Correlation study of biological characteristics of non-small cell lung cancer A549 cells after transfecting plasmid by microbubble ultrasound contrast agent
Xuan-Yan Guo, Man-Lu, Xue-Qing Chen, Fan-Ding He, Ang-Li*Ultrasonic Department, Academy of Sciences of Sichuan Province, People's Hospital of Sichuan Province, Sichuan Province, 6007, ChinaPancreatic Surgery, West China Hospital, Sichuan University, Sichuan Province, 6004, China
Correlation study of biological characteristics of non-small cell lung cancer A549 cells after transfecting plasmid by microbubble ultrasound contrast agent
Xuan-Yan Guo1, Man-Lu1, Xue-Qing Chen1, Fan-Ding He1, Ang-Li2*1Ultrasonic Department, Academy of Sciences of Sichuan Province, People's Hospital of Sichuan Province, Sichuan Province, 610072, China
2Pancreatic Surgery, West China Hospital, Sichuan University, Sichuan Province, 610041, China
ARTICLE INFO ABSTRACT
Article history:
in revised form 16 March 2016 Accepted 15 April 2016
Available online 20 June 2016
Microbubble ultrasound contrast agent
MiR-224
MiR-122a
Non-small cell lung cancer
Objective: To explore the role of the abnormal expression of miRNAs in the development process of non-small cell lung cancer and the feasibility of ultrasound microbubble-mediated gene therapy after transfecting antisense miRNA-224 and miRNA-122a plasmids into nonsmall cell lung cancer A549 cells. Methods: Antisense miRNA-224 and miRNA-122a plasmids were transfected into non-small cell lung cancer A549 cells on the optimal ultrasound microbubble mediated condition. We set up a control group. The cell proliferation activity,apoptosis, invasion ability were detected by MTT assay, Annexin V-PE, Transwell invasion experiment and colony formation assay, respectively. Results: The expression of miRNA-224 decreased and the expression of miRNA-122a rose after the plasmids of target genes were transfected into non-small cell lung cancer A549 cells, and there were significant differences when compared with those of the control group (P<0.05). After the plasmids of target genes were transfected into A549 cells, the growth of antisense miRNA-224 and miRNA-122a were inhibited, and the differences were significant as compared with the control group (P <0.05). Besides, the inhibition of miRNA-122a group was the most significant and there was statistically significant difference as compared with miRNA-224 group (t = -4.694, P = 0.009). After the plasmids of target genes were transfected into A549 cells, the proportion of apoptotic cells increased, the invasive cells were decreased and the clone ability reduced, and also there was a significant difference as compared with those of the control group (P < 0.05). What’s more, the apoptotic peak appeared in miRNA-122a group. Its invasion ability decreased most obviously (40.25 ± 3.97/visual field), the number of clone ability was 104.93 ± 4.87 and the inhibitory effect was the most obviously. There was statistically significant difference as compared with other groups (P < 0.05). Conclusions: A549 cells transfected by ultrasound microbubble-mediated antisense miRNA-224 and miRNA-122a plasmids possessed good transfection efficiency. The cell growth, invasion and colony forming abilities of transfected A549 cells were suppressed, which laid a solid foundation for the gene therapy of non-small cell lung cancer.
1. Introduction
Lung cancer is a kind of malignant tumor with high death rate. Non-small cell lung cancer (NSCLC) accounts for a high proportion and also possesses high death rate [1-4]. NSCLC can be caused by many factors, such as smoking, inheritance, chronic lung infection and so on. However, the exact pathogenesis of the disease still remains unknown [5]. At present, the idea that the abnormal expressions of tumor suppressors and cancer genes play an important role in the development process of cancer has been approved by many scientists [6]. MicroRNA (miRNAs) is a noncoding small RNAs which could regulate gene expression [7]. There are researches claiming that the related abnormal expression of miRNA can lead to the occurrence of NSCLC. Therefore, an intensive study of that kind of miRNA is of great significance to the exploration of the pathogenesis of NSCLC [8]. Recently,scientists have found that the expression of miR-224 increases in tissues of liver cancer, breast cancer and gastric cancer [9, 10], while the expression of miR-122a decreases in tissues of liver cancerand oral cancer [11, 12]. We predicted that miR-122a and miR-224 may work certainly in the occurrence and development of NSCLC. Microbubble ultrasound contrast agent is a new effective widelyused delivery vector, which can carry target genes and transfer them into cell nucleus smoothly [13-15]. The objectives of this study was to explore the role of the abnormal expression of miRNA-122a and miRNA-224 on the biological characteristics of NSCLC cells after transfecting antisense miRNA-122a and miRNA-224 plasmids into non-small cell lung cancer A549 cells.
