An Overall Green Process from Preparation of FeCl3 Modified β Zeolites to Its Use in Catalyzing Direct Hydroxylation of Benzene with Hydrogen Peroxide
ZHOU Jian-bo, FU Zai-hui, LIU Ya-chun, XU Chao
(1. Basic Medical College of Changsha Medical University, Changsha 410219, China;2. College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China )
An Overall Green Process from Preparation of FeCl3Modified β Zeolites to Its Use in Catalyzing Direct Hydroxylation of Benzene with Hydrogen Peroxide
ZHOU Jian-bo1, FU Zai-hui2*, LIU Ya-chun2, XU Chao1
(1. Basic Medical College of Changsha Medical University, Changsha 410219, China;2. College of Chemistry and Chemical Engineering, Hunan Normal University, Changsha 410081, China )
AbstractFeCl3modifiedβzeolite catalyst was prepared by using a convenient solid-state ion exchange instead of a traditional ion exchange and characterized by XRD, TG-DSC, and low temperature N2adsorption methods. The catalyst is active and selective for the direct hydroxylation of benzene to phenol with hydrogen peroxide (H2O2). And its phenol selectivity can be further improved through tailoring its surface hydrophobicity/hydrophilicity with dimethyldiethoxysilane (DDS) to restrain the sequent oxidation of phenol. In addition, it can be recovered and reused for three times with little loss of reactivity. Hence, an overall green process from the preparation of the catalyst to its application in catalytic reaction has been establised here.
Key wordsBenzene; FeCl3modifiedβzeolites; hydroxylation; phenol; solid-state ion exchange
Phenol is a very important organic intermediate in the field of fine chemicals production[1]. Its traditional production process, so-called cumene process[2], is environmentally unacceptable because it generally involves multi-step syntheses and generates large quantities of waste products. Therefore, one of the foremost challenges currently facing the chemical industry is to look for a cleaner, safer, and more environmentally friendly one-step process to produce phenol. Nowadays, the methods for direct hydroxylation of benzene to phenol with H2O2in the liquid phase[3-4], with N2O[5]or O2[6]in vapor phase have attracted much attention. The methods possess the outstanding merits such as short synthesis route, higher atom efficiency and near non-pollution. So they are considered to be clean and environmentally friendly and likely replace the cumene method in the future. Among the processes, direct hydroxylation of benzene with H2O2to phenol is one of the most promising routes due to water as the only byproduct and the mature technology for production of H2O2[7]. At present, the main attention is focused on seeking the efficient oxidation catalyst for the process. Various catalysts can be applied to the process, mainly including TS zeolite and Cu, Fe, and V containing catalysts[8-10]. The Fe-containing catalyst for the process is of great interest because of its low cost and high efficiency[11].
The preparation method of catalysts has a significant effect on the performance. The traditional methods such as the framework substitution[12], surface grafting[13], solution ion exchange[14]and impregnation[15]are usually employed to prepare transition metals modified zeolites. These methods commonly have some defects such as a tedious preparation process, low production efficiency and environmental pollution. The solid state dispersion or ion exchange of transition metal compounds on the porous metal oxides and zeolites, which has been widely reported[16], is a suitable preparation method for the unstable compounds in aqueous solution like some metal chlorides. And this modification method is convenient, efficient and environmentally friendly in comparison with the traditional methods. For example, ZnCl2modifiedβ-Al2O3and NaY catalysts prepared by this method were reported to be excellent for vapor phase O-alkylation of catechol with methanol and high regioselective Diels-Alder reaction of myrcene and acrolein, respectively[17].
In addition, hydroxylation of benzene is a potentially successive reaction, in which phenol as primary target product is more susceptible to oxidation than the reactant benzene. As a result, the selectivity to phenol is reduced. Although the selectivity of the catalysts can often be enhanced by modification of their shape selectivity, this approach is generally ineffective in the hydroxylation of benzene. So, how to control the successive oxidation reactions to increase the phenol selectivity remains to be a challenge for chemists. A method of controlling the selectivity of a successive oxidation process, named chemical affinity selectivity, has been reported in the literature[18]. In this approach, the affinity of the catalyst surface to substrates is controlled by tailoring surface hydrophobicity/hydrophilicy, thereby enhancing the product selectivity. However, the number of related reports on this approach is still very limited.

