were adjusted using the Hochberg25 procedure in order to protect the type I error rate at the 0.05 level.
RESULTS
Patient disposition, demographics, and disease characteristics
A total of 82 patients were randomized to the study. The demographics for all patients, 41 patients were treated with epalrestat (CG) and 41 patients were treated with Shutangluo fang with epalrestat (STG), are provided in Table 1. There were no signif i cant dif f erences between two groups.
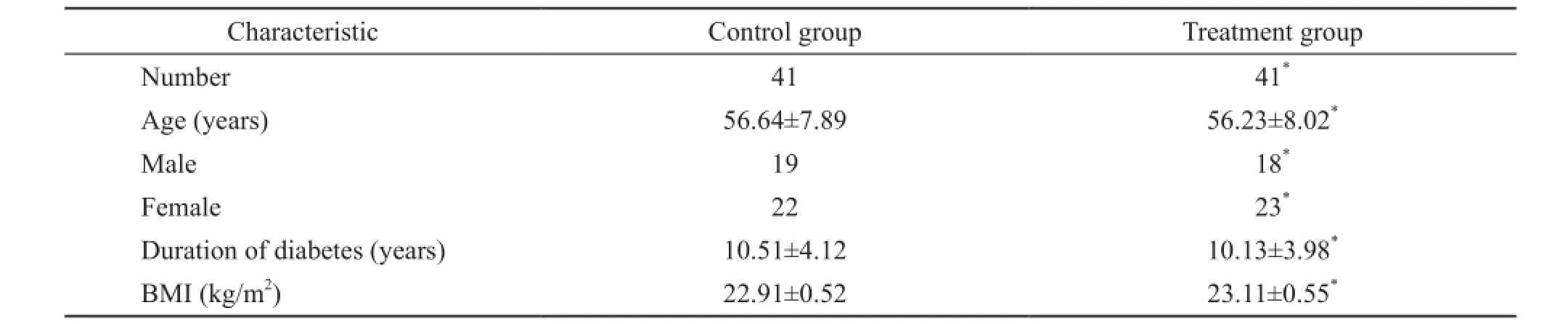
Table 1. Selected characteristics of subjects
Changes in glycemic control and lipid prof i le
Changes in glycemic control and lipid profile of the patients were followed up for 3 months. In the control group (n= 41), A1C (means ± SD) at baseline and 3 months was (7.71±1.05), and (7.91±1.02)%, respectively. Corresponding values in the Shutangluo fang group (n=41) were (7.82 ± 1.12), and (7.79±1.11)%, respectively. There were no signif i cant dif f erences between the two groups. Also, there were no significant differences in lipid profile between the two groups at any time points (Table 2).
Table 2. Mean change from baseline to endpoint of HbA1c and lipid pro fi le
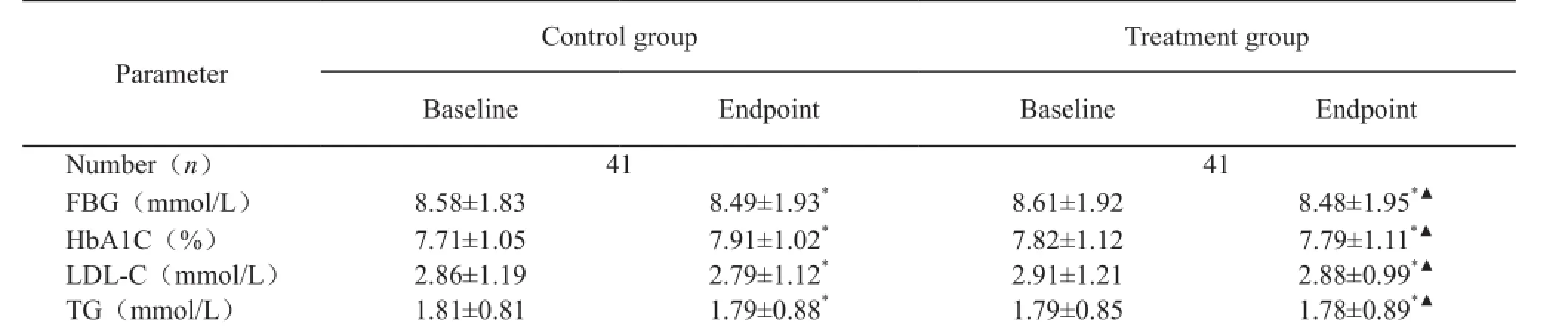
Table 2. Mean change from baseline to endpoint of HbA1c and lipid pro fi le
FBG: fasting blood glucose; LDL-C: low density lipoprotein cholesterol.HbA1C: glycosylated hemoglobin A1C; TG: total triglyceride;*P>0.05, versus baseline;▲P>0.05,versus control group.