2. Materials and methods
2.1. Groups and transfection
In this study, non-small cell lung cancer A549 cells were applied and cultured in 96-well plates with a cell density of 1 000/well. They were divided into four groups. The experimental groups included the antisense miR-224 group in which antisense miR-224 was transfected and the miRNA-122a group in which miRNA-122a was transfected, while the control groups had the control-antisense-miR-224 group in which antisense miRNA-224 plasmid was transfected and the control-miR-122a group in which miRNA-122aplasmid was transfected. Antisense miRNA-224 and miRNA-122a plasmids were mixed with microbubble suspension evenly, respectively, and placed in in the refrigerator at a temperature of 4 ℃. After the adhesion stabilized, they were put into the corresponding wells of the 96-well plates in accordance with the previous grouping for ultrasonic treatment.
2.2. Methods
2.2.1. Real-time PCR
Cells were transfected according to the above grouping. A blank control group was built. Then, the total RNA of each group was extracted according to the directions of Trizol kits. After that, the concentrations and purities of those RNA were detected. cRNA was obtained according to the directions of reverse transcriptase kits and was used as a template and amplified by corresponding primers. Then, the expression of the target gene was tested three times repeatedly.
2.2.2. MTT experiment
The cell suspension of A549 cells was inoculated in 96-well plates with a number of 1 000 cells and a volume of 100 μL in each well. Three ventral orifices and four 96-well plates were inoculated and cultured in an incubator. After 24 h, cells were transfected according to the above grouping. After transfected for 1, 2, 3 and 4 days, 20 μL of MTT solution was added into each well, respectively. After that,the culture solution was discarded after culturing 4 h continuously and 150 μL of DMSO dissolved crystal was added. Ten minutes later, the the absorbance value was test for three times and the data were analyzed.
2.2.3. Annexin V-PE experiment
Cells were transfected according to the corresponding groups. Then, cells were collected after digested by pancreatin (no EDTA)and washed by PBS. Binding Buffer with 7-AAD was added into the collected cells. After reacting for 5-15 min, 450 μL Binding Buffer was added sequentially and 1 μL of Annexin V-PE was added. After reacting for 5-15 min, flow cytometer was employed for detection which was repeated for three times.
2.2.4. Transwell assay
A549 cells were grouped into the antisense miR-224 group, miR-122a group, control-antisense miR-224 group and control-miR-122a group. A number of 100 μL cell suspension extracted from each group with a density of 5×105/mL was added into the invasion chamber. And then they were cultured in an incubator for one day and counted for three times.
2.2.5. Soft agar clone formation assay
Cells were transfected according to the previous grouping. An underlying agar was made in 6-well plates. Next, 100 μL of cell suspension with a concentration of 10000/mL was added into every 1 mL top agar and then paved on the underlying agar carefully. They were placed in an incubator for 10 days after solidification. After that, the number of cell colonies was calculated for three times.
2.3. Statistical methods
SPSS19.0 was used for statistical analysis. The cell proliferation activity, apoptosis, invasion ability of miRNA-224 and miENA-122a in A549 cells transfected by miR-122a group and antisense miR-224 group were detected by t-test. Differences were statistically significant when P< 0.05.
3. Results
3.1. The expressions of miRNA-224 and miRNA-122a of A549 after transfected
It was discovered by real-time PCR detection that the expression of miRNA-224 of A549 cells decreased significantly after transfected antisense miRNA-224 and miRNA-122a plasmids, and there were significant differences as compared with those of the control group (t = -4.634, P = 0.010); while the expression of miRNA-122a increased obviously and the differences were statistical significant (t= 6.448,P= 0.003) (Table 1).
