Electro-optical properties of high birefringence liquid crystal compounds with isothiocyanate and naphthyl group∗
Zeng-Hui Peng(彭增辉),Qi-Dong Wang(王启东),Shao-Xin Wang(王少鑫),Li-Shuang Yao(姚丽双), Yong-Gang Liu(刘永刚),Li-Fa Hu(胡立发),Zhao-Liang Cao(曹召良),Quan-Quan Mu(穆全全), Cheng-Liang Yang(杨程亮),and Li Xuan(宣丽)
State Key Laboratory of Applied Optics,Changchun Institute of Optics,Fine Mechanics and Physics, Chinese Academy of Sciences,Changchun 130033,China
Electro-optical properties of high birefringence liquid crystal compounds with isothiocyanate and naphthyl group∗
Zeng-Hui Peng(彭增辉),Qi-Dong Wang(王启东),Shao-Xin Wang(王少鑫),Li-Shuang Yao(姚丽双)†, Yong-Gang Liu(刘永刚),Li-Fa Hu(胡立发),Zhao-Liang Cao(曹召良),Quan-Quan Mu(穆全全), Cheng-Liang Yang(杨程亮),and Li Xuan(宣丽)‡
State Key Laboratory of Applied Optics,Changchun Institute of Optics,Fine Mechanics and Physics, Chinese Academy of Sciences,Changchun 130033,China
Liquid crystal(LC)compound with isothiocyanate and naphthyl group is an attractive high birefringence LC material, and can be used in optical devices.In this paper,the electro-optical properties of a series of this type of LC compounds were investigated.The melting points and enthalpy values of these LC compounds were higher than those of corresponding compounds with the phenyl group.These compounds exhibited high birefringence with a maximum value of 0.66.Fluorine substitution in the molecular almost does not affect the birefringence value.When these LC compounds with the naphthyl group were dissolved in a commercial LC mixture,the electro-optical properties depending on temperature were investigated.In the low-temperature region,LC mixtures with the naphthyl-group LC compounds exhibited higher viscosity than pure commercial LCs.In the high-temperature region,viscosity values very closely approached each other.When response performance was investigated,figure-of-merit(FoM)values were measured.The FoM values of LC mixtures containing LC compounds with naphthyl group were lower than those of reference benzene LCs in the low-temperature region.However,in the high-temperature region,the results were reversed.These isothiocyanate LC compounds with naphthyl group can be applied in special fast-response LC device,particularly the ones used under high-temperature conditions.
naphthalene,isothiocyanate liquid crystals,high birefringence,electro-optical property
1.Introduction
Liquid crystal(LC)devices can be used as display devices and optical modulators,and their electro-optical properties are mainly dominated by the characteristic of the LC material.[1]High-birefringence LC is a type of attractive optical material that can be used in color-sequential LC displays and fast spatial light modulators.In the above applications,a fast response time is very desirable,and the phase modulation needs to be sufficiently large to a certain value,such as 2π for wavefront corrector.In such conditions,the response time of the LC device was dominated by the birefringence(Δn)and viscoelastic coefficient,and the thickness of the LC layer becomes a constant controlled by phase modulation and Δn.Gauza et al. had defined the figure-of-merit(FoM)value to evaluate the response performance of LC materials.[2]LC material with high Δn and low viscosity will exhibit a high FoM value and can provide a short response time.
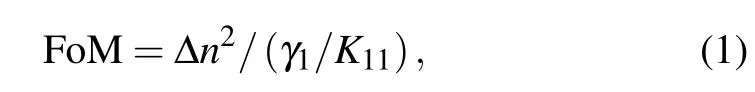
where Δn is the birefringence,γ1is the rotational viscosity,K11is the splay elastic constant of the LC material,and γ1/K11is defined as the viscoelastic coefficient.
In the LC materials research,high-birefringence LC should be designed with a long conjugation molecular structure,e.g.,biphenyl,terphenyl, tolane,phenyl-tolane,naphthalene group,double-bond and triple-bond structure.[3]To achieve high Δn(>0.3),some of these functional groups were combined in one LC molecular structure.The compounds with benzene isothiocyanate group(NCS)are important high Δn LCs and always exhibit relatively low viscosity.For example,the Δn values of representative LC compounds 4’-n-propyl-3- fluoro-4-isothiocyanato-biphenyl,[4]4’-n-propyl-3,5-di fluoro-4-isothiocyanato-terphenyl,[5]4’-n-propyl-3-fl uoro-4-isothiocyanato-tolane,[6]and 1-(3,5-di fluoro-4-iosthiocyanatophenyl)-2-(4-n-propyl-biphenyl)ethyne[7]are 0.26,0.44,0.38,and 0.54,respectively,as listed in Fig.1.
