三叶荚蒾中萜类化学成分的研究
胡疆+卯霞+景年华+史俊友+刘慧青
[摘要] 采用硅胶、反向硅胶、Sephadex LH-20及MCI柱色谱等多种色谱技术和方法对三叶荚蒾Viburnum ternatum进行化学成分研究,运用现代波谱学方法并结合文献对分离得到的化合物进行结构鉴定。从该植物枝叶70%丙酮水提取液的醋酸乙酯萃取部分中分离并鉴定了12个化合物,包括4个环烯醚萜类成分ternatumin A(1),2,9-dioxatricyclo[4.3.1.03,7]decanes (2),7,10,2′-triacetylsuspensolide F(3)和7,10,2′,3′-tetra-acetylsuspensolide F(4),5个环烯醚萜苷类成分viburtinoside IV(5),viburtinoside II(6),viburtinoside B(7),luzonoside A (8)和luzonoside B(9)以及3个三萜类成分2α,3β,24-trihydroxy-12-ursen-28-oic acid(10),6-hydroxy-20(29)-lupen-3-one(11)和pomalic acid(12)。其中,化合物1为新化合物,化合物2为新天然产物,化合物3~12为首次从该植物中分离得到。
[关键词] 三叶荚蒾; 忍冬科; 环烯醚萜; 环烯醚萜苷; 三萜
[Abstract] Four iridoids (1-4), five iridoid glucosides (5-9), and three triterpenoids (10-12) were isolated from the ethyl acetate soluble fraction of 70% Me2CO extract of the aerial parts of Viburnum ternatum through various column chromatographies over silica gel, ODS, Sephadex LH-20 and MCI. Their structures were elucidated as ternatumin A (1), 2,9-dioxatricyclo[4.3.1.03,7]decanes (2), 7,10,2′-triacetylsuspensolide F (3), 7,10,2′,3′-tetraacetylsuspensolide F (4), viburtinoside IV (5), viburtinoside II (6), viburtinoside B (7), luzonoside A (8), luzonoside B (9), 2α,3β,24-trihydroxy-12-ursen-28-oic acid (10), 6-hydroxy-20(29)-lupen-3-one (11), and pomalic acid (12) based on the their chromatographic properties, chemical and physicochemical methods, and spectroscopic data. Compound 1 was a new compound and compounds 3-12 were isolated from this plant for the first time. Furthermore, we note here the first isolation of compound 2 as a new natural product.
[Key words] Viburnum ternatum; Caprifoliaceae; iridoids; iridoid glucosides; triterpenoids
荚蒾属Viburnum植物属忍冬科Caprifoliaceae,全球约230余种,分布在温带和亚热带地区。我国约有74种,广布于全国各省区,尤以西南和中南地区(中高海拔)种类最多[1-2]。该属植物多为灌木或小乔木,落叶或常绿。许多种类是著名的园林观赏植物,也有部分种类的根、茎、枝叶或果实入药,具有镇静、利尿及解除子宫痉挛等药理活性[3-4]。该属植物化学成分结构类型多样,包括二萜类、三萜类、环烯醚萜类、倍半萜类、黄酮类、木脂素类、酚苷类及香豆素类等[5-6]。三叶荚蒾V. ternatum生于中国南方海拔650~1 400 m的山谷、山坡丛林或灌丛中[5]。