Tab.1 The preparation method of phenol
In this paper, we report an overall green process, which involves the preparation of FeCl3modifiedβzeolites by use of the simple solid state reaction and their catalytic application in the direct hydroxylation of benzene with H2O2as an green oxidant, and explore to improve the selectivity for phenol through tailoring their surface’s hydrophobicity/hydrophilicity with dimethyldiethoxysilane (DDS) and check their catalytic stability by recycling tests.
1Experimental
1.1Catalysts preparation
Hβzeolite support (the molar ratio of Si to Al for 30) was supplied by Changling Petroleum Chemical Engineering Company of Hunan Yueyang of China, and was first calcined in air at 500 ℃ for 6 h prior to use. FeCl3modifiedβcatalysts were prepared by solid state dispersion method with anhydrous ferric chloride (loading of FeCl3for 1.0 mmol.g-1) as the Fe(III) source. Mixing them up with mechanical grind, then calcining them in the nitrogen atmosphere at different temperature gave the modified catalysts (denoted as Fe-β(T)). After that, the Fe-β(500) catalyst calcined at 500 ℃ was further dealt with an appropriate amount of dimethyldiethoxysilane (DDS) in toluene solvent, and then extracted with toluene and washed with ethanol, with the obtained catalyst noted as Fe-β(500,SM).
1.2Catalysts characterization
The XRD measurements of the samples were carried out with a Dangong Y-2000 diffractometer with Cu Kα radiation (λ=1.541 75 Å) , a scan speed of 2°·min-1and a 0.06° step size from 4° to 40°. Their TG-DSC measurements were performed on a NETZSCH STA409PC from 25 ℃ to 1 000 ℃ with a heating rate of 10 ℃·min-1in the N2atmosphere (flow rate 20 mL/min). The specific surface area and pore volume of the samples were measured by MICROMERIPICS ASAP 2 400 low temperature N2adsorption apparatus on the basis of the China standard GB/T 5816-1995. The actual iron content of the calcined samples was measured by the chemical titration method.
1.3Catalytic testing
Hydroxylation of phenol was carried out in a 150 mL double-necked round-bottom flask fitted with a water condenser and kept in an oil bath. In a typical reaction, 0.025 g of catalysts, 2 mL (22.5 mmol) of benzene and 14 mL of acetonitrile were added successively into the reactor. After the mixture was heated to the reaction temperature (65 ℃) under vigorous magnetic stirring, 2.4 mL of 30 wt.% H2O2(22.5 mmol) was added into the reactor and the reaction was proceeded for 5 h. The reaction products were analyzed by Agilent 1 100 HPLC (Eclipse C18, 4.6×250 mm, eluent methanol/water 55/45, flow rate 0.8 mL/min, UV detectorλ272 and 254 nm).
2Results and discussion
2.1Catalyst characterization
The effects of calcination temperature on the crystal structure and physical properties of parent zeolite were checked by use of XRD and low temperature N2adsorption, and the obtained results were shown in Fig.1 and Tab.2, respectively. Decreasing trends in the characteristic diffraction peaks or the relative crystal degree (see Fig.1 and Tab.2) and the specific area (Sg) and porous volume (PV) of the parent zeolite with the calcination temperature of Fe-βwere observed. For example, when the calcination temperature of Fe-βincreased from 400 ℃ to 600 ℃, the crystal degree of the parent zeolite was reduced by about 80%, which is in accordance with the decrease in itsSgandPV. This indicates that structure deterioration of the zeolite has occurred in the overall solid state reaction process, which is likely due to the formation of HCl in the solid ion exchange process. In addition, the changes in itsSgandPVbefore and after the modification of FeCl3clearly indicate that FeCl3has been introduced inside the pores of Hβ. However, at too high calcination temperature (more than 600 ℃), characteristic diffraction peaks of the parent zeolite (see the Fe-β(950)) have disappeared, indicating that its crystal structure has completely disrupted. This can be further confirmed from the results that the measuredSgandPVare abnormally low (see Tab.2). From the Table, it is found that the lattice volume of all the Fe-βsamples before collapse was larger than that of the Hβand it increased with the increasing calcination temperature. This should be due to the framework incorporation of iron ions, leading to the crystal cell expansion of Hβ.