3.2. The cell proliferation detected by MTT experiment
Compared with the relative control group, the proliferation of A549 cells reduced distinctly after transfecting antisense miR-224 and miR-122a plasmids. Moreover, the proliferation of A549 cells transfected miR-122a plasmids slowed down most obviously. The differences from the first day to the fourth were statistically significant (P< 0.05). Besides, on the fourth day of transfection, the proliferation rate of A549 which transfected miR-122a plasmids was slower than that of A549 transfected antisense miR-224 plasmids. The differences were statistical significant (t= -4.694, P=0.009). After transfecting antisense miR-224 plasmids, the proliferation rates of the first two days remained no distinct differences as compared to the control group. However, the proliferation rates of the third and fourth days were slower evidently than those of the control group. The differences showed statistical significance (t= -3.913, P= 0.017,t = -4.146, P = 0.014) (Table 2).

Table 1The expressions level, apoptosis, number of invasion cells and change of the ability of cell clone formation of A549.
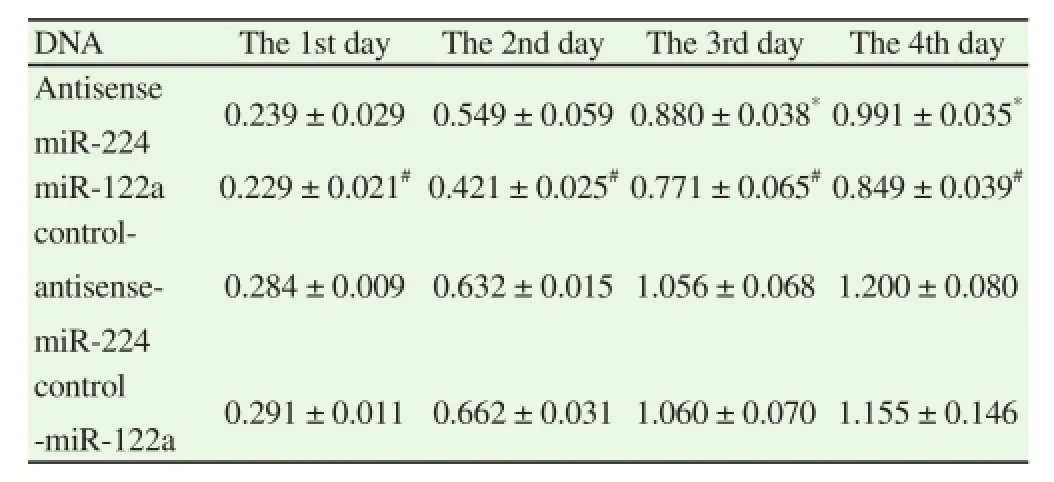
Table 2The cell proliferation of A549 after transfected plasmids.
3.3. Detection of the change of cell apoptosis by Annexin V-PE
The cell apoptosis of A549 cell was detected by Annexin V-PE. The results showed that the apoptosis rates of cells transfecting antisense miR-224 and miR-122a plasmids increased significantly. The cell apoptosis rate of the antisense miR-224 group increased obviously as compared with that of the control-antisense miR-224 group. The differences had statistical significances (t= 35.162, P= 0.000). The cell apoptosis rate of the antisense miR-122a group also increased obviously as compared with that of the control-miR-122a group. The differences showed statistical significances (t = 49.565, P = 0.000). In addition, the cell apoptosis rate of the miR-122a group was higher than that of the antisense miR-224 group and the differences were statistically significant (t = 8.404, P = 0.001) (Table 1).
3.4. The detection of the invasion ability of A549 cells by Transwell assay
The number of cells of every vision was observed by a microscope. The results showed that the invasion ability of A549 cells reduced significantly after transfecting target gene plasmids, and the differences remained statistically significant (P < 0.05). Besides, the invasion inhibitory ability of A540 cells which transfected miRNA-122a was better than that of the antisense miR-224 group, and the differences were also statistically significant (t = -9.259, P = 0.001)(Table 1).