国内外对该植物的化学成分研究报道较少,本课题组曾从三叶荚蒾分离得到2个新木脂素和1个新双黄酮[7],为进一步寻找其天然活性产物,对三叶荚蒾70%丙酮提取液的醋酸乙酯萃取部分的化学成分进行了系统研究,分离并鉴定了1个新的环烯醚萜ternatumin A(1),1个新天然产物的环烯醚萜2,9-dioxatricyclo[4.3.1.03,7]decanes (2),2个已知的环烯醚萜7,10,2′-triacetylsuspensolide F(3)和7,10,2′,3′-tetra-acetylsuspensolide F(4),5个已知环烯醚萜苷viburtinoside Ⅳ(5),viburtinoside Ⅱ(6),viburtinoside B(7),luzonoside A (8)和luzonoside B(9),以及3个三萜类成分2α,3β,24-trihydroxy-12-ursen-28-oic acid(10),6-hydroxy-20(29)-lupen-3-one(11)和pomalic acid(12)。化合物3~12为首次从该植物中分离得到。
1 材料
Jasco P-1020 全自动数字旋光仪(日本分光公司);Shimadzu UV-2401A 紫外分光光度仪(日本,岛津);Bruker Tensor-27 傅立叶变换中红外光谱仪(德国,布鲁克);API QSTAR Pulsar 液相四级杆飞行时间质谱仪(美国,应用系统公司);Bruker Avance 400型核磁共振仪,TMS内标(德国Bruker 公司);柱色谱硅胶(200~300目,青岛海洋化工有限公司);薄层色谱硅胶GF254(烟台江友硅胶开发有限公司);RP-18反相硅胶(50 μm,日本,YMC 公司);Sephadex LH-20(瑞典,Amersham);MCI柱填充材料为MCI-gel CHP-20P(75~150 μm,日本,三菱化学);柱色譜用试剂均为分析纯(国药集团化学试剂有限公司)。
药材于2015年7月采自于云南富源地区,经曲靖师范学院卯霞副教授鉴定为三叶荚蒾V.ternatum。样本保存于曲靖师范学院少数民族用资源研究所。
2 提取和分离
干燥的三叶荚蒾枝叶12.5 kg,经粉碎后在室温下用70%丙酮50 L提取3次,每次3 d,提取液减压回收溶剂得浸膏276 g。浸膏用水混悬,水混悬液用醋酸乙酯15 L萃取3次,得醋酸乙酯部分122 g。醋酸乙酯部分经硅胶柱色谱(200~300目)用氯仿-甲醇(100∶0~70∶30)进行梯度洗脱,经TLC检查后合并得到8个部分Fr. 1~Fr. 8。Fr. 2(15 g)经硅胶柱色谱用石油醚(30~60 ℃)-醋酸乙酯(8∶2)洗脱得到3个部分Fr. 2-1~Fr. 2-3,Fr. 2-1(1.1 g)经MCI柱用甲醇-水(80∶20)脱色后,用Sephadex LH-20氯仿-甲醇(1∶1)多次纯化得到化合物2(98 mg),Fr. 2-2(2.1 g)经硅胶柱色谱用氯仿-甲醇(90∶10)纯化得到化合物1(78 mg)和11(52 mg)。Fr. 3(8 g)经硅胶柱色谱用石油醚-丙酮(8∶2)反复纯化得到化合物6(57 mg)和12(63 mg)。Fr. 4(11 g)经硅胶柱色谱用氯仿-醋酸乙酯(3∶1)洗脱得到4个部分Fr. 4-1~Fr. 4-4。Fr. 4-1(1.3 g)经硅胶柱色谱用石油醚-醋酸乙酯(2∶1)洗脱后,用Sephadex LH-20柱(氯仿-甲醇 1∶1)纯化得到化合物3(73 mg)。Fr. 3-3(1.6 g)经反相柱RP-18柱用甲醇-水(80∶20)纯化得5(35 mg)和9(57 mg)。Fr. 5(13 g)经硅胶柱色谱用石油醚-丙酮(3∶7)多次纯化,得到化合物 4(38 mg)。Fr. 7(9 g)经硅胶柱色谱用氯仿-甲醇(1∶9)洗脱得到3个部分Fr. 7-1~Fr. 7-3。Fr. 7-1(987 mg)经Sephadex LH-20柱用甲醇多次纯化得到化合物7(47 mg),Fr. 7-2(1.1 g)经硅胶柱色谱用氯仿-甲醇(85∶15)反复纯化得到化合物8(55 mg)。Fr. 7-3(1.7 g)经RP-18反相柱用甲醇-水(8∶2)纯化得到化合物10(102 mg)。
3 结构鉴定
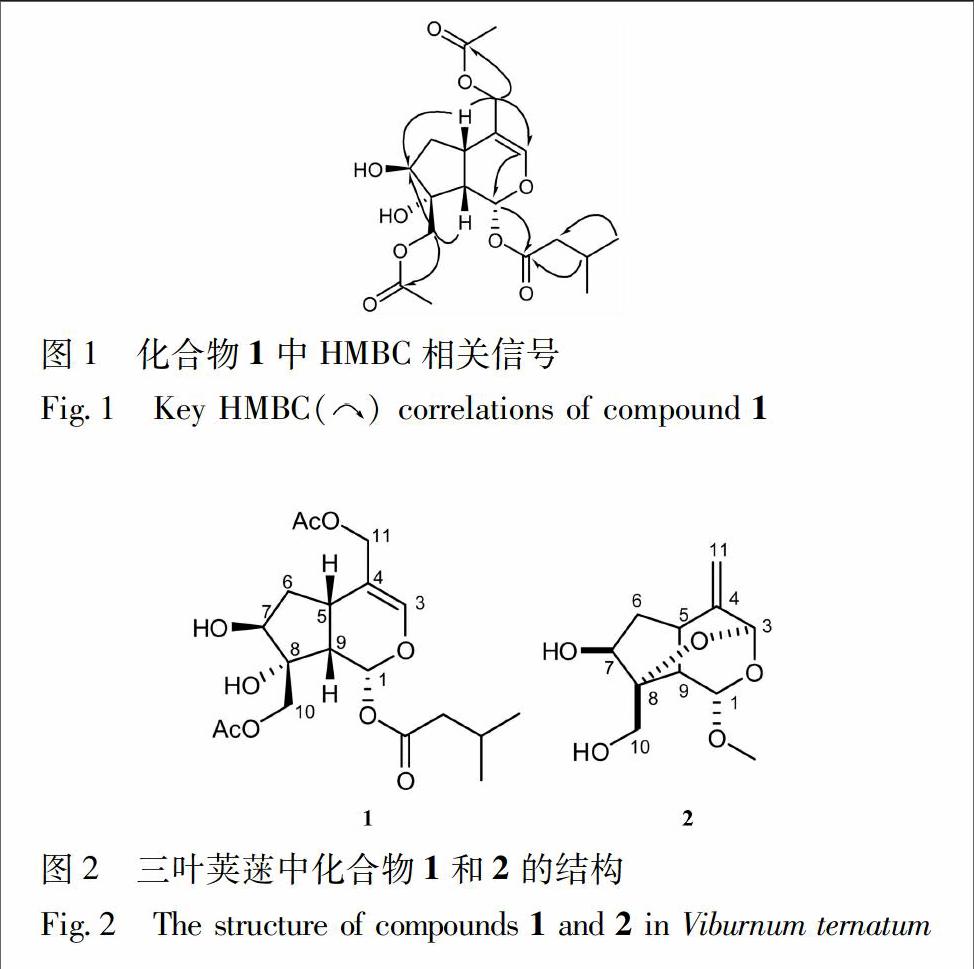
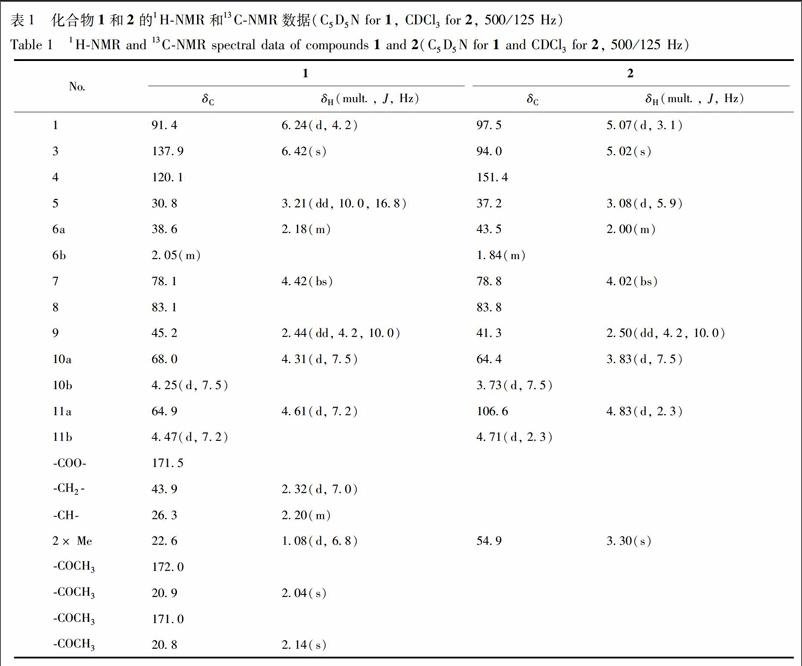