To reduce the viscosity of high Δn LC compound,some multifluorine-substituted phenyl-tolane isothiocyanate compounds,including laterally[7]and bilaterally[8]fluorinated compounds,were synthesized.These compounds exhibited low viscosity and high FoM value.Moreover,the viscosity value also could be calculated via a theoretical molecular dynamics method.[9]The LC compounds and mixtures with high birefringence have been reviewed in detail by Dabrowski et al.[10]
Furthermore,the naphthyl group can produce a longer conjugation length than the benzene group,and the LC compound with the naphthyl group will exhibit a high Δnvalue.Some LC compounds with naphthyl and isothiocyanate groups,such as naphthyl-benzene isothiocyanate[11]and naphthyl-ethynyl-benzene isothiocyanate,[12]have been studied,and the Δn value of these compounds are larger than 0.40.In this paper,we investigated the properties of three new fluorine-substituted benzene isothiocyanate LC compounds with the naphthyl group.The LC-phase transition properties and electro-optical properties of the investigated LC compounds and a few reference compounds are compared and discussed.
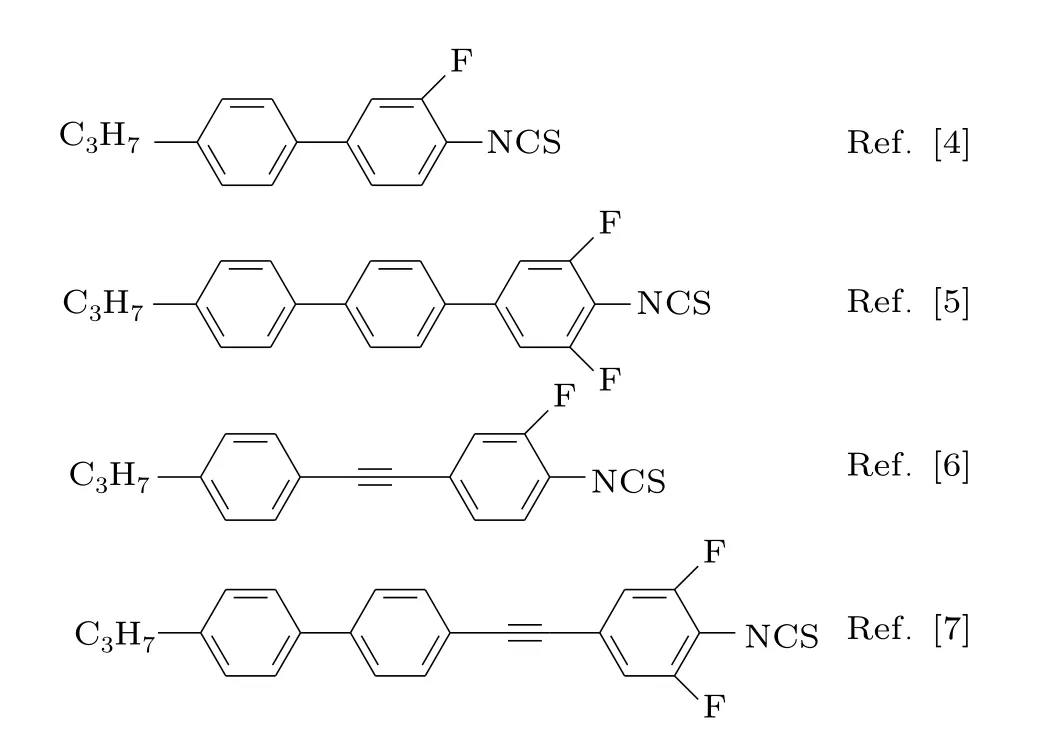
Fig.1.Common chemical structures of NCS LC compounds.