3.5. The change of the ability of cell clone formation
The results of the colony-forming unit assays revealed that the clone formation ability of D549 declined after transfecting target gene plasmids as compared with the control group, and the differences had statistical significance (P< 0.05). Besides, the inhibitory effect of A549 cells group on its clone formation transfecting miRNA-122a was better than that of the antisense miR-224 group. The differences were also statistically significant (t = -5.807, P = 0.004) (Table 1).
4. Discussion
NSCLC is kind of tumor with a high grade of malignancy which severely threatens human health. Surgery, radiotherapy and chemotherapy do not work effectively, so a new treatment plan is needed to be developed [16-19]. At present, gene therapy has raise passions and concerns, which might provide a new way for the treatment of the disease. In the occurrence and development of tumor, miRNA, a kind of small endogenous, plays an important role[20, 21]. The expression quantity of miRNA can be detected by many ways. Among them, real-time RT-PCR is used most commonly because of its excellent specificity, sensitivity and accuracy [22-24]. However, owing to the lack of gene vectors, researches on miRNA become difficult. Recently, researches have shown that ultrasound microbubble with target gene injected into bodies for ultrasound irradiation could lead to favorable transfection effect [25, 26]. The main objective of this study was to explore the role of the abnormal expression of miRNA-122a and miRNA-224 on the biological characteristics of NSCLC A549 cells transfecting miRNA-122a and antisense miRNA-224 plasmids.
MiRNA could regulate many expressions of mRNA, which is a key substance of the upstream of the signal pathways in cells. Hence,the research on miRNA was of great importance to the study of the disease [27]. The results of this study showed that the expression level of A549 cells transfecting antisense miR-224 decreased significantly and the expression level of miR-122a in non-small cell lung cancer A549 cells increased obviously as compared with the control group. It is indicated that ultrasound microbubble possessed an excellent transfection effect, which could be used for follow-up studies.
In this study, MTT assay was employed to detect the proliferation condition of non-small cell lung cancer A549 cells. The results showed that there were distinct differences of the proliferation conditions between the experiment group and control group. The proliferation of A549 of the experiment group was inhibited evidently. In previous studies, mir-244 has over-expressed in some tumors and improved proliferation. However, miR-122a did the opposite [10, 11]. Therefore, when plasmids of antisense miR-224 and over-expressed miR-122a were transfected, the proliferation of A549 was inhibited effectively.
Annexin V-PE experiment was used to determine the apoptosis of non-small cell lung cancer A549 cells. The results showed that the apoptosis rate of the experiment group was raised significantly as compared with that of the control group. Besides, the apoptosis rate of the miR-122a group was more than 10%, which might be related to the wonderful effect of the ultrasound microbubble mediatedtransfection and its biological function. There were researches claiming that miR-122a could promote the apoptosis of hepatoma cells by regulating the expressions of genes such as Bcl-2, Mcl-1. Plasmids transfected in non-small cell lung cancer cells played a similar role in the process [28-30].
The results of Transwell assay revealed that after transfecting the target gene, the invasion ability of the experiment group declined significantly as compared to the control group. After transfected antisense miR-224 and miR-122a plasmids, the expression of miR-224 of A549 cells reduced and the expression of miR-122a increased. They might work on the direct target gene PTEN to increase the expression quantity of PTEN and weaken the invasion ability of A549n cells [31].
Results of soft agar clone formation assay declared that the clone formation capacity of the expression group reduced significantly and cells transfecting miR-122a showed the best clone formation capacity. This may be because the decrease of the expression of miR-224 and the increase of the expression of miR-122a caused the change of the expressions of substances such as HIF-1a, CDKNI B/ p27, CDKNI A/p21 and so on, which, as a result, decreased its clone formation capacity.
To sum up, in non-small cell lung cancer A549 cells, the proliferation, invasion ability and clone formation capacity of 549 cells were inhibited obviously after transfeting antisense miR-224 and miR-122a plasmids by microbubble ultrasound contrast agent. In addition, the number of apoptosis cells increased distinctly. This analysis of the biological characteristics of non-small cell lung cancer A549 cells by miRNAs provided a new way for the gene therapy of NSCLC.
Declare of interest statement
We declare that we have no conflict of interest conflict.
