Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
Cheng-yun Gong,Zhi-gng Geng,An-le Dong,Xin-xin Ye,Guo-zhong Wng,Yun-xi Zhng∗.Key Lbortory of Mterils Physics,Centre for Environmentl nd Energy Nnomterils,Anhui Key Lbortory of Nnomterils nd Nnotechnology,Institute of Solid Stte Physics,Chinese Acdemy of Sciences,Hefei 230031,Chin.b.Nno Science nd Technology Institute,University of Science nd Technology of Chin,Suzhou 215123,Chin
Highly Efficient and Selective Removal of Pb(II)ions by Sulfur-Containing Calcium Phosphate Nanoparticles
Cheng-yun Gonga,b,Zhi-gang Genga,An-le Donga,Xin-xin Yea,Guo-zhong Wanga,Yun-xia Zhanga∗
a.Key Laboratory of Materials Physics,Centre for Environmental and Energy Nanomaterials,Anhui Key Laboratory of Nanomaterials and Nanotechnology,Institute of Solid State Physics,Chinese Academy of Sciences,Hefei 230031,China.
b.Nano Science and Technology Institute,University of Science and Technology of China,Suzhou 215123,China
A facile one-step co-precipitation method was demonstrated to fabricate amorphous sulfurcontaining calcium phosphate(SCP)nanoparticles,in which the sulfur group was in-situ introduced into calcium phosphate.The resulting SCP exhibited a noticeable enhanced performance for Pb(II)removal in comparison with hydroxyapatite(HAP),being capable of easily reducing 20 ppm of Pb(II)to below the acceptable standard for drinking water within less than 10 min.Remarkably,the saturated removal capacities of Pb(II)on SCP were as high as 1720.57 mg/g calculated by the Langmuir isotherm model,exceeding largely that of the previously reported absorbents.Significantly,SCP displayed highly selective removal ability toward Pb(II)ions in the presence of the competing metal ions(Ni(II),Co(II),Zn(II), and Cd(II)).Further investigations indicated that such ultra-high removal efficiency and preferable affinity of Pb(II)ions on SCP may be reasonably ascribed to the formation of rodlike hydroxypyromorphite crystals on the surface of SCP via dissolution-precipitation and ion exchange reactions,accompanied by the presence of lead sulfide precipitates.High removal efficiency,fast removal kinetics and excellent selectivity toward Pb(II)made the obtained SCP material an ideal candidate for Pb(II)ions decontamination in practical application.
Sulfur-containing calcium phosphate,Pb(II)ions,Selective removal
I.INTRODUCTION
Heavy metals exist extensively in natural and contaminated waters due to the excessive discharge from the electroplating,polishing,mining,metallurgy,printing and dyeing industries into the aquatic bodies, which constitutes a persistent threat towards public health and the natural ecosystem because of their nonbiodegradablility,accumulation in humans and other living organisms via food chains,as well as potentially high toxicity even at very low concentrations.As a representative member of notorious heavy metals,Pb(II) has been arousing much attention,which not only interferes with the metabolism of human bodies,but also indirectly damages the central nervous system,as well as causes various diseases and disorders when its concentration exceed permissible limits[1−3].In view of the increasingly Pb(II)pollution issues,the World Health Organization(WHO)has set the stringent standard for the maximum allowable lead level in drinking water (10µg/L).Consequently,it is of particular significance to develop highly efficient and economical remediation techniques to reduce the toxic heavy metals in natural waters and industrial waste-water to below acceptable levels.In an attempt to achieve the goal,various remediation techniques have been developed,including chemical precipitation,coagulation-flocculation,membrane separation,ion exchange,reverse osmosis,electrochemical reduction,and so on.However,most of these procedures usually suffer from unavoidable disadvantages,such as high cost,unsatisfactory removal efficiency,especially for the heavy metals with trace levels, and the secondary generation of toxic sludge or waste effluents that usually need more problematic subsequent treatment.
Among the various techniques,adsorption is found to be quite promising alternative to other conventional techniques for the decontamination of dissolved heavy metal ions from wastewater due to its high efficiency, flexible operation and low cost. The traditional adsorbents,such as activated carbon,clays and zeolites have been commonly utilized to remove heavy metal ions from contaminated waters because of their high surface area,hydrophilic character and low cost[4−12]. However,these conventional materials possess some in-trinsic shortcomings,e.g.poor removal abilities,low selectivity,and relativity weak affinity for the target heavy metal ions because of their physical adsorption driven by electrostatic attraction or van der Waals forces interaction. As a family of the environmentally benign adsorbents,hydroxyapatite(HAP, Ca10(PO4)6(OH)2)is a better choice for the immobilization of heavy metals because of its high sorption capacity,fast kinetics,low solubility,high stability under reducing and oxidizing conditions,ready availability,biodegradability and low cost[13−19].It has turned out that HAP was able to capture and store a large variety of heavy metals from the aqueous environment through ionic exchange reaction,surface complexation, precipitation of new metal phosphates with a lower solubility,and substitution of Ca in HAP by other metals during recrystallization[14,20−22].However,to date, some problems are still unresolved,which are associated with their applications towards Pb(II)removal,for example,low adsorption capacities,and poor selectivity over other heavy metal ions of HAP.
∗Author to whom correspondence should be addressed.E-mail: yxzhang@issp.ac.cn,Tel.:+86-551-65592145,FAX:+86-551-65591434
From the viewpoint of practical application,the nonspecific uptake for heavy metal ions is extremely adverse,particularly in the presence of coexisting cations at high levels,which strongly competes against the target heavy metals and thus results in a dramatic decrease in effective removal capacity towards the target heavy metal ions.Therefore,an ideal adsorbent should not only have the feature of high adsorption capacity but also possess strong affinity for the target heavy metal ions,which could specifically remove these heavy metal ions without the interference of other metal ions.One of the most effective strategies to improve sorption selectivity of the absorbents toward the target contaminants is surface modification.For the soft heavy metal ions, such as Pb(II)ions,the optimal candidate of the functional groups is thiol group on the basis of the principles of hard and soft acids and bases,in which sulfur atom can easily realize the specific capture towards the soft lewis acid metal ions.For instance,thiol-functionalized magnetic mesoporous silica,montmorillonite,clay minerals,kaolinite and zeolite were reported and exhibited a highly selective adsorption capability for heavy metal ions[23,24].Nevertheless,thiol modification usually existed some unavoidable drawbacks,such as expensive, too many chemicals,severe and complicated reaction conditions[25].Even if a high level of thiol functionalization was achieved,less than stoichiometric adsorption took place in these hybrid materials due to limited accessibility to the thiol groups[26].In addition, thiol-functionalized sorbents were usually not suitable for long-term use because the thiol groups had a great tendency to be oxidized to disulfide ones under ambient conditions,causing a gradual loss of the capacity of these sorbents[27].Considering these limits of the grafting thiol groups,there is an urgent need to develop the ideal materials to realize the removal of Pb(II)ions from contaminant waters in simple,efficient and economically practicable ways.