化合物1 为无色油状物质,ESI-MS谱指出其准分子离子峰m/z 435 [M + Cl]-,结合13C-NMR和DEPT谱数据,推测其分子式为C19H28O9,不饱和度为6,并且经HR-ESI-MS谱(m/z 435.142 5 [M+Cl]-(C19H28O9Cl,计算值 435.142 2) 验证。化合物1的13C-NMR和DEPT谱中除了2个乙酰基[δC172.0,20.9; 171.0,20.8]和1个异戊酸片段-OCOCH2CH(CH3)2[δC171.5,43.9,26.3,22.6]的信号外,还显示该化合物的母核有3个亚甲基,其中2个为被氧取代亚甲基[δC38.6(C-6),68.0(C-10)和64.9(C-11)];5个次甲基,包括1个烯碳[δC 137.9(C-3)]和2个被氧取代次甲基[δC 91.4(C-1)和δC 78.1(C-7)],2个季碳 [δC 83.1(C-8)和δC 120.1(C-4)]。H-1(δH6.24,d,J=4.2 Hz)与异戊酸片段中C=O(δC 171.5)的HMBC相关信号说明异戊酸与1位羟基成酯。以上光谱数据表明,化合物1为10碳的环烯醚萜。与已知化合物suspensolide A的苷元具有相似的結构[8],不同之处在于2个乙酰基的取代位置。在化合物1的HMBC谱中可以看到,H-10(δH4.25,4.31,each,d,J=7.5 Hz)和H-11(δH4.47,4.61,each,d,J=7.2 Hz)分别与2个乙酰基羰基碳(δC171.0 和 172.0)有明显的相关信号,由此可以确定2个乙酰基的连接位置分别为C-10和C-11见图1。其相对构型通过ROESY谱并与已知化合物suspensolide A的苷元进行比较得到确定。在ROESY谱中,H-5和H-10与H-9的相关信号表明H-10为β构型。H-6a/H-5和H-6b/H-7的ROESY的相关信号表明C-7上的羟基为β构型。另外,H-1/H-9的ROESY相关信号以及H-1/H-9的耦合常数(J=4.2 Hz)说明H-1为β构型。根据以上数据可以推断化合物1结构,见图2,并命名为ternatumin A。
化合物1 无色油状物C19H28O9,[α] -30.23(c 0.10,MeOH)。UV(MeOH) λmax (log ε): 191(2.68),195(2.72),206(3.05) nm。IR(KBr) νmax: 3 435,2 960,1 738,1 255,1 100 cm–1。1H-NMR和13C-NMR数据见表1。
化合物2 无色油状物。EI-MS m/z 228 [M]+。HR-ESI-MS m/z 251.089 2 [M+Na]+(C11H16O5Na,计算值251.089 5)。[α] +15.30(c 0.02,MeOH)。UV(MeOH)λmax (log ε): 292(4.15) nm。IR(KBr) νmax: 3 075,1 740,1 660,948 cm-1。1H-NMR和13C-NMR数据见表1。以上数据与文献[9]对照,鉴定该化合物为2,9-dioxatricyclo[4.3.1.03,7]decanes。
化合物3 白色无定形粉末。FAB-MS m/z 627 [M+Na]+。1H-NMR(CD3OD,500 Hz) δ: 6.14(1H,d,J=5.3 Hz,H-1),6.38(1H,bs,H-3),2.90(1H,m,H-5),2.10~2.12(2H,overlapped,H-6a,-CH2CHMe2),1.98(1H,m,H-6b),5.02(1H,t,J=2.9 Hz,H-7),2.39(1H,dd,J=5.3,9.9 Hz,H-9),4.27(1H,d,J=11.5 Hz,H-10a),4.20(1H,d,J=11.5 Hz,H-10b),4.25(1H,d,J=11.7 Hz,H-11a),4.11(1H,d,J=11.7 Hz,H-11b),4.50(1H,d,J=8.0 Hz,H-1′),4.71(1H,dd,J=8.0,9.4 Hz,H-2′),3.51(1H,t,J=9.4 Hz,H-3′),3.34(1H,m,H-4′),3.27(1H,m,H-5′),3.88(1H,dd,J=1.7,12.0 Hz,H-6′a),3.68(1H,dd,J=5.6,12.0 Hz,H-6′b),2.22(2H,d,J=6.9 Hz,-CH2CHMe2),0.95(6H,d,J=6.6 Hz,-CH2CHMe2),2.09,2.05 和2.03(each 3H,s,3×-COCH3)。13C-NMR(CDC13,125 MHz) δ: 91.3(C-1),140.9(C-3),115.4(C-4),33.9(C-5),36.5(C-6),80.8(C-7),82.2(C-8),45.8(C-9),67.9(C-10),69.3(C-11),100.4(C-1′),75.3(C-2′),76.1(C-3′),71.7 (C-4′),78.0(C-5′),62.6(C-6′),172.9(-COO-),42.2 (-CH2CHMe2),26.8(-CH2CHMe2),22.6 和22.7 (-CH2CHMe2),172.6,171.7和171.6(3×-COCH3),21.1,21.0和20.7(3 × -COCH3)。以上數据与文献[10]对照,鉴定该化合物为7,10,2′-triacetylsuspensolide F。