2.Experiment
2.1.LC materials
Three target LC compounds with the naphthyl group were synthesized via common organic synthesis methods.[13]Table 1 lists the chemical structures and mesomorphic properties of these compounds which are named as S1,S2,and S3.The data of chemical structure identification are listed as follows: S1:1H NMR(CDCl3,δ/ppm):1.480–1.535(m,3H),4.148–4.199(m,2H),7.144(s,1H),7.178–7.200(d,1H),7.281–7.288(m,1H),7.430–7.496(m,2H),7.607–7.628(m,1H), 7.776–7.797(m,2H),and 7.921(s,1H).S2:1H NMR(CDCl3, δ/ppm):0.939–0.973(t,3H,J=13.6),1.410–1.532(m, 4H),1.532–1.896(m,2H),4.067–4.099(t,2H,J=12.8), 7.111–7.121(d,2H,J=4.0),7.142(s,1H),7.166–7.189(d, 1H,J=9.2),7.478–7.499(d,1H,J=8.4),7.677–7.724(t, 2H,J=18.8),7.960(s,1H).S3:1H NMR(CDCl3,δ/ppm): 0.974–1.010(t,3H,J=14.4),1.679−−1.735(m,2H),2.661 (t,2H,J=15.6 Hz),7.055–7.072(m,1H),7.141–7.190(m, 1H),7.297–7.317(m,2H),7.531–7.572(m,1H),7.630–7.667 (m,2H),7.748–7.778(m,1H),7.852–7.968(m,2H),7.995–8.059(m,2H).
Three reference isothiocyanate LC compounds(Ref-1, Ref-2,and Ref-3)without the naphthyl group were prepared and investigated;the molecular structure analysis data can be found in the literature.[14–16]
2.2.Test method
Differential scanning calorimetry(TA Instrument, Q2000)was used to determine the phase transition points and enthalpy values of LC compounds in the temperaturerising procedure.Results were obtained from∼3 mg samples at a rising rate of 10°C/min.The LC texture was used to confirm the phase state under polarized light microscopy (Olympus,BX-51)with a thermal stage.Each compound was dissolved into a commercial host LC(SLC9023,ChengzhiY-onghua Display Materials Co.)with 10.00 wt%concentration for measurement of the Δn value and γ1/K11value.In optical measurement,[17]a homogeneous LC cell with 4.90µm thickness and∼1.0°pretilt angle was used.The Δn value of the LC mixture was calculated from the total phase retardation of the LC cell.The γ1/K11value was calculated from the response time derived by Wu etal.[18]The detailed measurement method is given as follows.Under a small driving voltage,the phase decay time of the homogeneous LC cell is expressed as δ(t)=δ0exp(−2t/τ0),where δ0is the total phase change of the LC cell without driving voltage.At t=0,the driving voltage is removed instantaneously.Through the fitting of the phase change(δ)depending on time(t),the LC director response time τ0can be calculated.According to the famous LC device response time formula expressedas τ0=γ1d2/K11π2, the γ1/K11value can be calculated with known τ0and d.The Δn of SLC9023 is 0.241 at λ=589 nm,and the γ1/K11is 13.57 ms/µm2at room temperature.
3.Results and discussion
3.1.Phase transitions and mesomorphic properties
Table 1 summarized the melting points and enthalpy values for three investigated compounds and their reference compounds.The investigated compounds contain a similar structure with reference compounds,in which the naphthyl group was replaced with the benzene group.First,the melting point changing with length of LC unit was investigated,and the sequence is
Usually,the melting point will become high with the long mesomorphic group.Therefore,S3 shows the highest melting point.Compound S2 is close to S1,and this is probably because S2 has a long flexible terminal carbon chain,showing the low melting point.
Then,NCS compounds with the naphthyl group and benzene group were further compared here.The results show that the naphthyl compounds have a higher melting point than the corresponding benzene compounds:

The molecular weight is probably the reason for the high melting point in the comparison of the similar structure,and theincreasing of length and width of the mesomorphic unit is another possible factor.
