Peripheral SLC6A4 gene expression in obsessive-compulsive disorder in the Han Chinese population
Xuemei WANG, Qing ZHAO , Wen CHEN, Shunying YU,3, Zhen WANG,3*, Zeping XIAO*
Peripheral SLC6A4 gene expression in obsessive-compulsive disorder in the Han Chinese population
Xuemei WANG1#, Qing ZHAO2#, Wen CHEN1, Shunying YU2,3, Zhen WANG2,3*, Zeping XIAO4*
OCD, SLC6A4, gene expression, RT-PCR, Yale–Brown Obsessive-Compulsive Scale score(Y-BOCS).
1. Introduction
Obsessive-compulsive disorder (OCD) is a psychiatric disorder characterized by unwanted thoughts(obsessions) and repetitive behaviors (compulsions).Twin and family studies suggest that OCD has a genetic basis, but a major causative gene(s) for the disorder has not yet been identified.[1-3]
There is significant research surrounding genes carrying an increased risk of OCD.[4]The gene SLC6A4(serotonin transporter, 5-HTT or SERT), located on chromosome 17q11.1–q12, has been studied extensively for genetic association with OCD. A 5-HTT linked polymorphic region (5-HTTLPR) has two common variants, short (S) and long (L). McDougle et al. found a strong relationship between the L allele and OCD.[5]Voyiaziakis and his colleagues also found evidence of genetic association at the SLC6A4 locus with OCD, and rare individual haplotypes containing L(A) with P<0.05 were observed.[6]However, Dickel and his colleges failed to detect the association with OCD when examining 5-HTTLPR.[7]Lin expanded the scope of investigation from 13 independent case–control association studies with 3445 subjects (1242 OCD patients and 2203 controls) in a meta-analysis, and there was no association detected for the LL genotype or solely for the allelic L variant.[8]Bolch et al. have also reported that there was no association between genetic variation in the 5-HTTLPR locus and OCD in their meta-analysis.[9]A study by Tibrewal et al. also failed to find a relationship between the L allele and OCD.[10]Overall , molecular genetic association studies have yielded inconsistent results. Variation may be due to lack of OCD subtype classification and sample size.
In addition, there have been suggestions of sexspecific OCD findings for SLC6A4, and for the LPR. Xu et al. did not withstand multiple-marker nor multipleanalysis corrections[11], whereas the Dutch sample may have.[12]Voyiaziakis and his colleagues found evidence of genetic association at the SLC6A4 locus with OCD and a possible gender effect.[6]Dickel et al. also found nominally significant excess transmission of the L allele in females, but after correction, there was no significant association remaining.[7]A recent metaanalysis included a total of 1991 participants with OCD and their 5-HTTLPR allele status was examined. They found that OCD was not associated with the S-allele of the 5-HTTLPR polymorphism. Moreover, late-onset OCD was not associated with the S-allele. However,when stratified by sex, there was an emerging sexspecific relationship. There was a trending association between the S-allele and OCD status in females but not in males.[13]Future studies should clearly examine sex differences and OCD age-of-onset. In the present study,we measured gene expression levels of SLC6A4 in the peripheral blood of Han Chinese individuals with OCD.We hypothesized that the genetic loci for OCD may be sex specific.
2. Methods
2.1 Subjects
Chinese outpatients 18-65 years of age with a clinical diagnosis of OCD were recruited at the Shanghai Mental Health Center from 2010 to 2014. The institutional review board at Shanghai Mental Health Center approved the study protocol and consent processes. All patients met DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4thedition,American Psychiatry Association, 1994) criteria for OCD, as determined by the Chinese version of the Mini-International Neuropsychiatric Interview (MINI)for DSM-IV.[14]The validity of the Chinese version of the MINI was 0.76–0.88 when compared with the Chinese version of the SCID-P, and the inter-rater and test–retest reliabilities of the two measures were 0.94 and 0.97–1.00, respectively.[15]Clinical verification of the diagnoses were made by a senior psychiatrist. Healthy controls were screened using the MINI to exclude major Axis I disorders, including substance dependence and psychotic disorders (including schizophrenia or schizophrenia-like disorders). There are 227 OCD patients and 260 healthy controls in our sample.
In the present study, the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) was used to assess the severity of obsessive-compulsive symptoms of the patients.[16]The Chinese version of Y-BOCS has good content validity and construct validity[12], good internal consistency(alpha=0.75), good inter-rater reliability (ICC=0.82), and good test-retest reliability (ICC=0.75). All patients were assessed by two or more consultant psychiatrists.
Participants (OCD patients and healthy controls)were included if they were at least 18 years of age,without neurological, endocrinological or other serious physical disorders, were not using psychotropic agents(antidepressants, anxiolytics, antipsychotics) and/or analgesics (including nonsteroidal anti-inflammatory drugs).