化合物4 白色无定形粉末。FAB-MS m/z 669 [M+Na]+。C29H42O16。1H-NMR(CD3OD,500 Hz) δ: 6.13(1H,d,J=5.2 Hz,H-1),6.40(1H,bs,H-3),2.92(1H,m,H-5),2.12(1H,m,H-6a),1.97(1H,m,H-6b),5.02(1H,m,H-7),2.38(1H,dd,J=5.2,6.0 Hz,H-9),4.22(2H,br s,H-10),4.14(1H,d,J=11.6 Hz,H-11a),4.28(1H,d,J=11.6 Hz,H-11b),4.64(1H,d,J=7.8 Hz,H-1′),4.79(1H,dd,J=7.8,9.0 Hz,H-2′),5.07(1H,t,J=9.0 Hz,H-3′),3.57(1H,t,J=9.0 Hz,H-4′),3.43(1H,ddd,J=2.2,5.6,9.0 Hz,H-5′),3.71(1H,dd,J=5.6,12.0 Hz,H-6′a),3.88(1H,dd,J=2.2,12.0 Hz,H-6′b),2.25(2H,d,J=7.8 Hz,-CH2CHMe2),2.15(1H,m,-CH2CHMe2),0.95(6H,d,J=6.6 Hz,-CH2CHMe2),2.06,2.03,2.02和2.00(4×-COCH3)。13C-NMR(CDC13,125 MHz) δ: 91.1(C-1),140.8(C-3),115.0(C-4),33.8 (C-5),36.3(C-6),80.5(C-7),82.0(C-8),45.5 (C-9),67.6(C-10),69.3(C-11),99.6(C-1′),73.1(C-2′),76.8(C-3′),69.2(C-4′),77.3(C-5′),62.1(C-6′),172.6(-COO-),44.0(-CH2CHMe2),27.5(-CH2CHMe2),22.5(-CH2CHMe2),171.0,171.4,171.9和172.3(4×-COCH3),20.6,20.7,20.7和20.9(4×-COCH3)。以上数据与文献[11]对照,鉴定该化合物为7,10,2′,3′-tetra-acetylsuspensolide F。
化合物5 白色无定形粉末。FAB-MS m/z 585 [M+Na]+。1H-NMR(CD3OD,500 Hz) δ: 6.17(1H,d,J=4.5 Hz,H-1),6.32(1H,bs,H-3),2.93(1H,bq,H-5),1.93(2H,m,H-6),3.94(1H,bt,J=3.4 Hz,H-7),2.32(1H,dd,J=4.5,10.0 Hz,H-9),4.22(2H,br s,H-10),4.07(1H,d,J=11.2 Hz,H-11a),4.23(1H,d,J=11.2 Hz,H-11b),4.49(1H,d,J=8.0 Hz,H-1′),4.70(1H,dd,J=8.0,9.3 Hz,H-2′),3.53(1H,t,J=9.3 Hz,H-3′),3.36(1H,t,J=9.3 Hz,H-4′),3.29(1H,m,H-5′),3.69(1H,dd,J=5.4,12.0 Hz,H-6′a),3.88(1H,dd,J=2.4,12.0 Hz,H-6′b),2.20(2H,d,J=7.2Hz,-CH2CHMe2),2.05(3H,s,Ac),2.08(3H,s,Ac),2.06(1H,m,-CH2CHMe2),0.94(6H,d,J=6.6 Hz,-CH2CHMe2)。13C-NMR(CDC13,125 MHz) δ: 91.6(C-1),140.1(C-3),116.0(C-4),33.0(C-5),38.4(C-6),79.2(C-7),82.7(C-8),45.4(C-9),68.8(C-10),69.6(C-11),100.7(C-1′),75.2(C-2′),76.0(C-3′),71.5(C-4′),77.8(C-5′),62.5(C-6′),173.0,(-COO-),44.0(-CH2CHMe2),26.5(-CH2CHMe2),22.4(-CH2CHMe2),171.6和172.9(2×-COCH3),20.7和21.0(2×-COCH3)。以上数据与文献[12]对照一致,鉴定该化合物为viburtinoside IV。