References
[1] Hao Z, Tian CY, Yang FT, Zhang JH. Correlation between expression of epidermal growth factor receptor and adverse reactions after chemotherapy of advanced non-small-cell lung cancer. Pak J Med Sci 2015; 31(5): 1115-1120.
[2] Chen QQ, Ji XX, Zhou X, Shi QL, Yu HM, Fu HQ, et al. Clinical observation of docetaxel or gemcitabine combined with cisplatin in the chemotherapy after surgery for stage II-III non-small cell lung cancer. Contemp Oncol (Pozn) 2015; 19(4): 323-326.
[3] Kriegsmann M, Muley T, Harms A, Tavernar L, Goldmann T, Dienemann H, et al. Differential diagnostic value of CD5 and CD117 expression in thoracic tumors: A large scale study of 1465 non-small cell lung cancer cases. Diagn Pathol 2015; 10(1): 210.
[4] Yan L, Yao Y, Wang LH, Wang ML, Fu XH. Detection of CK19, LUNX,and KS1/4 mRNA expression in the peripheral blood for diagnosis of micrometastases in patients with non-small cell lung cancer and their clinical implications. Genet Mol Res 2015; 14(4): 15090-15097.
[5] Wei H, Lu WP, Li M, Zhang QP, Lu S. Concomitance of P-gp/LRP expression with EGFR mutations in Exons 19 and 21 in non-small cell lung cancers. Yonsei Med J 2016; 57(1): 50-57.
[6] Moon H, Zheng XX, Loh TJ, Jang HN, Liu YC, Jung DW, et al. Identification of regulatory-RNAs for alternative splicing of Ron Proto-Oncogene. J Cancer 2015; 6(12): 1346-1351.
[7] Melo SF, Barauna VG, Neves VJ, Fernandes T, Lara Lda S, Mazzotti DR,et al. Exercise training restores the cardiac microRNA-1 and -214 levels regulating Ca2+handling after myocardial infarction. BMC Cardiovasc Disor 2015; 15(1): 166.
[8] Wang C, Ding M, Xia M, Chen S, Van Le A, Soto-Gil R, et al. A fivemiRNA panel identified from a multicentric case-control study serves as a novel diagnostic tool for ethnically diverse non-small-cell lung cancer patients. EBio Med 2015; 2(10): 1377-1385.
[9] An F, Olaru AV, Mezey E, Xie Q, Li L, Piontek KB, et al. MicroRNA-224 induces G1/S checkpoint release in liver cancer. J Clin Med 2015; 4(9): 1713-1728.
[10] Chen W, Fan XM, Mao L, Zhang JY, Li J, Wu JZ, et al. MicroRNA-224: as a potential target for miR-based therapy of cancer. Tumor Biol 2015;36(9): 6645-6652.
[11] Diao S, Zhang JF, Wang H, He ML, Lin MC, Chen Y, et al. Proteomic identification of microRNA-122a target proteins in hepatocellular carcinoma. Proteomics 2010; 10(20): 3723-3731.
[12] Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012; 122(8): 2884-2897.
[13] Khokhlova TD, Haider Y, Hwang JH. Therapeutic potential of ultrasound microbubbles in gastrointestinal oncology: recent advances and future prospects. Therapeut Adv Gastroenterol 2015; 8(6): 384-394.
[14] Meairs S. Facilitation of drug transport across the blood-brain barrier with ultrasound and microbubbles. Pharmaceutics 2015; 7(3): 275-293.
[15] Malhi H, Grant EG, Duddalwar V. Contrast-enhanced ultrasound of the liver and kidney. Radiol Clin N Am 2014; 52(6): 1177-1190.
[16] Joo JH, Song SY, Kim SS, Jeong Y, Jeong SY, Choi W, et al. Definitive radiotherapy alone over 60 Gy for patients unfit for combined treatment to stage II-III non-small cell lung cancer: retrospective analysis. Radiat Oncol 2015; 10(1): 250.
[17] Yokouchi H, Kanazawa K. Revisiting the role of COX-2 inhibitor for non-small cell lung cancer. Transl Lung Cancer Res 2015; 4(5): 660-664.