Herein,we tried to use trisodium monothiophosphate (Na3PO3S)as the sulfur source to synthesize sulfurcontaining calcium phosphate(SCP)nanoparticles,in which the sulfur group was introduced directly to the structure of calcium phosphate with no need of functional groups modification.The removal efficiency of Pb(II)and corresponding removal kinetics for the asobtained nanoparticles were demonstrated as compared to the commercial HAP particles in order to gain a better understanding of the role of the intrinsic sulfur group.The selective removal of SCP towards Pb(II) ions was evaluated in the presence of the interfering metal ions.The underlying mechanisms for Pb(II)removal were proposed based on X-ray diffraction(XRD), field emission scanning electron microscope(FESEM), Fourier transform infrared spectroscopy(FT-IR)and X-ray photoelectron spectroscopy(XPS)analyses of SCP before and after Pb(II)treatment.
II.EXPERIMENTS
A.Chemicals
Pb(NO3)2,CdCl2,Co(NO3)2,Ni(NO3)2,Zn(NO3)2, Ca(NO3)2·4H2O,NH3·H2O,and HNO3were of analytic grade and purchased from Sinopharm Chemical Reagent Co.Ltd.(Shanghai,China).The commercial HAP was provided by Aladdin Reagent Co.Ltd. (Shanghai,China).Na3PO3S was obtained from Alfa Aesar(Beijing,China). Deionized water with a resistivity of above 18.2 MΩ·cm was obtained using a JL-RO100 Millipore-Q Plus water purifier and used throughout the experiments.All chemicals were used directly without any further purification during the experiments.
B.Synthesis of SCP
SCP were prepared by one-step precipitation method with Ca(NO3)2·4H2O and Na3PO3S as starting materials,in which ammonia solution was used for pH adjustment. In a typical procedure,18.16 mmol of Ca(NO3)2·4H2O was dissolved in 160 mL of deionized water,followed by adding 120 mL of Na3PO3S solution(0.0997 mol/L)with vigorous mechanical stirring at room temperature(~25°C),in which pH of solution was adjusted to 10.5±0.1 by adding ammonia solution. The reaction was continued for 2 h before being aged for 24 h at room temperature.Then,the suspensions were separated by centrifugation and washed with deionized water and ethanol for several times to remove the unwanted ions(Na+and NO3−).The resultant samples were then freeze-dried for further use.
C.Characterization
The crystalline structure of the obtained sample was identified by XRD(Philips X'pert PRO)analysis using Ni-filtered monochromatic Cu Kα radiation at 40 kV and 40 mA.The morphology and microstructure examinations were conducted using a FESEM(Sirion 200 FEG,USA)atan accelerating voltage of 10 kV. The compositions of resulting products were tested by energy-dispersive X-ray spectroscopy(EDS)attached to FESEM.Nitrogen adsorption-desorption isotherms were measured using an automated gas sorption analyzer(Autosorb-iQ-Cx).FT-IR spectra of the samples were recorded on a Thermo Nicolet NEXUS FIIR spectrophotometer using the KBr pellet method in the wavenumber range 400−4000 cm−1at a resolution of 4 cm−1.Raman spectra were measured on a Renishaw Micro-Raman Spectroscopy(Renishaw inVia Reflex)using 532 nm laser excitation.Zeta potential of the obtained suspension was determined using a zeta potential analyzer(Zetasizer3000HSa,Malvern,UK). XPS analyses were conducted on a Thermo Scientific ESCALAB 250 equipped with a focused monochromatic Al Kα X-ray source,in which all of the binding energies were calibrated with reference to the C 1s peak(284.8 eV). Quantitative determination of the metal ions content was performed by inductively coupled plasma optical emission spectrometer(ICP-OES, ICP-6300 Thermo Fisher Scientific,USA).
D.Batch treatment experiments
To determine the optimum pH for Pb(II)removal, the influence of solution pH on the uptake of Pb(II)was examined first.10 mg of samples were added to 500 mL of Pb(II)solution(50 mg/L)with varying pH ranging from 2 to 6,which was adjusted using 0.1 mol/L HNO3or 0.1 mol/L NaOH solution.After shaken at 25°C for 12 h,the suspensions were filtered by a 0.22µm filter membrane and the obtained supernatant was used for Pb(II)analysis.
Kinetic experiments for Pb(II)removal were conducted as follows.10 mg of the samples were added to 500 mL of Pb(II)solution(20 mg/L,pH=5.5)under continuous stirring at 25°C.At the given time intervals,the suspensions were withdrawn and then immediately filtrated through a 0.22µm filter membrane.The obtained supernatant was then used for ICP-OES measurements to determine Pb(II)ions concentration.The filtered samples were then freeze dried and investigated to identify the removal mechanisms.
Removal isotherm experiments were conducted by adding 10 mg of samples to 500 mL of Pb(II)solution with varying concentrations(40−100 mg/L for SCP, 3−60 mg/L for HAP(Aladdin))in different conical flasks.The suspensions were shaken for 12 h to attain the adsorption equilibrium.The amount of Pb(II)immoblized on the samples under equilibrium conditions was obtained from the following equation[28]:
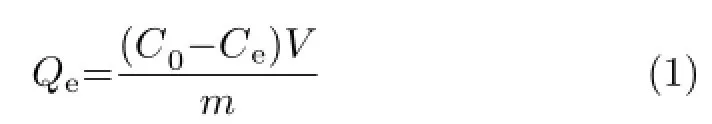
where C0and Ceare the initial and equilibrium concentrations of Pb(II)solution,respectively.V is the volume of Pb(II)solution,m denotes the mass of the samples.