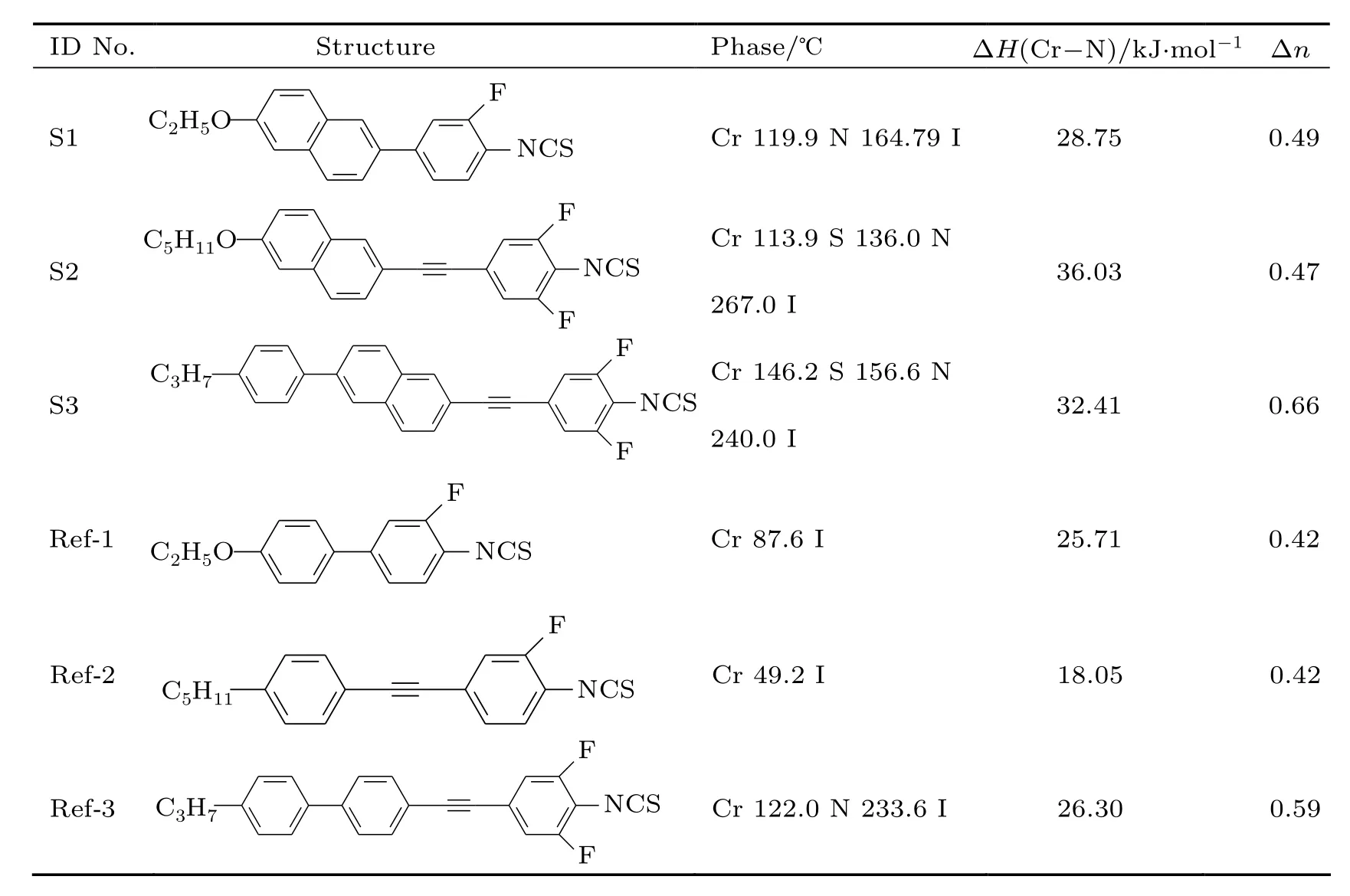
Table 1.Molecular structures,phase transition properties,and birefringence of LC compounds.
The melting enthalpy of these LC compounds was also investigated here.Similar to melting tests,the naphthyl compounds have higher melting enthalpy than benzene compounds:
Test results show that the investigated compounds S1,S2, and S3 all have LC phase,but reference compounds Ref-1 and Ref-2 do not have any LC phase.Therefore,these investigated compounds could achieve a wider nematic range than the reference compounds for new LC mixture exploration.
3.2.Electro-optical properties
When the Δn value of the LC mixture is obtained with the optical method,theΔn value ofa single LC compound at25°C can be extrapolated according to the guest–host equation

where gh,g,and h denote the guest–host LC solution,guest compound,and host LCs respectively,and x is the mass concentration of the guest LC compound.The extrapolated Δn values of target LC compounds are 0.49,0.47,and 0.66,as shown in Table 1.