The solid state reaction of Hβwith FeCl3was further studied by use of TG-DSC apparatus.Typical TG-DSC curves of uncalcined Fe-βand Hβcalcined at 500 ℃ were presented in Fig. 2. The significant loss of weight in the TG-DSC curves of Fe-βcould be observed in 100~600 ℃, suggesting that the solid state reaction of Hβwith FeCl3mainly occured before 600 ℃. The curves could be clearly divided into three stages (denoted as a1-b1/ a-b, b1-c1/b-c and c1-d1/c-d stages). The first stage (a1-b1/ a-b) with about 5% loss of weight, which is the fast exothermal process, appears in the low temperature range less than the melting point of FeCl3(301 ℃), corresponding to the pure solid state ion exchange process. The second stage (b1-c1/b-c) with about 1% loss of weight, which is the exothermic-endothermic balance process, just appears in the melting range of FeCl3, indicating that the melting, dispersion and ion exchange processes of FeCl3inside the pores of Hβsimultaneously were taking place in this stage. The third stage has the biggest loss of weight (about 9%) and broader and stronger exothermic peak. In this stage, the most important modified process was happening, which corresponded to the melting state ion exchange process. However, after the temperature goes beyond 600 ℃, a broadest and strongest exothermic peak within 650~1 048 ℃ for the Fe-βand 800~1 129 ℃ for the Hβcan be observed in the DSC curves of two samples, and they should correspond to the framework collapse process of the zeolite. But this peak on the Fe-βbecame shifted to the lower temperature range compared to that of Hβ, further confirming that the introduction of FeCl3easily causes the framework collapse of Hβ. Therefore, it should be reasonable to conclude that a relative low calcination temperature (500 ℃) is needed to prepare the catalysts, which can not only enhance the solid state reaction but also efficiently reduce the drop in crystal degree of the Hβ.

Fig.1 XRD patterns of Fe-β calcined at (a) 400 ℃, Fig.2 The TG and DSC curves of Hβ (1-1 and 1-2) and (b) 500 ℃, (c) 600 ℃ and (d) 950 ℃ uncalcined Fe-β (2-1 and 2-2)
The measured actual iron contents of calcined catalysts are listed in Tab.2. The iron content of the catalyst decreased with the increase of calcination temperature, and the reason may be that FeCl3with a boiling point of 315℃ was volatile during the calcination process, especially when the calcination temperature became higher.
2.2Hydroxylation of benzene
Hydroxylation of benzene with H2O2was employed to examine the catalytic property of FeCl3modified catalysts. The obtained results are shown in Tab.2. The pure Hβwas found to be inactive for this reaction, but, after the introduction of FeCl3, it became both reactive and selective to phenol, indicating that the iron site on the Fe-βplayed a key role in the hydroxylation of benzene. And the reactivity and selectivity of the Fe-βare dependent of its preparation temperature. Among them, Fe-βcalcined at 500 ℃ gave the highest reactivity (ETOF of the Fe-β(500) for 84). However, the catalysts prepared at the higher calcination temperature showed poor ETOFs, likely, due to the structure collapses of these samples to make iron active sites embedded, as shown by the above characterized peaks.
Noteworthy, phenol selectivity is not very excellent over the Fe-βcatalyst because of its deep oxidation. In order to improve phenol selectivity, the Fe-β(500) was further treated with DDS. The results are shown in Tab.2 as well. An obviously improved performance, in which benzene conversion only slightly decreased but phenol selectivity increased about 15%, was observed over the Fe-β(500,SM), indicating that the increase in its surface hydrophobicity should have played a significant role in restraining the deep oxidation of phenol and improving phenol selectivity in agreement with the previous report[20].

Tab.2 Characterized and benzene hydroxylation results of Hβ and FeCl3 modified catalysts
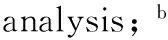
2.3Effects of process parameters
Fe-β(500,SM) with the maximum ETOF was employed to examine the impact of various process parameters such as its amount, molar ratio of benzene to oxidant, reaction temperature and addition of water on its catalytic properties. The results are presented in Tables 3 and 4. A general increasing trend in reactivity with catalyst and oxidant amounts, as well as temperature was observed. Too much catalyst or H2O2or too high temperature does not necessarily lead to the increase in reactivity.In some cases, they even inversely caused slightly worse results. The impact of catalyst amount and temperature on phenol selectivity has similar change patterns. That is, the selectivity firstly ascended and then descended as these process parameters were increased. This implies that these parameters all possess an optimal value (catalyst for 0.025 g and temperature for 65 ℃) for obtaining the highest phenol selectivity. And another decreasing trend in selectivity with increasing H2O2amount is observed. Considering phenol yield and H2O2effective conversion, H2O2amount with equal molar ratio to benzene is found to be suitable. Besides, the effect of adding water on benzene hydroxylation is apparent (shown as Tab.4), and it can result in an increase in conversion but it also lead to the decrease in phenol selectivity with a significant increase in catechol and hydroquinone formed by further hydroxylation of phenol. It is well known that the mechanism of aromatics hydroxylation over the transition metal iron catalysts is a typical free radical one[20], and water is an excellent solvent of phenol hydroxylation because it can play a key role in stabilizing the hydroxyl radicals (·OH) produced by H2O2. Therefore, this can easily be comprehended why adding water could considerably enhance the sequent hydroxylation of the formed phenol.