FIG.1(a)XRD,(b)SEM image,and(c)EDS spectrum of SCP.
To evaluate the removal preference for the target Pb(II)ions in the presence of competing ions,10 mg of samples were added into 500 mL of multi-metals (Pb(II),Cd(II),Ni(II),Co(II),and Zn(II))solution with equal initial concentration(20 mg/L),the other experimental details were similar to the above description.For comparison,each mono-metal removal experiment was separately also conducted under the similar conditions.The distribution coefficient Kdused for determining the selectivity of absorbents for heavy metals was given by the equation:
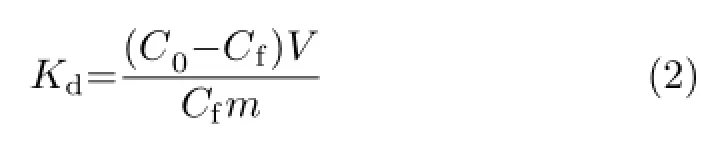
where C0and Cfare the initial and local concentrations of metal ions(ppm)after the treatment,respectively. V is the volume of the testing solution,and m is the amount of the solid sorbent used in the experiment.In our case,the ratio of V to m was 50 L/g.
III.RESULTS AND DISCUSSION
A.Characterization of SCP
As shown in Fig.1(a),XRD pattern of the as-prepared samples consisted of two fairly broad,ill-defined diffraction peaks,indicating their amorphous characteristicand small sizes.And the morphology of SCP samples was investigated by SEM observation.From Fig.1(b), it can be seen that the synthesized samples were irregular particles with sizes from 60 nm to 100 nm,which was in consistence with the XRD data.EDS analysis in Fig.1(c)revealed the existence of Ca,P,S,Si,and O species,in which Si signal was originated from the substrate.
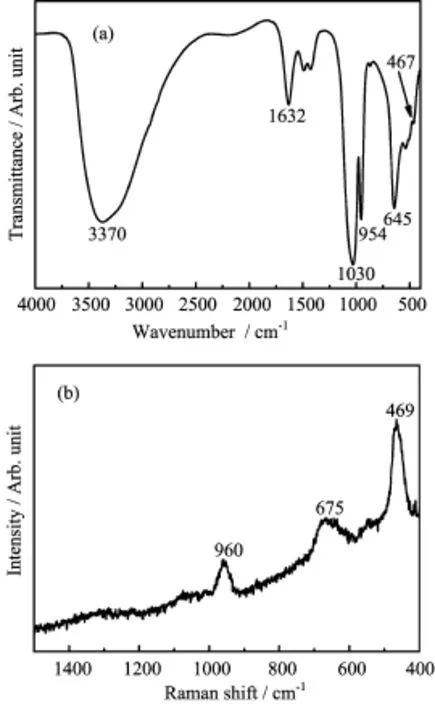
FIG.2(a)FT-IR and(b)Raman spectra of the obtained SCP.
FT-IR and Raman spectra shown in Fig.2 provided further supporting evidence for the successful incorporation of sulfur group in the framework of SCP. The broad absorption at 3370 cm−1(Fig.2(a))was assigned to stretching vibrations ν(OH)of the crystal water molecules.And the bending mode σ(OH) of water molecules was observed at 1632 cm−1.As expected,the characteristic bands at 1030,954,and 645 cm−1were attributed to νas(PO3),νs(PO3)and δas(PO3)vibrations of(PO3S)3−,respectively. In addition,the weak ν(PS)vibration was detected at 467 cm−1[29].The Raman spectrum(Fig.2(b))displayed intense bands between 1200 and 400 cm−1.The strongest emission at 469 cm−1originated from ν(PS), and the emissions at 675 and 960 cm−1can be assigned to the δas(PO3)and νs(PO3)vibrations,respectively,in accordance with the previous report about thiophosphates NaM(PO3S)·9H2O(M=Ca,Ba)[29]. The above-mentioned analyses indicated the presence of sulfur groups in the as-synthesized SCP samples in the form of monothiophosphate.
N2adsorption/desorption analysis was carried out to characterize the specific surface area and porous structures of the as-prepared samples.Figure 3 displayed the adsorption/desorption isotherms and the corresponding pore size distribution curve.A typical type IV hysteresis loop(Fig.3(a))in the relative pressure(P/P0) range of 0.7−1.0 was observed for the obtained SCP samples,indicating the existence of a mesoporous structure,which was attributed to aggregates of spherical particles.The pore size distribution derived from the desorption branch was determined by Barret-Joyner-Halenda(BJH)method(as shown in Fig.3(b)),in which the mean pore diameter was ca.18.74 nm with the total pore volume of 0.27 cm3/g.And the corresponding Brunauer-Emmett-Teller(BET)surface area was calculated to be 55.28 m2/g,providing abundant active sites for the following Pb(II)removal process.
B.Removal experiment of Pb(II)from aqueous solution
In order to demonstrate the potential applications in wastewater treatment of the as-prepared SCP,the various adsorption parameters,such as solution pH,the contacting time,initial Pb(II)ions concentration,competing ions,were studied to find out the best conditions for the maximum removal efficiency of Pb(II)ions from aqueous solution.In addition,a series of contrast experiments were also carried out to reveal the difference of Pb(II)removal capabilities between the as-synthesized SCP and commercial HAP(purchased from Aladdin, the corresponding XRD and SEM image of HAP were given in Fig.S1 in supplementary material)under the similar treatment conditions.