Apparently,the Δn value of target LCs is 0.05–0.07 larger than reference compounds in Table 1: The phenomenon can be attributed to the large polarity of naphthyl groups.Hird et al.previously reported NCS compounds with the naphthyl benzene group without substituted fluorine,and its structure is similar to S1 and the Δn value is 0.44.[11]Zhao et al.synthesized NCS compounds with the naphthyl-ethynyl-benzene group,similar to S2 and with Δn value of 0.45.[12]All these compounds show a close Δn value to the investigated compounds in this work.Hence,it shows that substituted fluorine almost does not affect the Δn value of the compound.
Figure 2(a)shows the Δn value changing with temperature for LC mixture of three investigated compounds dissolved in commercial LC(SLC9023).It shows that Δn values for three investigated compounds decrease with increasing temperature and become zero at approximately the clearing point temperature.The Δn value of each LC compound mixture is larger than pure host LCs because Δn of target LC is far larger than that of pure commercial LC material.At most temperature ranges,S3 shows a higher Δn value than the others.
Figure 2(b)shows the compared result of the Δn value for investigated and reference compounds.At each temperature range,investigated compounds show a higher Δn value than reference compounds.For the high temperature range, the Δn values for Ref-1 and Ref-2 decrease faster than that of S1 and S2 because Ref-1 and Ref-2 do not have a nematic phase.Hence,the LC mixtures with dissolved Ref-1 and Ref-2 show a lower clearing point.The temperature-dependent Δn of an LC material can be described as
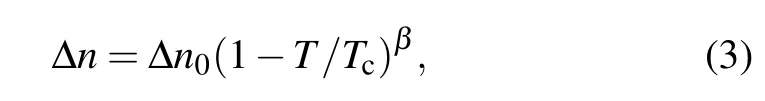
where Δn0is the birefringence at T=0 K,β is a material constant,and Tcis the clearing point of the LC material.According to Eq.(3),Δn values of Ref-1 and Ref-2 decrease quickly within the high-temperature range.The decreasing speeds for S3 and Ref-3 are almost the same and it is possible that they show a similar LC phase range.
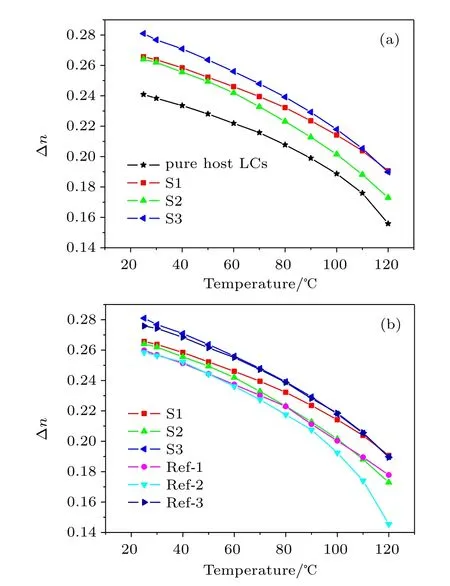
Fig.2.(color online)Temperature-dependent birefringence of different LC mixtures.(a)Results of pure host LCs and host LCs doped with compounds S1,S2,and S3.(b)Results of host LCs doped with naphthyl compound(S1,S2,and S3)and its reference compound(Ref-1, Ref-2,and Ref-3).The symbols are experimental data,and the lines are connecting lines.There are some reduplicative data in panels(a)and (b).