Parameter Control group Treatment group Baseline EndpointBaseline Endpoint Number(n)4141 FBG(mmol/L)8.58±1.838.49±1.93*8.61±1.928.48±1.95*▲HbA1C(%)7.71±1.057.91±1.02*7.82±1.127.79±1.11*▲LDL-C(mmol/L)2.86±1.192.79±1.12*2.91±1.212.88±0.99*▲TG(mmol/L)1.81±0.811.79±0.88*1.79±0.851.78±0.89*▲
Change in subjective symptoms
At baseline, the pain scores based on visual analogue score (VAS) had no significant difference between the two groups (5.73±1.17 VS 5.68±1.19, P>0.05). At the endpoint, both groups signif i cantly reduced pain compared to the baseline. The endpoint mean pain score, was signif i cantly improved by Shutangluo fang when compared to the control group (2.21±0.82 VS 4.13±0.97,P<0.05).
The proportion of patients achieving a >15% reduction in pain was signif i cantly higher in Shutangluo fang group than the control group (Table 3).

Table 3. Analysis of the proportion of responders
Change in sensory nerve conduction velocity(SNCV)
Table 4 shows each electrophysiological measure (means ± SD) at the baseline and end-of-treatment time points. At baseline, no statistically signif i cant deteriorations in median SNCV were observed in the two groups. The electrophysiological measure of sensory function measures demonstrated signif i cant improvement (P<0.05) over the 3-month treatment period in both groups. At the endpoint, mean SNCV was significantly improved by Shutangluo fang when compared to the control group (P<0.05).
Table 4. Analysis of the nerve conduction velocity
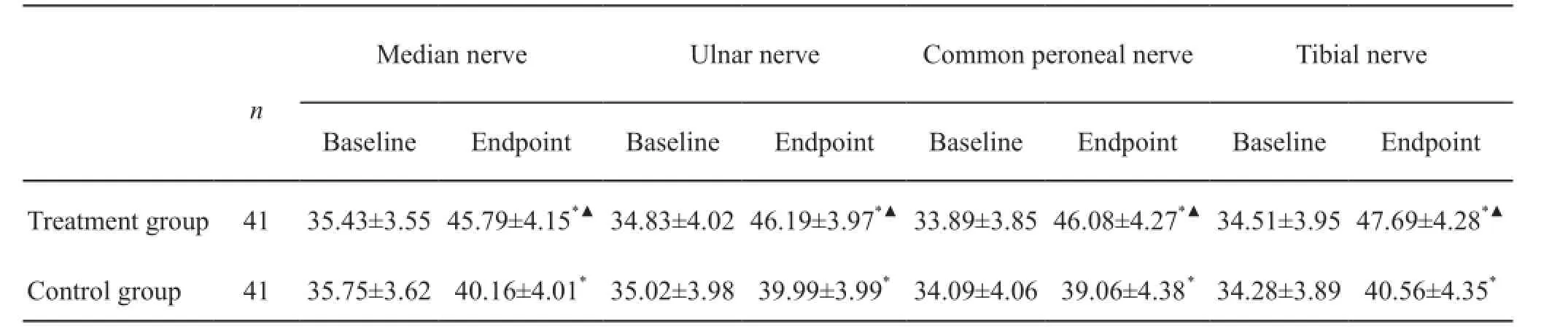
Table 4. Analysis of the nerve conduction velocity
*P<0.05, versus baseline;▲P<0.05, versus control group.
Median nerveUlnar nerveCommon peroneal nerveTibial nervenBaselineEndpointBaselineEndpointBaselineEndpointBaselineEndpoint Treatment group4135.43±3.55 45.79±4.15*▲34.83±4.02 46.19±3.97*▲33.89±3.85 46.08±4.27*▲34.51±3.95 47.69±4.28*▲Control group4135.75±3.6240.16±4.01*35.02±3.9839.99±3.99*34.09±4.0639.06±4.38*34.28±3.8940.56±4.35*
DISCUSSION
Painful diabetic peripheral neuropathy represents a diffuse symmetric and length-dependent injury to patients and it has bad ef f ect on quality of life, morbidity, and costs from a public health perspective6. Although several pivotal trials have shown that strict glycemic control reduces the occurrence and progression of diabetes-related complications, it is difficult to restore nerve function7. So far no ideal drug has been available for its management. In this trial, Shutangluo fang was effective on PDPN.