1.Effect of pH value in aqueous solution
The solution pH was one of the most important factors for the uptake of heavy metals,which might significantly affect both the hydrolytic species of heavy metal ions and the surface charge of adsorbent.In order to identify the optimal PH value of the uptake of Pb(II), the removal experiments were carried out using 50 mg/L of Pb(II)aqueous solution with initial pH values ranging from 2.0 to 6.0,in view of the fact that high pH (over 6.0)resulted in the formation of Pb(II)hydroxide precipitation.As presented in Fig.4,there was a strong pH dependent on the removal capacity of Pb(II) on SCP,accompanied by the increased removal capacity with the increase of pH.The uptake of Pb(II)almost kept constant over the pH range 2.0−4.0,and increased dramatically from 1085.5 mg/g to 1570.5 mg/g with the rising pH from 4.0 to 6.0,which could be reasonably explained as follows.At lower pH,the increased net positive charge of SCP surface may be the main cause of low removal capacity due to the competition between Pb(II)ions and protons,in which the dissolution of SCP and formation of new precipitations were responsible for the uptake of Pb(II).With the increase of pH,the negative charge density on SCP surface increased,and thereby enhancing the electrostatic attraction of Pb(II)ions on the absorbents surface and ion exchange reaction between Ca(II)of SCP and Pb(II)in aqueous solution [30].Based on the above analyses,the following batch experiments were undertaken at pH=5.5 in this study, in which the dissolution of SCP and Pb(II)hydroxide precipitation can be avoided to the maximum extent.
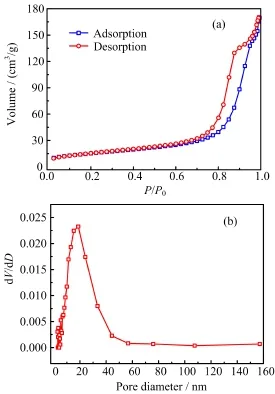
FIG.3 N2adsorption desorption isotherm(a)and the corresponding BJH pore size distribution curve(b)of the synthesized SCP.
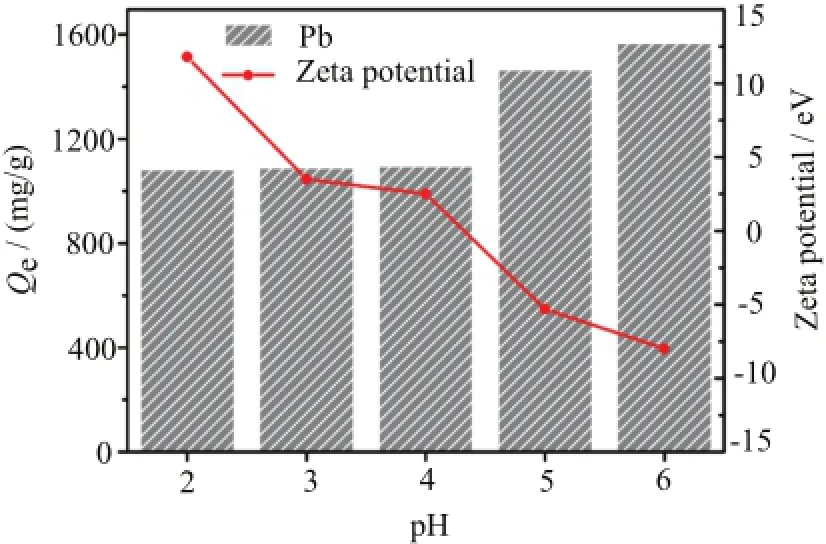
FIG.4 Effect of initial pH on the Pb(II)removal by SCP and zeta potential of SCP under different pH.Experimental conditions: 50 mg/L of initial Pb(II)concentration (500 mL),10 mg of samples,12 h of equilibrium time and temperature 25°C.
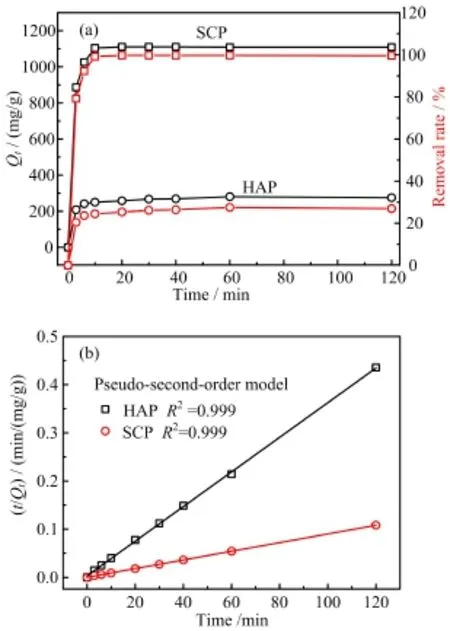
FIG.5(a)Removal kinetics of Pb(II)on SCP and HAP (Aladdin).Experimental conditions:initial Pb(II)concentration 20 mg/L(500 mL),sorbent 10 mg,pH 5.5,contact time 120 min,and temperature 25°C.(b)Pseudo-secondorder kinetic fitting of Pb(II)removal by SCP and HAP (Aladdin).
2.Removal kinetics
In order to gain an insight into the removal kinetics,Pb(II)removal experiments were carried out and the removal capacities(Qt)of Pb(II)were determined at different time intervals,in which the initial concentration of Pb(II)was set as~20 mg/L with 10 mg of the sorbents dosage.To elucidate superiority of SCP towards Pb(II)removal,HAP was also involved here for comparison.As illustrated in Fig.5(a),the removal rate was rather rapid at the first 5 min,and thereafter reached a plateau after 10 min for SCP and 20 min for HAP.Accordingly,the Pb(II)removal capacity at equilibrium on SCP was as high as 1108 mg/g and the removal efficiency of Pb(II)ions approached 100%,indicating a complete removal of Pb(II)from the treated solution.That is,SCP was capable of effectively reducing 20 ppm of Pb(II)contaminated water to below the acceptable limit for drinking water within 10 min with a dosage of 20 mg/L,indicating its huge potential as a highly effective lead capture.By comparison,the adsorption capacity at equilibrium and removal efficiency of Pb(II)on HAP were only 275.5 mg/g and 22.78%,respectively.The removal kinetics of Pb(II)ions were well fitted with pseudo-second-order kinetic model with theexcellent correlation coefficients(R2=0.999)(shown in Fig.5(b)),indicating that the removal of Pb(II)ions was based on the chemisorption process.Similar results was reported for the Pb(II)adsorption behavior by hydroxyapatite[31].As seen in Table S1(supplementary material),the rate constants k2values of Pb(II)adsorption on SCP(0.0206 g/(mg·min))were significantly greater than that on HAP(0.0033 g/(mg·min)).These results indicated that the as-prepared SCP had much higher removal activity for Pb(II)ions than HAP,which can be attributed to the unique“soft”Lewis basic character of SCP.