Tab.3 Effects of Fe-β(500,SM) and H2O2 amounts as well as reaction temperature on benzene hydroxylation

Tab.4 Effects of H2O2 amount on benzene hydroxylation over Fe-β(500,SM)a
aThe typical reaction conditions described in the experimental section were employed for benzene hydroxylation, the obtained products mainly included the aimed product phenol and the by-products such as quinone derivatives, unidentified products and a trace amount of catechol and hydroquninone.
Finally, the possibility of recycling Fe-β(500,SM) was also checked under the optimal reaction conditions with acetonitrile as solvent. The recycling results showed that about 36.1% of benzene conversion and 89.0% of phenol selectivity could be maintained after three cycles. These results are similar to those over the fresh catalyst, indicating that the active sites (iron ions) on the catalyst are very stable and their leaching occurs little. This could be proved by the measured iron contents of fresh catalyst (0.62 mmol/g) and recycled catalyst (0.60 mmol/g) nearly being the same. This also implies that FeCl3is mainly exchanged in the cationic sites inside the pores of H-βwith abundant exchanged cationic sites, and these exchanged iron ions are not easily washed away in the reaction process. As a result, it can be recovered and reused for three times without observable loss of reactivity.
3Conclusions
The solid state ion exchange method as a convenient, high-efficient and practical modification approach has been successfully employed to prepare the FeCl3modified beta zeolite catalyst (Fe-β). XRD, TG-DSC and low temperature N2adsorption measurements all confirmed that the key factor of preparing an excellent Fe-βcatalyst is to select a suitable calcination temperature. And the incorporation of iron ions into the framework ofβzeolite has occurred in the solid-state reaction process. These Fe-βcatalysts are reactive and selective in hydroxylation of benzene to phenol with H2O2. Among them, the Fe-βcalcined at 500 ℃ gives the highest ETOF (84), and its phenol selectivity can further be increased by about 15% after it is treated with DDS, suggesting that the increase in hydrophobicity on the DDS treated catalyst’s surface played a key role in restraining the successive oxidation of phenol and increasing its selectivity. Furthermore, the Fe-βcatalyst is very stable and its active iron sites are little leached away in the reaction process. As a result, it can be recovered and reused for three times without significant loss of reactivity.
References:
[1]HOCKING M B, INTIHAR D J. Oxidation of phenol by aqueous hydrogen peroxide catalyzed by ferric ion-catechol complexes [J]. J Chem Technol Biotechnol, 1985,35(7):365-381.
[2]朱丽娜,李洪涛,姜道华,等.我国苯酚丙酮生产技术及市场[J].化工技术与开发, 2014,43(1):35-37.
[3]MIYAKE T, HAMADA M, SASAKI Y,etal. Direct synthesis of phenol by hydroxylation of benzene with oxygen and hydrogen [J]. Appl Catal A: Gen, 1995,131(1):3342.
[4]ANTONYCAJ A, SRINIVASAN K. One-step hydroxylation of benzene to phenol over layered double hydroxides and their derived forms[J] .Catal Surv Asia, 2013,17(2):47-70.
[5]YURANOV I, BULUSHEV D A, RENKEN A,etal. Benzene to phenol hydroxylation with N2O over Fe-Beta and Fe-ZSM-5: Comparison of activity per Fe-site[J]. Appl Catal A: Gen, 2007,319(1):128-136.
[6]GE H Q , LENG Y, ZHOU C J,etal. Direct hydroxylation of benzene to phenol with molecular oxygen over phase transfer catalysts: cyclodextrins complexes with vanadium-substituted heteropoly acids[J]. Catal Lett, 2008,124(3):324-329.
[7]RENUKA N K. A green approach for phenol synthesis over Fe3+/MgO catalysts using hydrogen peroxide[J].Mol Catal A: Chem, 2010,316(1-2):126-130.