In general,the viscoelastic coefficient decreases with increasing temperature and reaches a constant value when close to the clearing point,as shown in Fig.3(a).At 25°C,the viscoelastic coefficients for pure host LC material,S1,S2, and S3 are 13.57,16.05,16.39,and 19.33 ms/µm2,respectively.Naphthyl compounds dissolved in the commercial host LC material will cause an enhanced viscoelastic coefficient. The viscoelastic coefficient of the S3 mixture is approximately 42%higher than that of the pure host LC material.If we supposed that similar LC mixtures have close elastic coefficient, the enhancement of the viscoelastic coefficient was probably caused by viscosity increment.Therefore,S1,S2,and S3 show larger viscosity than the others.Among them,S3 shows the largest viscosity due to its longest molecular chain.At approximately 100°C,all LC compounds show almost the same viscoelastic coefficient:
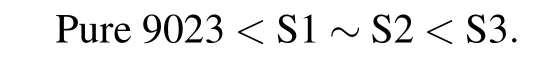
Figure 3(b)gives the comparison result of viscoelastic coefficients for investigated and reference compounds dissolved in LC mixture.As shown,the naphthyl LC mixture has a larger viscoelastic coefficient than that of the corresponding benzene LC mixture within the low-temperature range.It shows that the viscosity coefficient for naphthyl compounds is larger than that of benzene compounds:
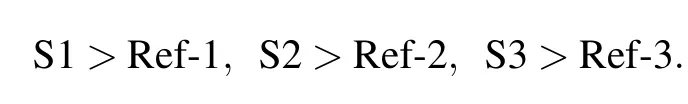
This result is derived from the longer and wider molecular structure of the naphthyl group than the benzene group. Among these compounds,S1 is almost the same as S2,and so is Ref-1 and Ref-2.This shows that the alkyne bond does not show any significant influence on the viscosity of high-birefringence LC compounds.Within the high-temperature range,these compounds show a close viscoelastic coefficient, except S1 which has a slightly higher viscosity.

Fig.3.(color online)Temperature-dependent viscoelastic coefficient of different LC mixtures.(a)Results of pure host LCs and host LCs doped with compounds S1,S2,and S3.(b)Results of host LCs doped with naphthyl compound(S1,S2,and S3)and its reference compound(Ref-1,Ref-2,and Ref-3).The symbols are experimental data,and the lines are connecting lines.There are some reduplicative data in panels(a) and(b).
The FoM value,which is calculated according to Eq.(1) using Δn and γ1/K11and considering the effects of birefringence and viscoelastic coefficient,can be used to compare the response performance of LC materials.Figure 4(a)gives the FoM change curve with temperature for investigated LC mixture and pure commercial LC.Four sets of FoM data all increase first and then decrease with increasing temperature. They obtained a maximum value at approximately 100°C.At 25°C and 30°C temperatures,they all show the same FoM value as pure commercial LC material,and this is because Δn and viscoelastic coefficient both increase with increasing temperature.At temperatures higher than 40°C,the FoM values of these compounds show different order:

This phenomenon is due to the fact that at high temperatures, the investigated compounds give the same viscoelastic coefficient as pure 9023 but with high Δn value,and hencethey show a larger FoM value than pure 9023.At 100°C,LC mixture S3 achieves the highest FoM value among all these compounds.
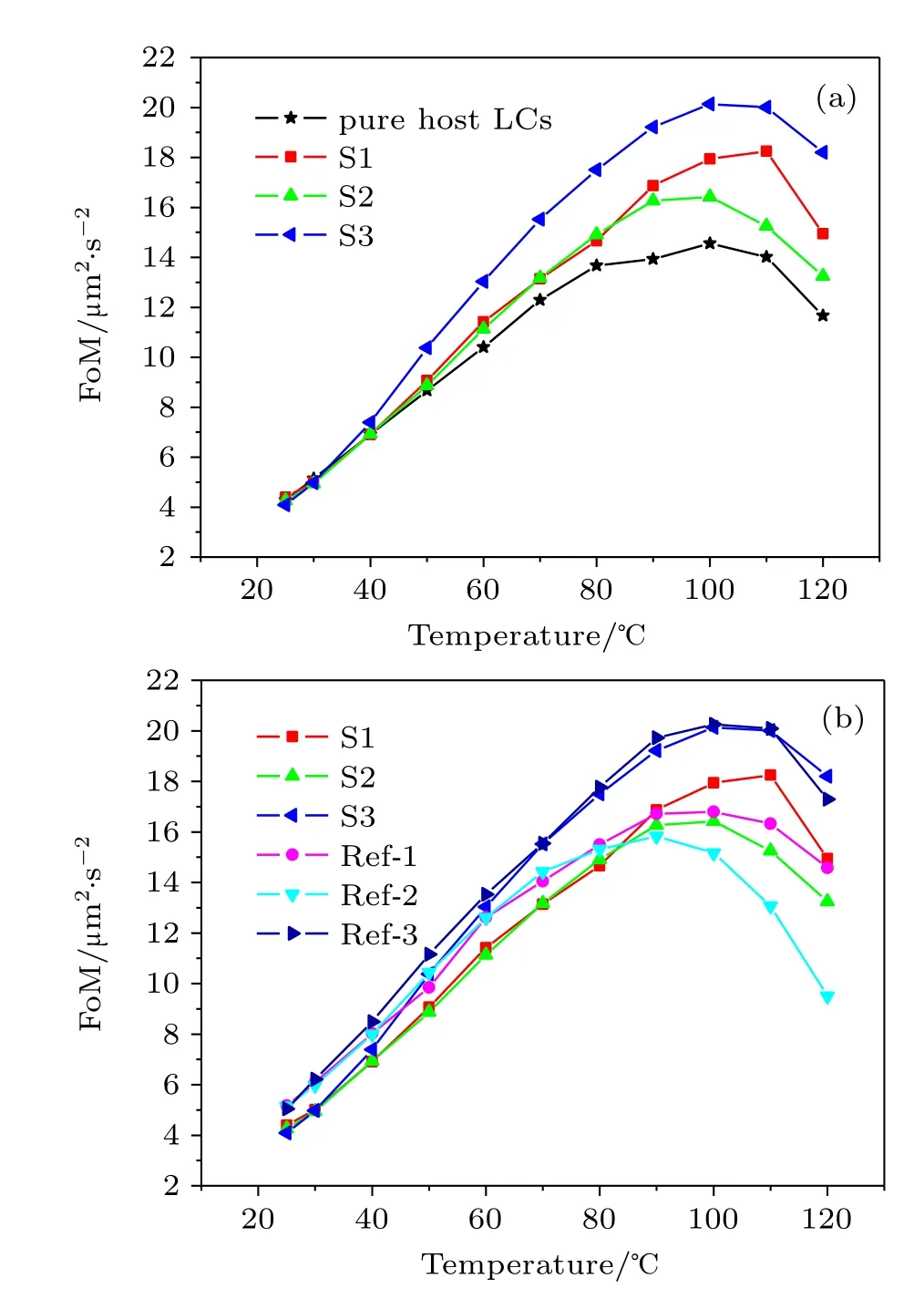
Fig.4.(color online)Temperature-dependent FoM value of different LC mixtures.(a)Results of pure host LCs and host LCs doped with compounds S1,S2,and S3.(b)Results of host LCs doped with naphthyl compound(S1,S2,and S3)and its reference compound(Ref-1, Ref-2,and Ref-3).The symbols are experimental data,and the lines are connecting lines.There are some reduplicative data in panels(a)and (b).
Figure 4(b)shows the FoM curve with temperature for investigated LC mixture and reference LC mixture.Within the low-temperature range of 25–30°C,the FoM curve is divided into two groups due to different response properties. One group is S1,S2,and S3,and the other is Ref-1,Ref-2, and Ref-3 with the order
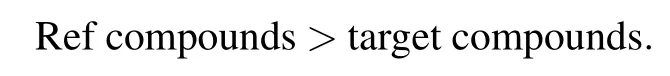
This is because the investigated compounds show larger viscosity than reference compounds.However,within the high temperature range,the rising slope of FoM value for investigated compounds is larger than that of reference compounds, and FoM data for both investigated and reference compounds gradually get close to each other.For S1 and S2,they show the same FoM value as the reference compound at approximately 80–90°C.At higher temperatures,the investigated compounds show a better response property because the clearing point and Δn value of investigated compounds are higher than those of the reference ones.For S3 and Ref-3,they obtained the same FoM value at 60°C and so is the maximum value.
4.Conclusion
We have developed three new fluorinated benzene isothiocyanate LC compounds with the naphthyl group and three reference compounds without the naphthyl group,and investigated their phase transition properties and electro-optical properties.The investigated naphthyl LC compounds all exhibit higher melting points than the corresponding reference compounds.The Δn values of investigated LC compounds were tested with the guest–host method,and the values were 0.49,0.47,and 0.66,which were higher than the reference LC compounds.When the target LC compounds were dissolved in a commercial LC material,the response properties of the LC mixtures were measured.The viscoelastic coefficients of the LC mixtures containing the target compounds were significantly larger than that of LC mixtures with reference compounds in the low-temperature region,and the compound S3 with the highest molecular weight exhibits the largest viscoelastic coefficient.The viscoelastic coefficients of these LC mixtures were similar in the high-temperature region.The LC mixtures containing the target compounds exhibit larger FoM values than the corresponding reference LC mixtures in the high-temperature region.However,at low temperatures,the results were reversed.Therefore,useful application of these naphthylLC compounds in high-speed display devices and optical devices are foreseeable.