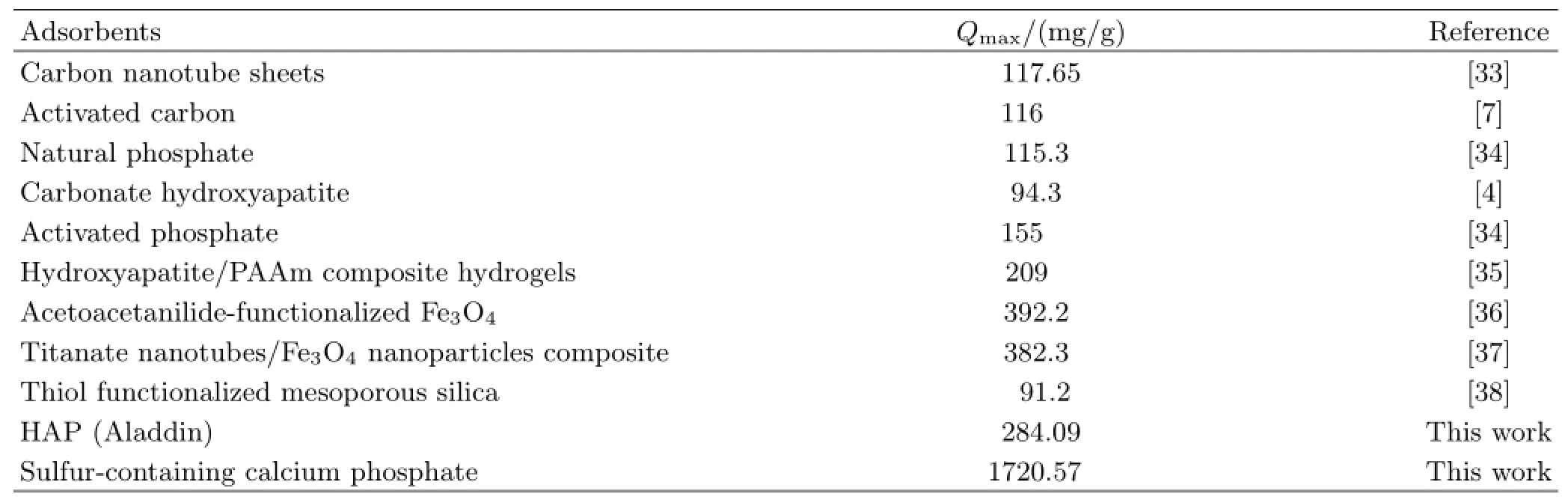
TABLE I Summary of the maximum Pb(II)removal capacities by various adsorbents.
C.Removal isotherm
In order to examine the maximum removal capacity, the removal isotherm studies were performed at equilibrium conditions with the different initial concentration of heavy metal ions.As illustrated in Fig.6(a),the effect of the initial Pb(II)ion concentration on the removal performance of the absorbents was investigated, in which the solution pH value was controlled to be around 5.5.The removal amount of Pb(II)ions by the absorbents increased with increasing the initial concentration of Pb(II)ions in aqueous solution.At all initial concentrations,SCP had a much higher removal amount for Pb(II)ions in comparison with HAP(Aladdin),indicating that the former had a better affinity ability for heavy metal Pb(II)ions than the latter,which was consistent with the removal kinetics results.And the removal isotherms can be well fitted via Langmuir model with 0.999 of the correlation coefficient(R2)values(Fig.6(b)),which may be attributed to the reason that the active sites were homogeneously distributed on the surface of the sorbents[32].And the saturated removal capacities of Pb(II)ions(Table S2 in supplementary materials)calculated from the Langmuir model were 1720.57 for SCP,which was far superior to the commercial HAP(284.09 mg/g)and the other sorbent materials are listed in Table I,revealing that SCP in this study was an effective adsorbent for Pb(II)from wastewater.

FIG.6(a)Removal isotherms of Pb(II)on SCP and HAP (Aladdin),(b)Langmuir model fitting of Pb(II)on SCP and HAP(Aladdin).Experimental conditions:initial Pb(II) concentration 40-100 mg/L for SCP and 20-60 mg/L for HAP(Aladdin),sorbent dosage of 10 mg/500 mL,pH=5.5, contact time for 12 h,and temperature at 25°C.
D.Effect of competing metal ions
In addition to sufficiently rapid binding rates,high removal capacity,an ideal wastewater treatment agentsshould have features of strong affinity for the target metals in the presence of the interfering metal ions so as to resist the nonspecific adsorption of the coexisting ions.Taken into account the fact that different heavy metal ions always coexisted at high levels in natural waters and industrial effluents,it was significant to elucidate removal selectivity of a given sorbent toward the target heavy metal ions,in which the competitive adsorption of coexisting ions to the binding sites may affect the removal efficiency of target ions and thus result in a dramatic decrease in working capacity.In order to investigate the selective binding characteristics of SCP towards Pb(II)ions,the removal experiments both in a single metal ion solution(Pb(II),Cd(II),Ni(II),Co(II), and Zn(II))and a mixed solution containing the five metal ions were conducted,in which HAP was also involved here for comparison.As demonstrated in Fig.7, SCP exhibited a higher removal efficiency of Pb(II)over other metal ions no matter in single metal solution or in multi-metal system,whereas,the removal rate of metal ions in mono/multi-metal system on HAP decreased dramatically under the identical conditions.Particularly,the removal efficiency of Pb(II)ions was almost not affected in the presence of the interfering cations (Cd(II),Zn(II),Ni(II),and Co(II)),indicating a high selectivity of SCP for Pb(II)over other metal ions.