化合物6 白色无定形粉末。FAB-MS m/z 689 [M+Na]+。1H-NMR(CD3OD,500 Hz) δ:6.18(1H,d,J=4.0 Hz,H-1),6.30(1H,br s,H-3),2.90(1H,bq,H-5),1.89(2H,m,H-6),3.92(1H,br s,H-7),2.26(1H,dd,J=4.5,9.6 Hz,H-9),4.18(2H,bs,H-10),4.05~4.25(2H,m,H-11),4.55(1H,d,J=8.2 Hz,H-1′),4.81(1H,dd,J=8.0,9.0 Hz,H-2′),3.58(1H,t,J=9.0 Hz,H-3′),3.39(1H,t,J=9.0 Hz,H-4′),3.27(1H,m,H-5′),3.70(1H,dd,J=5.4,12.0 Hz,H-6′a),3.88(1H,dd,J=1.8,12.0 Hz,H-6′b),7.47(2H,d,J=8.0 Hz,H-2″,H-6″),6.80(2H,d,J=8.0 Hz,H-3″,H-5″),6.40(1H,d,J=16.3 Hz,H-7″),7.64(1H,d,J=16.3 Hz,H-8″),2.15(2H,d,J=8.0 Hz,-CH2CHMe2),2.04(1H,m,-CH2CHMe2),0.93(6H,d,J=6.6 Hz,-CH2CHMe2),2.01(3H,s,-COCH3)。13C-NMR(CDC13,125 MHz) δ:91.5(C-1),140.1(C-3),115.5(C-4),32.4(C-5),38.4(C-6),78.9(C-7),82.5(C-8),45.5(C-9),68.9(C-10),69.7(C-11),101.2(C-1′),75.2(C-2′),76.0(C-3′),71.7(C-4′),77.9(C-5′),62.6(C-6′),127.2 (C-1″),131.2(C-2″,C-6″),116.8(C-3″,C-5″),161.1(C-4″),115.8(C-7″),146.8(C-8″),167.6 (C-9″),173.0,(-COO-),44.0(-CH2CHMe2),26.5 (-CH2CHMe2),22.4(-CH2CHMe2),172.8 (-COCH3),20.9(-COCH3)。以上數据与文献[3]对照,鉴定该化合物为viburtinoside Ⅱ。
化合物7 白色无定形粉末。ESI-MS m/z 707 [M-H]-。1H-NMR(CD3OD,500 Hz) δ:6.15(1H,d,J=5.2 Hz,H-1),6.34(1H,br s,H-3),2.91(1H,bq,H-5),2.03(2H,m,H-6),5.01(1H,br s,H-7),2.36(1H,dd,J=5.4,9.6 Hz,H-9),4.15(2H,br s,H-10),4.07(2H,m,H-11),4.55(1H,d,J=8.2 Hz,H-1′),4.85(1H,dd,J=8.1,9.0 Hz,H-2′),3.50(1H,t,J=9.1 Hz,H-3′),3.40(1H,t,J=9.1 Hz,H-4′),3.27(1H,m,H-5′),3.70(1H,dd,J=5.4,12.0 Hz,H-6′a),3.84(1H,dd,J=1.8,12.0 Hz,H-6′b),7.76(2H,d,J=8.0 Hz,H-2″,H-6″),6.82(2H,d,J=8.0 Hz,H-3″,5″),6.88(1H,d,J=12.4 Hz,H-7″),5.78(1H,d,J=12.4 Hz,H-8″),2.19(2H,d,J=8.0 Hz,-CH2CHMe2),2.12(1H,m,-CH2CHMe2),0.93(6H,d,J=6.2 Hz,-CH2CHMe2),1.98(6H,s,2×-COCH3)。13C-NMR(CDC13,125 MHz) δ: 90.3(C-1),139.7(C-3),115.3(C-4),32.9(C-5),35.8(C-6),80.2 (C-7),81.6(C-8),45.1(C-9),67.2(C-10),68.7(C-11),100.5(C-1′),74.7(C-2′),75.9(C-3′),71.8(C-4′),77.1(C-5′),62.8(C-6′),126.8 (C-1″),133.6(C-2″,C-6″),115.2(C-3″,C-5″),159.6(C-4″),144.2(C-7″),114.7(C-8″),166.4 (C-9″),171.3(-COO-),43.5(-CH2CHMe2),26.0 (-CH2CHMe2),22.4(-CH2CHMe2),170.2和170.0(2× -COCH3),20.5和20.2(2×-COCH3)。以上数据与文献[6]对照,鉴定该化合物为viburtinoside B。