[1]Wu S T and Yang D K 2014 Fundamentals of Liquid Crystal Devices, 2nd edn.(:Wiley)
[2]Gauza S,Wang H Y,Wen CH,Wu S T,Seed A J and Da¸browski R 2003 Jpn.J.Appl.Phys.42 3463
[3]Zhang R,Peng Z H,Liu Y G,Zheng Z Z and Xuan L 2009 Chin.Phys. B 18 4380
[4]Peng Z H,Liu Y G,Cao Z L Mu Q Q,Lu X H,Hu L F,Yu Z and Xuan L 2010 Chin.J.Liq.Cryst.Disp.25 622(in Chinese)
[5]Parish A,Gauza S,Wu ST,Dziaduszek J and Da¸browski R 2008 Liq. Cryst.35 79
[6]Gauza S,Li J,Wu ST,Spadło A,Da¸browski R,Tzeng Y N and Cheng K L 2005 Liq.Cryst.32 1077
[7]Gauza S,Parish A,Wu ST,Spadło A and Da¸browski R 2008 Liq.Cryst. 35 483
[8]Peng Z H Wang Q D,Liu Y G,Mu Q Q,Cao Z L,Xu H Y,Zhang P G, Yang C L,Yao L S,Xuan L and Zhang Z Z 2016 Liq.Cryst.43 276
[9]Wang Q D,Peng Z H,Liu Y G,Yao L S Ren G and Xuan L 2015 Acta Phys.Sin.64 126102(in Chinese)
[10]Dabrowski R,Kula P and Herman J 2013 Crystals 3 443
[11]Hird M,Toyne K J,Goodby J W,Gray G W,Minter V,Tuffin R P and McDonnell D G 2004 J.Mater Chem.14 1731
[12]Zhao Y Z,Wang D,He Z M,Chen G,Zhang L Y,Zhang H Q and Yang H 2015 Chin.Chem.Lett.26 785
[13]Catanescu O,Wu S T andChien L C 2004 Liq.Cryst.31 541
[14]Urban S,Czupryński K,Da¸browski R,Gestblom B,Janik K,Kresse H and Schmalfuss H 2001 Liq.Cryst.28 691
[15]Spadło A,Da¸browskiR,Filipowicz M,Stolarz Z,Przedmojski J,Gauza S,Fan Y H and Wu S T 2003 Liq.Cryst.30 191
[16]Catanescu O andChien L 2006 Liq.Cryst.33 115
[17]Peng Z H,Liu Y G,Yao L S,Cao Z L,Mu Q Q,Hu L F,Lu X H and Xuan L 2011 Chin.Phys.Lett.28 94207
[18]Wu S T and Wu C S 1990 Phys.Rev.A 42 2219
28 March 2017;revised manuscript
18 April 2017;published online 31 July 2017)
10.1088/1674-1056/26/9/094210
∗Project supported by the National Natural Science Foundation of China(Grant Nos.61378075,61377032,11604327,and 61475152)and the Science Foundation of State Key Laboratory of Applied Optics,China.
†Corresponding author.E-mail:yaols@ciomp.ac.cn
‡Corresponding author.E-mail:xuanli@ciomp.ac.cn
©2017 Chinese Physical Society and IOP Publishing Ltd http://iopscience.iop.org/cpb http://cpb.iphy.ac.cn
- Chinese Physics B的其它文章
- Improved control for distributed parameter systems with time-dependent spatial domains utilizing mobile sensor actuator networks∗
- Geometry and thermodynamics of smeared Reissner–Nordström black holes in d-dimensional AdS spacetime
- Stochastic responses of tumor immune system with periodic treatment∗
- Invariants-based shortcuts for fast generating Greenberger-Horne-Zeilinger state among three superconducting qubits∗
- Cancelable remote quantum fingerprint templates protection scheme∗
- A high-fidelity memory scheme for quantum data buses∗