[8]KROMER A, RODUNER E. Catalytic oxidation of benzene on liquid ion-exchanged Cu,H(Na)/ZSM-5 and Cu,H(Na)/Y zeolites: spin trapping of transient radical intermediates[J]. Chem Plus Chem, 2013,78(3):268-273.
[9]GOPALAKRISHNAN S, ZAMPIERI A, W. Schwieger.Mesoporous ZSM-5 zeolites via alkali treatment for the direct hydroxylationof benzene to phenol with N2O[J]. Catalysis, 2008,260(1): 193-197.
[10]高丙莹,吴娟,何红运. 新型 Ti-Co-β沸石的合成、表征及催化性能的研究[J]. 湖南师范大学自然科学学报, 2014,37(2):40-46.
[11]IMRE B, HALASZ J, FREY K,etal. Oxidative hydroxylation of benzene and toluene by nitrous oxide over Fe-containing ZSM-5 zeolites[J]. React Kinet Catal Lett, 2001,74(2):377-383.
[13]GANESAN V, PAL M, TIWARI M. Manganese-Schiff base complex immobilized silica materials for electrocatalytic oxygen reduction[J]. Bull Mater Sci, 2014,37(3):623-628.
[14]SHERRY H S, WALTON H F. The ion-exchange properties of zeolites. II. Ion exchange in the synthetic zeolite Linde 4A[J]. J Phys Chem, 1967, 71(5):1457-1465.
[15]DORADO F, ROMERO R, CANIZARES P,etal. Influence of palladium incorporation technique onn-butane hydroisomerization over HZSM-5/bentonite catalysts[J]. Appl Catal A: Gen, 2004,274(1/2):79-85.
[16]DIMITROVA R, NEINSKA Y, MIHLYI M,etal. Reductive solid-state ion exchange as a way to vanadium introduction in BZSM and BBeta zeolites[J]. Appl Catal A: Gen, 2004,266(1):123-127.
[17]FU Z H, YU Y, YIN D L,etal.Vapor-phase highly selective O-methylation of catechol with methanol over ZnCl2modifiedγ-Al2O3catalysts[J]. Mol Catal A Chem, 2005,232(1):69-75 .
[18]HE J, GUO Z Y, MA H,etal. Enhancing the selectivity of benzene hydroxylation by tailoring the chemical affinity of the MCM-41 catalyst surface for the reactive molecules[J].J Catal, 2002,212(1):22-32.
[19]FUJIMOTO K, TOKUDA Y, AEKAWA M,etal. ChemInform abstract: selective and one-pot formation of phenols by anodic oxidation[J].Tetrahedron, 1996,52(11):3889-3896
[20]LIU C B, SHAN Y K, YANG X G,etal. Iron(II)-8-quinolinol/MCM-41-catalyzed phenol hydroxylation and reaction mechanism[J]. J Catal, 1997,168(1):35-41.
(编辑WJ)
DOI:10.7612/j.issn.1000-2537.2016.04.007
收稿日期:2016-05-03
基金项目:湖南省自然科学 项目(10JJ2007;11JJ6008);湖南省教育厅自然科学 项目(13C1127)
*通讯作者,E-mail:fzhhnu@tom.com
中图分类号TQ203.2;O643.32
文献标识码A
文章编号1000-2537(2016)04-0041-06
FeCl3改性β沸石的制备过程及在苯的羟基化催化反应的应用研究
周建波1, 伏再辉2*, 刘亚纯2, 徐超1
(1.长沙医学院基础医学院,中国 长沙410219;2.湖南师范大学化学化工研究院,中国 长沙410081)
摘要利用固态离子交换的方法制备出FeCl3改性的β沸石固相催化剂.采用XRD, TG-DSC及低温N2吸附法对所制的催化剂进行了表征.用H2O2作氧化剂将苯催化氧化成苯酚考察了催化剂的催化活性和选择性.通过调节二甲基二乙氧基硅烷(DDS)表面的亲水基和疏水基可以阻止苯酚进一步发生氧化反应从而提高催化反应的选择性.此外,在催化剂的回收实验中发现催化剂可以重复使用3次,而其催化活性没有太大的变化.所以从催化剂的制备到催化剂的催化过程都是绿色环保的.
关键词苯;FeCl3改性的β分子筛;羟基化;苯酚;固态离子交换