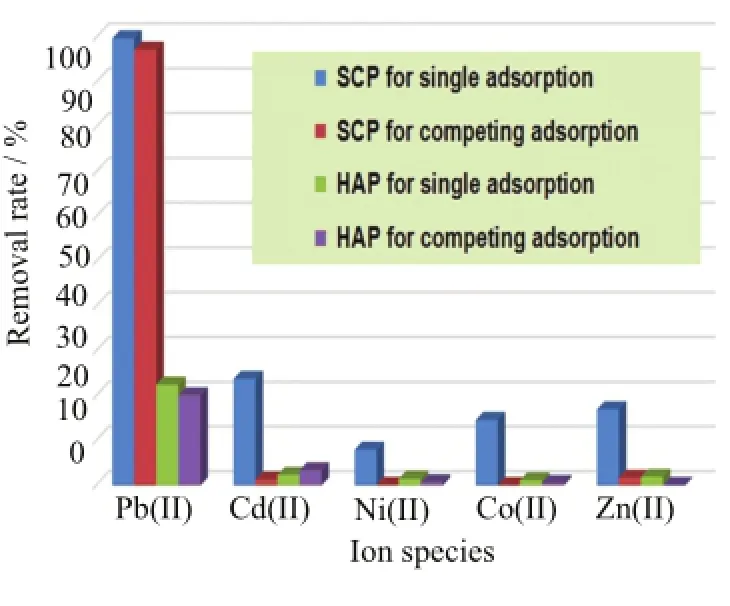
FIG.7 Removal rate of each metal ion in single metal ion solution and selective removal of Pb(II)by SCP and HAP (Aladdin)in a mixed solution containing the five metal ions. Experimental conditions:initial metal ions concentration 20 mg/L,sorbent dosage 10 mg/500 mL,pH=5.5,contact time 12 h,and temperature 25°C.
Typically,the affinity of sorbent for the metal ions was reflected in the distribution coefficient Kdvalues [39].The removal results of each metal ion by SCP and HAP(Aladdin)in an individual or mixed system were summarized in Table S3(supplementary materials).As expected,SCP presented substantially higher Kdvalues than HAP in both the two systems mentioned above, further verifying the preferential removal performance for the former.It was noted that the high selectivity of SCP toward Pb(II)was well maintained in the presence of the interfering metal ions with the same initial concentration,as reflected by the very high distribution coefficient Kdvalue(1.84×106mL/g),which was remarkably higher than that of other metal ions,consistent with a tremendous affinity for Pb(II)ion.These results indicated the potential of SCP as a kind of highly effective sorbent for Pb(II)decontamination.
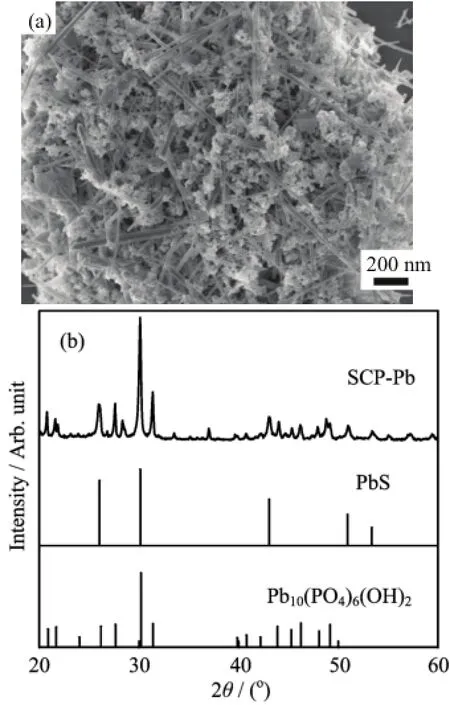
FIG.8(a)SEM and(b)XRD pattern of SCP after Pb(II) loading.
E.Removal mechanisms
To elucidate the removal mechanisms of Pb(II)ions by the obtained SCP,the morphologies and chemical compositions after Pb(II)treatment were characterized by SEM and XRD pattern.As displayed in Fig.8(a), interestingly,there was an obvious change in typical morphology from the pristine spherical nanoparticles to rod-like particles,indicating the formation of new products related to the Pb-loaded SCP.EDS spectrum (Fig.S2 in supplementary materials)confirmed the existence of Pb,S,P,and O elements after the Pb(II) loading on SCP,in which the signal of Ca element disappeared,indicating the ion-exchange between Pb(II) and Ca(II)ions.In addition,the homogeneous distribution of each element over the whole samples can be obtained from EDS mapping(Fig.S3 in supplementary materials).The phase structure of the Pb-loaded SCP was further identified by XRD analysis(Fig.8(b)). Different from the amorphous structure of the pristine SCP,a large number of peaks appeared in the spectrum of the Pb(II)-loaded SCP,in which the main peaks can be attributed to hydroxypyromorphite(HPy, Pb10(PO4)6(OH)2)and the diffraction peaks at 25.96°,30.07°,43.06°,50.98°,and 53.41°matched well with the(111),(200),(220),(311),and(222)planes of the standard XRD data for galena PbS(JCPDS 5-592), suggesting that the new products were mostly composed of HPy and PbS.The aforementioned analyses revealed that phosphates and sulfur groups performed the synergistic functions in Pb(II)removal,in which the lower solubility of HPy(Ksp=1.58×10−77)and PbS(Ksp=3.4×10−28)could promote the ion-exchange and dissolution-precipitation reaction between SCP and Pb(II).
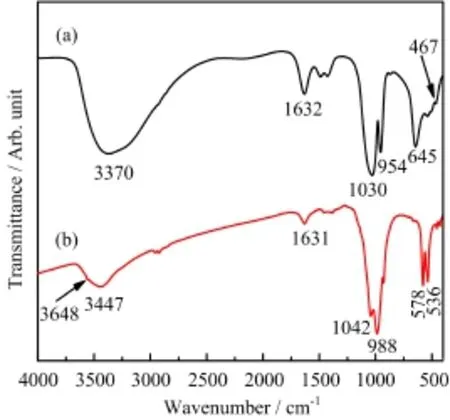
FIG.9 FT-IR spectra of SCP samples before(a)and after (b)Pb(II)treatment.
To gain insight into removal mechanism of heavy metals onto SCP,FT-IR study of SCP samples before and after Pb(II)loading was performed.As illustrated in Fig.9,the weak band at about 3648 cm−1,which was overlapped with the broad band of adsorbed water at about 3447 cm−1,was attributed to the hydroxyl group [40].After Pb(II)loading,the characteristic bands at 1030,954,and 645 cm−1associated with νas(PO3), νs(PO3)and δas(PO3)vibrations of(PO3S)3−shifted to 1042,988,and 578 cm−1,respectively,which were attributed to the stretching motions of the phosphate groups in the crystal lattice of the HPy[41],suggesting the involvement of these groups in Pb(II)adsorption.Additionally,the band at 467 cm−1originated from ν(PS)vibration disappeared due to the interactions of Pb(II)with phosphate and sulfur groups.It should be noted that not any characteristic band associated with Pb-S stretching and bending vibrations was observed,though confirmed by the XRD analysis,because galena,an ionic material,showed weak infrared features[42].