As we all know, hyperglycemia-induced hyperactivity of polyol pathway links to the augmentation of the metabolic disorders, like oxidative stress, and others, contributing to the deterioration of diabetic neuropathy. Suppression of this pathway may be an important key to efficient treatment for DPDN. The key enzyme of the polyol pathway is aldose reductase (AR). There are many reports supporting the beneficial effects of AR inhibitors in animal models for diabetic neuropathy and diabetic patients8. In our clinical trial, epalrestat which is the only ARI currently available commercially, is ef f ective on PDPN. But we also fi nd its ef f ect for symptoms (e.g. pain) is poor in clinical practice.
There is no painful diabetic peripheral neuropathy in terms of traditional Chinese medicine (TCM). However, according to its clinical manifestations, the disease may belong to the categories ofarthralgia zheng. Its etiology and pathogenesis mainly lie in uncontrolled diet or interior injury by seven emotions, which result in extreme heat with yin asthenia and stagnation of blood, failure of the transport nutrients to extremities, ultimately led to the symptoms including pain.
Many studies have demonstrated the efficacy of Chinese medicine in painful diabetic peripheral neuropathy9. Shutangluo fang is the clinical experienced prescription of our department. Euonymus alatus is the principal drug of Shutangluo fang. Its effects include disintegrating blood stasis and dredging collaterals, eliminating stasis to activate blood circulation and relieve pain. The ministerial drug is trumpetcreeper which is added to strengthen the effects of promoting blood circulationand remove blood stasis. Rehmannia, rhizoma coptidis and radix puerariae lobatae are used to clear away heat, nourishing Yin and cooling blood. The results presented here indicate that Shutangluo fang can relieve the pain and improve the sensory nerve conduction velocity.
In conclusion, Shutangluo fang is ef f ective on painful diabetic peripheral neuropathy. But we need long-term clinic trial to assess the efficacy and safety of Shutangluo fang in the treatment of painful diabetic neuropathy.
REFERENCES
1 Argof f CE, Cole BE, Fishbain DA, et al. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc,2006(81): S3-S11.
2 Casandra JR, James CW. Treatment of painful diabetic peripheral neuropathy. Prosthet Orthot Int,2015,39(1):17-28.
3 Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med,2000(108):2-8.
4 Chinese Diabetes Society. Guidelines for prevention and treatment of type 2 diabetes in China. Chin J Endocrinol Metab,2014,30(10):893-942.
5 Wernicke JF, Pritchett YL, D’Souza DN, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology, 2006,67(8):1411-1420.
6 Sadosky A, Schaefer C, Mann R, et al. Burden of illness associated with painful diabetic peripheral neuropathy among adults seeking treatment in the US: results from a retrospective chart review and cross-sectional survey. Diabetes Metab Syndr Obes,2013(6):79-92.
7 Fullerton B, Jeitler K, Seitz M, et al. Intensive glucose control versus conventional glucose control for type 1 diabetes mellitus. Cochrane Database Syst Rev,2014(2):CD009122.
8 Li Ping, Ma Jianhua, Gao Jialin, et al. Clinical efficacy and safety of epalrestat in diabetic neuropathy-A multicenter randomized controlled clinical trial. Chin J Endocrinol Metab,2015,31(9):743-747.
9 Pang GM, Zhu P, Han JT, et al. Clinical research on treatment of 60 patients with Diabetic peripheral neuropathy by Jiangtang Tongluo tablets. Clinical Journal of Chinese Medicine,2014,6(30):3-6.
(Accepted: June 29, 2016)
*Corresponding author: E-mail:liuchao@hotmail.com;Mobile phone:+86-258-5608735
METHODS:Adopting stratified randomized and controlled design,82 patients with PDPN were assigned to two groups. Both groups continued conventional therapy. Epalrestat was administered to patients in both groups,and Shutangluo fang was given to patients in the treatment group.The treatment course lasted for 3 months. The changes before and after treatment of symptoms and signs, electrophysiological assessments were observed.
RESULTS:The efficacy of the treatment group was better than the control group. The sensory nerve conduction velocity was significantly improved in the treatment group than the control group.
CONCLUSIONS:Shutangluo fang is ef f ective on PDPN.
 World Journal of Integrated Traditional and Western Medicine2016年3期
World Journal of Integrated Traditional and Western Medicine2016年3期
- World Journal of Integrated Traditional and Western Medicine的其它文章
- Meta-analysis about Western Medicine combined with activating blood drugs on modulating blood glucose and lipids in diabetic patients
- Clinical ef f ects of acupoints moxibustion therapy on postsurgical gastroparesis syndrome after resection of esophageal cardia cancer
- A medical understanding on the wuxing theory in cell
- Analysis of the features of TCM and western medicine in the diagnosis and treatment of subclinical hypothyroidism characteristics
- INSTRUCTION FOR AUTHORS