化合物8 白色无定形粉末。FAB-MS m/z 647 [M + Na]+。1H-NMR(CD3OD,500 Hz) δ:6.21(1H,d,J=4.5 Hz,H-1),6.41(1H,bs,H-3),3.10(1H,bq,H-5),2.23(1H,m,H-6a),2.17(1H,m,H-6b),5.09(1H,bs,H-7),2.45(1H,dd,J=4.5,9.8 Hz,H-9),3.67(1H,d,J=11.5 Hz,H-10a),3.72(1H,d,J=11.5 Hz,H-10b),4.12(1H,d,J=11.5 Hz,H-11a),4.28(1H,d,J=11.5 Hz,H-11b),4.30(1H,d,J=8.2 Hz,H-1′),3.19(1H,dd,J=8.1,9.0 Hz,H-2′),3.34(1H,t,J=9.2 Hz,H-3′),3.26(1H,t,J=9.0 Hz,H-4′),3.27(1H,m,H-5′),3.75(1H,dd,J=5.4,12.0 Hz,H-6′a),3.85(1H,dd,J=1.8,12.0 Hz,H-6′b),7.48(2H,d,J=8.0 Hz,H-2″,H-6″),6.81(2H,d,J=8.0 Hz,H-3″,H-5″),7.61(1H,d,J=8.9 Hz,H-7″),6.35(1H,d,J=8.9 Hz,H-8″),2.21(2H,d,J=8.0 Hz,-CH2CHMe2),2.10(1H,m,-CH2CHMe2),0.97(6H,d,J=6.5 Hz,-CH2CHMe2)。13C-NMR(CDC13,125 MHz) δ: 91.5(C-1),140.7(C-3),115.9(C-4),33.0(C-5),35.9(C-6),81.6(C-7),83.4(C-8),45.3(C-9),66.0(C-10),69.8(C-11),103.4 (C-1′),75.2(C-2′),78.1(C-3′),71.7(C-4′),78.0(C-5′),62.8(C-6′),127.8(C-1″),131.3 (C-2″,C-6″),116.8(C-3″,C-5″),161.4(C-4″),146.9 (C-7″),115.2(C-8″),167.7(C-9″),173.1 (-COO-),44.2(-CH2CHMe2),26.8(-CH2CHMe2),22.7(-CH2CHMe2)。以上數据与文献[12]对照,鉴定该化合物为luzonoside A。
化合物9 白色无定形粉末。FAB-MS m/z 647 [M+Na]+。1H-NMR(CD3OD,500 Hz) δ:6.17(1H,d,J=4.5 Hz,H-1),6.35(1H,br s,H-3),2.92(1H,bq,H-5),2.11(1H,m,H-6a),2.17(1H,m,H-6b),5.04(1H,br s,H-7),2.28(1H,dd,J=4.4,9.7 Hz,H-9),3.56(1H,d,J=11.3 Hz,H-10a),3.60(1H,d,J=11.3 Hz,H-10b),4.11(1H,d,J=11.7 Hz,H-11a),4.28(1H,d,J=11.7 Hz,H-11b),4.31(1H,d,J=8.2 Hz,H-1′),3.20(1H,dd,J=8.1,9.0 Hz,H-2′),3.34(1H,t,J=9.2 Hz,H-3′),3.26(1H,t,J=9.0 Hz,H-4′),3.27(1H,m,H-5′),3.65(1H,dd,J=5.4,12.0 Hz,H-6′a),3.87(1H,dd,J=1.8,12.0 Hz,H-6′b),7.54(2H,d,J=8.3 Hz,H-2″,6″),6.77(2H,d,J=8.3 Hz,H-3″,H-5″),6.91(1H,d,J=12.7 Hz,H-7″),5.79(1H,d,J=12.7 Hz,H-8″),2.22(2H,d,J=8.0 Hz,-CH2CHMe2),2.08(1H,m,-CH2CHMe2),0.96(6H,d,J=5.8 Hz,-CH2CHMe2)。13C-NMR(CDC13,125 MHz) δ: 91.4(C-1),140.5(C-3),115.9(C-4),33.0(C-5),36.0(C-6),81.3(C-7),83.4(C-8),45.3(C-9),65.8(C-10),69.8(C-11),103.4 (C-1′),75.1(C-2′),78.1(C-3′),71.8(C-4′),78.0(C-5′),62.8(C-6′),128.0(C-1″),133.4(C-2″,C-6″),115.9(C-3″,C-5″),161.4(C-4″),145.4 (C-7″),116.9(C-8″),168.7(C-9″),173.1 (-COO-),44.2(-CH2CHMe2),26.8(-CH2CHMe2),22.7 (-CH2CHMe2)。以上数据与文献[12]对照,鉴定该化合物为luzonoside B。