Based on the aforementioned analyses about SEM, XRD,FT-IR and XPS of SCP before and after Pb(II) treatment,the Pb(II)removal on SCP may be reasonably ascribed to the following factors.Firstly,the formation of rod-like HPy crystals on the surface of SCP was responsible for the removal of Pb(II)via dissolution-precipitation and ion exchange reactions, which was similar to the previous reports about the formation of HPy precipitation on HAP/polyurethane composite foams after Pb(II)immobilization[35].Secondly,sulfur group may display specific interaction with Pb(II)ions,accompanied by the formation of lead sulfide precipitates,in agreement with the deposition of PbS on Fe3S4after the uptake of Pb(II)[50].In addition,SCP with amorphous structure possessed a large amount of lattice defects and high degradability,facilitating the dissolution of SCP and the following precipitation together with ion exchange reactions,which was another reason for the ultra-high removal efficiency of Pb(II)by the obtained SCP.Similar phenomenon was found for HAP with low crystallinity,in which the calcium ions in HAP were more easily replaced by other metal ions[51].
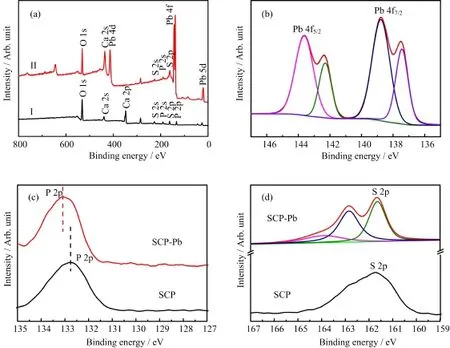
FIG.10 XPS spectra of SCP before(I)and after(II)Pb treatment:(a)survey spectra,(b-d)high-resolution of Pb 4f, P 2p and S 2p,respectively.
IV.CONCLUSION
In summary,amorphous SCP nanoparticles were synthesized via a facile co-precipitation route and their removal performance toward Pb(II)ions was also investigated. It turned out that the resulting SCP material exhibited fast adsorption kinetics and enhanced removal capacity(1720.57 mg/g)toward Pb(II)ions in comparison with the most absorbents reported,indicating its huge potential as a highly effective Pb(II)capture. More significantly,these amorphous SCP nanoparticles possessed preferable selectivity toward the target Pb(II)ions over other coexisting metal ions,as reflected by the very high distribution coefficient Kdvalue(1.84×106mL/g)of SCP towards Pb(II)ions(even several orders higher than HAP),which guaranteed their great application prospect in wastewater treatment.The characteristic XRD peaks,FT-IR and XPS analyses,as well as the morphology transformation from the primitive spherical nanoparticles to rod-like particles after Pb(II) treatment confirmed that the Pb(II)immobilization on SCP occurred mainly via HPy and PbS precipitation, surface adsorption and ion exchange.Therefore,the fabricated SCP nanoparticles in the present study were expected to have potential applicability for the effective detoxication of trace Pb(II)from the contaminated water.
Supplementary materials:XRD and SEM image of HAP from Aladdin,adsorption kinetics parameters of SCP and HAP(Aladdin)toward Pb(II),Langmuir and Freundlich model adsorption isotherm parameters and correlation coefficients of SCP and HAP(Aladdin) toward Pb(II),removal selectivity of SCP and HAP (Aladdin)toward single metal ion solution of Pb(II), Cd(II),Ni(II),Co(II),and Zn(II)and a mixed metal ions solution with the five metal ions(20 ppm per ion), EDS spectrum of SCP after Pb(II)treatment,SEM image and EDS mapping of SCP after Pb(II)treatment are all given.
V.ACKNOWLEDGMENTS
This work was supported by the National Basic Research Program of China(No.2013CB934302), the National Natural Science Foundation of China (No.51272255,No.51572263,and No.51472246),Scientific and Technical Key Research Program of Auhui Province(the Research on the Key Techniques of the Detection and Remediation for Soil Heavy Metal)and CAS/SAFEA International Partnership Program for Creative Research Teams of Chinese Academy of Sciences,China.
[1]B.Volesky,Hydrometallurgy 59,203(2001).
[2]S.Han,L.Hu,N.Gao,A.A.Al-Ghamdi,and X.Fang, Adv.Funct.Mater.24,3725(2014).
[3]H.A.Godwin,Curr.Opin.Chem.Biol.5,223(2001).
[4]D.Liao,W.Zheng,X.Li,Q.Yang,X.Yue,L.Guo, and G.Zeng,J.Hazard.Mater.177,126(2010).
(1)四种中心锥结构水力旋流器内压力场与速度场均呈轴对称分布。适当的减小上锥段底角能增大径向压力梯度和最大切向速度,有利于固液分离从而提高分离效率。
[5]H.Bedelean,A.Maicaneanu,S.Burca,and M.Stanca, Clay Miner.44,487(2009).
[6]M.Ceglowski and G.Schroeder,Chem.Eng.J.259, 885(2015).
[7]L.Largitte,T.Brudey,T.Tant,P.C.Dumesnil,and P.Lodewyckx,Micropor.Mesopor.Mater.219,265 (2016).
[8]A.Mishra,B.D.Tripathi,and A.K.Rai,Int.J.Environ.Sci.Technol.12,3443(2015).
[9]H.Z.Mousavi and A.Asghari,Asian J.Chem.21,2881 (2009).
[10]P.Tzvetkova,P.Vassileva,and R.Nickolov,J.Porous Mater.17,459(2010).
[11]L.Zhang,M.Fang,Nano Today.5,128(2010).
[12]L.L.Wang,L.Shen,H.Y.Jin,L.P.Zhu,and L.J. Wang,Chin.J.Chem.Phys.27,327(2014).
[13]P.Chand and Y.B.Pakade,Environ.Sci.Pollut.R. 22,10919(2015).
[14]L.M.Cui,Y.G.Wang,L.H.Hu,L.Gao,B.Du,and Q.Wei,RSC Adv.5,9759(2015).