[18] Souglakos J. Customizing chemotherapy in non-small cell lung cancer: the promise is still unmet. Transl Lung Cancer Res 2015; 4(5): 653-655.
[19] Chuang JC, Neal JW. Crizotinib as first line therapy for advanced ALK-positive non-small cell lung cancers. Transl Lung Cancer Res 2015; 4(5): 639-641.
[20] Ling H, Krassnig L, Bullock MD, Pichler M. MicroRNAs in testicular cancer diagnosis and prognosis. Urol clin North Am 2016; 43(1): 127-134.
[21] Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in alcoholinduced multi-organ injury. Biomolecules 2015; 5(4): 3309-3338.
[22] Yang CH, Li K, Pfeffer SR, Pfeffer LM. The type I IFN-Induced miRNA,miR-21. Pharmaceuticals (Basel) 2015; 8(4): 836-847.
[23] Thomas J, Ohtsuka M, Pichler M, Ling H. MicroRNAs: Clinical Relevance in Colorectal Cancer. Int J Mol Sci2015; 16(12): 28063-28076.[24] Yang ZH, Cappello T, Wang L. Emerging role of microRNAs in lipid metabolism. Acta pharmaceutica Sinica B 2015; 5(2): 145-150.
[25] Ramaswamy K, Marx V, Laser D, Kenny T, Chi T, Bailey M, et al. Targeted microbubbles: a novel application for the treatment of kidney stones. BJU Int 2015; 116(1): 9-16.
[26] Tranquart F, Arditi M, Bettinger T, Frinking P, Hyvelin JM, Nunn A,et al. Ultrasound contrast agents for ultrasound molecular imaging. Z Gastroenterolo 2014; 52(11): 1268-1276.
[27] Wu XL, Tan XH, Fu SW. May Circulating microRNAs be gastric cancer diagnostic biomarkers? J Cancer 2015; 6(12): 1206-1213.
[28] Gao LW, Ai JH, Xie ZD, Zhou CY, Liu C, Zhang H, et al. Dynamic expression of viral and cellular microRNAs in infectious mononucleosis caused by primary Epstein-Barr virus infection in children. Virol J 2015;12(1): 208.
[29] Song JH, Yang J, Pan F, Jin B. Differential expression of microRNAs may regulate pollen development in Brassica oleracea. Genet Mol Res 2015; 14(4): 15024-15034.
[30] Hu JS, Xu Y, Cai SJ. Specific microRNAs as novel biomarkers for combination chemotherapy resistance detection of colon adenocarcinoma. Eur J Med Res 2015; 20: 95.
[31] Akouchekian M, Hemati S, Kachoei ZA. Analysis of PTEN in two BRCA1 and BRCA2 wild-type familial breast cancer patients. J Res Med Sci 2015; 20(6): 629-630.
Document heading 10.1016/j.apjtm.2016.04.007
15 February 2016
*Corresponding author: Corresponding author: Ang-Li, Associate Chief Physician,Pancreatic Surgery, West China Hospital, Sichuan University, Sichuan Province,610041, China.
Tel: 028-85422323
E-mail: angli1979@hotmail.com
Foundation project: This study was supported by Science and technology plan projects of Sichuan Province (Grant No. 2015SZ0074).
 Asian Pacific Journal of Tropical Medicine2016年6期
Asian Pacific Journal of Tropical Medicine2016年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Experiment research of cisplatin implants inhibiting transplantation tumor growth and regulating the expression of KLK7 and E-cad of tumor-bearing mice with gastric cancer
- Study on prevention effect of Zishen Yutai pill combined with progesterone for threatened abortion in rats
- Expression and significance of angiostatin, vascular endothelial growth factor and matrix metalloproteinase-9 in brain tissue of diabetic rats with ischemia reperfusion
- Change of the peripheral blood immune pattern and its correlation with prognosis in patients with liver cancer treated by sorafenib
- Comparative study of the chitooligosaccharides effect on the proliferation inhibition and radiosensitization of three types of human gastric cancer cell line
- Are efforts up to the mark? A cirrhotic state and knowledge about HCV prevalence in general population of Pakistan