化合物10 白色无定形粉末。ESI-MS m/z 489 [M + H]+。1H-NMR(CDCl3,500 MHz): δ: 4.27(1H,dd,J=2.8,11.8 Hz,H-2),3.68(1H,d,J=11.8 Hz,H-3),5.18(1H,m,H-12),1.05(3H,s,H-23),3.79(1H,d,J=9.8 Hz,H-24a),3.87(1H,J=9.8 Hz,H-24b),0.91(3H,s,H-25),0.73(3H,s,H-26),1.08(3H,s,H-27),0.95(3H,d,J=6.0 Hz,H-29),0.93(3H,d,J=5.8 Hz,H-30)。13C-NMR(CDCl3,125 MHz) δ: 47.9(C-1),67.6(C-2),83.9(C-3),44.9(C-4),56.6(C-5),18.3(C-6),35.3(C-7),43.2(C-8),48.9(C-9),38.9 (C-10),24.7(C-11),128.3(C-12),138.9 (C-13),42.6(C-14),26.3(C-15),25.5(C-16),49.2 (C-17),53.2(C-18),43.6(C-19),41.6 (C-20),30.8(C-21),33.3(C-22),29.9(C-23),65.7(C-24),16.9(C-25),17.7(C-26),24.6 (C-27),180.7 (C-28),19.9(C-29),21.9(C-30)。以上数据与文献[13]对照,鉴定该化合物为2α,3β,24-trihydroxy-12-ursen-28-oic acid。
化合物11 白色无定形粉末。ESI-MS m/z 471 [M + H]+。1H-NMR(CDCl3,500 MHz): δ: 3.96(1H,m,H-6),1.10(3H,s,H-23),1.03(3H,s,H-24),1.06(3H,s,H-25),0.76(3H,s,H-26),1.34(3H,s,H-27),4.77(1H,bs,H-29a),4.67(1H,br s,H-29b),1.69(3H,s,H-30)。13C-NMR(CDCl3,125 MHz) δ: 38.6(C-1),33.0(C-2),219.8(C-3),43.4(C-4),57.5(C-5),67.9 (C-6),44.5(C-7),39.6(C-8),48.7(C-9),32.9(C-10),21.9(C-11),25.4(C-12),38.3(C-13),40.5(C-14),29.8(C-15),32.5(C-16),55.1 (C-17),48.2(C-18),47.5(C-19),154.7(C-20),30.4 (C-21),37.3(C-22),22.3(C-23),19.8 (C-24),16.2(C-25),17.5(C-26),14.6(C-27),180.9(C-28),110.5(C-29),19.8(C-30)。以上数据与文献[14]对照,鉴定该化合物为6-hydroxy-20(29)-lupen-3-one。
化合物12 白色无定形粉末。ESI-MS m/z 473 [M+H]+。1H-NMR(CDCl3,500 MHz) δ: 3.51(1H,dd,J=5.2,10.8 Hz,H-3),5.69(1H,t,J=4.0 Hz,H-12),1.31(3H,s,H-23),1.10(3H,s,H-24),0.98(3H,s,H-25),1.21(3H,s,H-26),1.81(3H,s,H-27),1.53(3H,s,H-29),1.19(3H,d,J=4.4 Hz,H-30)。13C-NMR(CDCl3,125 MHz) δ:39.3(C-1),28.4(C-2),78.5(C-3),39.7(C-4),56.2(C-5),19.2(C-6),33.9(C-7),40.7(C-8),48.1(C-9),37.7(C-10),24.3(C-11),128.3(C-12),140.3(C-13),42.7(C-14),29.6(C-15),26.7 (C-16),48.6(C-17),54.9(C-18),73.0(C-19),42.4(C-20),27.2(C-21),38.8(C-22),29.1 (C-23),15.9(C-24),16.8(C-25),17.5(C-26),25.0(C-27),180.9(C-28),27.4(C-29),17.1 (C-30)。以上數据与文献[15]对照,鉴定该化合物为pomalic acid。