[15]M.S.Fernando,R.M.de Silva,and K.N.de Silva, Appl.Surf.Sci.351,95(2015).
[16]X.Y.Zhao,Y.J.Zhu,J.Zhao,B.Q.Lu,F.Chen,C. Qi,and J.Wu,J.Colloid Interface Sci.416,11(2014).
[17]F.Zhuang,R.Tan,W.Shen,X.Zhang,W.Xu,and W.Song,J.Alloys Compd.637,531(2015).
[18]S.Hokkanen,E.Repo,L.J.Westholm,S.Lou,T. Sainio,and M.Sillanp¨a¨a,Chem.Eng.J.252,64(2014).
[19]X.Ye,W.Dazhi,T.Honggao,and W.Huixin,Chin.J. Chem.Phys.14,340(2001).
[20]E.Mavropoulos,A.M.Rossi,A.M.Costa,C.A.Perez, J.C.Moreira,and M.Saldanha,Environ.Sci.Technol. 36,1625(2002).
[21]D.P.Minh,N.D.Tran,A.Nzihou,and P.Sharrock, Chem.Eng.J.232,128(2013).
[22]D.P.Minh,H.Sebei,A.Nzihou,and P.Sharrock, Chem.Eng.J.198,180(2012).
[23]O.Fardmousavi and H.Faghihian,CR.Chim.17,1203 (2014).
[24]G.Li,Z.Zhao,J.Liu,and G.Jiang,J.Hazard.Mater. 192,277(2011).
[25]L.C.Lin,M.Thirumavalavan,and J.F.Lee,Clean-Soil Air Water 43,775(2015).
[26]R.C.Schroden,M.Al-Daous,S.Sokolov,B.J.Melde, J.C.Lytle,A.Stein,M.C.Carbajo,J.T.Fernandez, and E.E.Rodriguez,J.Hazard.Mater.12,3261(2002).
[27]J.Lee,S.Gomez-Salazar,and L.Tavlarides,Reac. Funct.Polym.49,159(2001).
[28]A.Aklil,M.Mouflih,and S.Sebti,J.Hazard.Mater. 112,183(2004).
[29]K.Kazmierczak,J.Heck,and H.H¨oppe,Z.Anorg.All. Chem.636,409(2010).
[30]L.Xiong,C.Chen,Q.Chen,and J.Ni,J.Hazard. Mater.189,741(2011).
[31]S.H.Jang,Y.G.Jeong,B.G.Min,W.S.Lyoo,and S.C.Lee,J.Hazard.Mater.159,294(2008).
[32]K.Foo and B.Hameed,Chem.Eng.J.156,2(2010)
[33]M.A.Tofighy and T.Mohammadi,J.Hazard.Mater. 185,140(2011).
[34]M.Mouflih,A.Aklil,and S.Sebti,J.Hazard.Mater. 119,183(2005).
[35]S.H.Jang,B.G.Min,Y.G.Jeong,W.S.Lyoo,and S.C.Lee,J.Hazard.Mater.152,1285(2008).
[36]R.Sharma,A.Puri,Y.Monga,and A.Adholeya,J. Mater.Chem.A 2,12888(2014).
[37]F.Liu,Y.Jin,H.Liao,L.Cai,M.Tong,and Y.Hou, J.Mater.Chem.A 1,805(2013).
[38]G.Li,Z.Zhao,J.Liu,and G.Jiang,J.Hazard.Mater. 192,277(2011).
[39]S.L.Ma,Q.M.Chen,H.Li,P.L.Wang,S.M.Islam, Q.Y.Gu,X.J.Yang,and M.G.Kanatzidis,J.Mater. Chem.A 2,10280(2014).
[40]N.F.Mohammad,R.Othman,and F.Y.Yeoh,Ceram. Int.41,10624(2015).
[41]S.Ramesh,N.Rameshbabu,R.Gandhimathi,M.S. Kumar,and P.Nidheesh,Appl.Water Sci.3,105 (2013).
[42]I.Chakraborty and S.P.Moulik,J.Nanopart.Res.7, 237(2005).
[43]A.Lobo,T.M¨oller,M.Nagel,H.Borchert,S.Hickey, and H.Weller,J.Phys.Chem.B 109,17422(2005).
[44]H.Zhao,M.Chaker,and D.Ma,Phys.Chem.Chem. Phys.12,14754(2010).
[45]H.Zhao,D.Wang,T.Zhang,M.Chaker,and D.Ma, Chem.Commun.46,5301(2010).
[46]W.E.Morgan and J.R.Van Wazer,J.Phys.Chem. 77,964(1973).
[47]A.M.Puziy,O.I.Poddubnaya,R.P.Socha,J.Gurgul, and M.Wisniewski,Carbon 46,2113(2008).
[48]X.R.Yu,F.Liu,Z.Y.Wang,and Y.Chen,J.Electron. Spectrosc.Relat.Phenom.50,159(1990).
[49]L.Hern´an,J.Morales,L.S´anchez,J.Tirado,J.Espinos,and A.Gonz´alez Elipe,Chem.Mater.7,1576 (1995).
[50]L.Kong,L.Yan,Z.Qu,N.Yan,and L.Li,J.Mater. Chem.A 3,15755(2015).
[51]Y.Lei,J.J.Guan,W.Chen,Q.F.Ke,C.Q.Zhang, and Y.P.Guo,RSC Adv.5,25462(2015).
(Dated:Received on March 11,2016;Accepted on April 22,2016)
 CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
CHINESE JOURNAL OF CHEMICAL PHYSICS2016年5期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Hydrodeoxygenation of Anisole over Ni/α-Al2O3Catalyst
- Structural,Magnetic and Optical Properties of BiFe1−xNbxO3
- Surface Chemistry of Ga(CH3)3on Pd(111)and Effect of Pre-covered H and O
- Photo-depositing Ru and RuO2on Anatase TiO2Nanosheets as Co-catalysts for Photocatalytic O2Evolution from Water Oxidation
- Efficient Removal Phenol Red over Ternary Heterostructured Ag-Bi2MoO6/BiPO4Composite Photocatalyst
- Combination Computing of Support Vector Machine,Support Vector Regression and Molecular Docking for Potential Cytochrome P450 1A2 Inhibitors