[参考文献]
[1] 中国科学院中国植物志编辑委员会. 中国植物志. 第72卷 [M]. 北京:科学出版社,2004.
[2] 浙江植物志编辑委员会. 浙江植物志. 第6卷 [M]. 杭州:浙江科学技术出版社,1993.
[3] Mohamed M A, Marzouk M S A, Moharram F A, et al. Phytochemical constituents and hepatoprotective activity of Viburnum tinus [J]. Phytochemistry, 2005, 66(23): 2780.
[4] Lobstein A, Weniger B, Malécot V, et al. Polyphenolic content of two Colombian Viburnum species(Caprifoliaceae) [J]. Biochem Syst Ecol, 2003, 31(1): 95.
[5] Wang X Y, Shi H M, Li X B. Chemical constituents of plants from the genus Viburnum [J]. Chem Biodivers, 2010, 7(3): 567.
[6] Fukuyama Y, Fujii H, Minami H, et al. Neovibsanin F and its congeners, rearranged vibsane-type diterpenes from Viburnum suspensum [J].J Nat Prod, 2006, 69(7): 1098.
[7] Hu J, Shi X D, Mao X, et al. Chemical constituents from the ethanol extract of Viburnum ternatum [J].
J Asian Nat Prod Res, 2014, 16(7): 703.
[8] Hase T, Iwagawa T. Bitter principles of Viburnum suspensum [J]. Chem Lett, 1982, 11(1): 13.
[9] Peter Willibrord T, Samuel D. Esters of 4-hydroxy-2,9-dioxatricyclo[4.3.1.03,7]decanes and processes for their production: US, 4016176[P].1979.
[10] Tomassini L, Gao J J, Serafini M, et al. Iridoid g1ucosides from Viburnum sargenti [J]. Nat Prod Res, 2005, 19(7): 667.
[11] Tomawni L, Foddai S, Nicoletti M, et al. Iridoid glucosides from viburnum ayavacense [J]. Phyrochemistry, 1997, 46(5): 901.
[12] Fukuyama Y, Minoshima Y, Kishimoto Y, et al. Iridoid glucosides and p-coumaroyl iridoids from Viburnum luzonicum and their cytotoxicity [J].J Nat Prod,2004, 67(11): 1833.
[13] Wang B H, Polya G M. Selective inhibition of cyclic AMP-dependent protein kinase by amphiphilic triterpenoids and related compounds [J]. Phytochemistry, 1996, 41(1): 55.
[14] Dantanarayana A P, Kumar N S, Muthukuda P M, A lupane derivative and the 13C-NMR chemical shifts of some lupanols from Pleurostylia opposite [J]. Phytochemistry, 1982, 21(8): 2065.
[15] 雷軍,肖云川,刘淼,等. 糯米藤化学成分研究 [J]. 中成药,2013,35(7): 1489.
[责任编辑 丁广治]