2.2 Sample collection
Fasting blood samples were obtained from all subjects for extraction of mRNA. Total RNA was extracted from peripheral whole blood using the QIAamp RNA blood Mini Kit (QIAGEN, Chatsworth, CA, USA) following the manufacturer’s standard protocol. To ensure no DNA contamination, clean-up of the RNA was performed using QIAGEN spin columns with an additional DNAase step (QIAGEN, Chatsworth, CA, USA). Reverse transcription was performed using TaqMan reverse transcription reagents with random primers (Takara Bio, Kyoto, Japan). Samples were stored at -80oC prior to further use. 6 OCD patients and 4 healthy controls did not have mRNA successfully extracted. In all, 50 first episode and drug-free patients and 60 matched healthy controls were included.
2.3 Real-time quantitative RT-PCR
Real-time quantitative RT-PCR was performed in triplicate for each sample on an ABI Prism 7900 sequence detection system with TaqMan Universal PCR mastermix (Applied Biosystems, Foster City,CA, USA) according to the manufacturer’s protocol.An Assay- By-Demand probe/primer set specific to SLC6A4 (Hs00169010_m1) and housekeeping control gene (glyceraldehyde-3-phosphate dehydrogenase,GAPDH) probe were purchased from Applied Biosystems (Applied Biosystems, Foster City, CA,USA). The expression data produced were analyzed and converted into threshold cycle values (-values)using SDS 2.0 (Applied Biosystems, Foster City, CA,USA). Value was defined as the cycle number at which the fluorescence exceeds the preset fluorescence threshold. The average -value of GAPDH and SLC6A4 was calculated from triplicate results for each sample.The ∆∆CTanalysis method was used to measure the relative gene expression.[17]The comparative method is a quantitative approach to assess relative changes in mRNA levels between two samples. The ∆value of each sample (patients and controls) was obtained by subtracting the average GAPDH -value of each sample from the average SLC6A4 -value of each sample (i.e.,SLC6A4 expression normalized by GAPDH). The gene expression levels were calculated using the equation.[18,19]
2.4 Statistical analysis
Statistical analyses were conducted using SPSS for Windows (Version 19.0). Continuous variables were presented as mean and standard deviation (SD). The demographic and clinical data for the groups were compared using t-tests, chi-square tests, or Mann Whitney U-tests, as appropriate. Spearman’s correlation test was used to test the interaction between clinical variables and the gene expression of SLC6A4. For all statistical tests, the critical level of significance was set at 0.05 (two-tailed) .

Figure 1. Enrollment of cases and controls
3. Results
3.1 Demographics
The groups were matched for age and male:female ratio (p > 0.05). Demographic and clinical characteristics of the patients are shown in Table 1. The mean (SD) Y-BOCS total score was 22.5(6.4) (minimum 16, maximum 40). The mean age of onset of OCD was 22.8(SD=9.16).
3.2 SLC6A4 gene expression levels between patients with OCD and controls
Gene expression levels of SLC6A4, which were nonnormally distributed variables, were compared using Mann-Whitney U-test between groups. As shown in Table 2, no significant differences between patients with OCD and healthy controls (Z=-0.79, p=0.428)were found in our study.
3.3 SLC6A4 gene expression levels between female and male patients with OCD
Gene expression levels of SLC6A4 in female and male OCD are non-normally distributed, therefore Mann-Whitney U-test was used to compare gender differences.
As shown in Table 3, there were no significant group differences between female and male OCD patients for SLC6A4 gene expression levels (Z=-1.66, p=0.096) and other demographic and clinical characteristics, except the age at onset of OCD[t(df=39.74)=-2.07, p=0.045].
3.4 Correlations between SLC6A4 gene expression levels and OCD
In this study, we did not find a significant correlation between SLC6A4 expression levels and OCD (spearman correlation coefficient r=0.16, p=0.272) .There is no significant correlation between SLC6A4 expression levels and obsessive subscore (r=0.05, p=0.746) or compulsive subscore (r=0.10, p=0.050). We also did not find a significant correlation between SLC6A4 expression levels and the age onset of OCD (r=0.04, p=0.771).
4. Discussion
4.1 Main findings
SLC6A4 is the molecular target of the selective serotonin reuptake inhibitors (SSRIs), namely fluoxetine, fluvoxamine, sertraline, paroxetine and citalopram. SSRIs collectively represent the most clinically effective and widely studied pharmacological treatment for OCD; they are effective in reducing both
the thought (obsessions) and behavioral (compulsions)components of OCD.[20,21]Imaging studies have suggested that individuals with OCD have decreased serotonin transporter availability in the midbrain/brainstem.[22-24]The changes in serotonin transporter protein levels alter the amounts of serotonin receptors and serotonin synthesis and metabolism.[25]
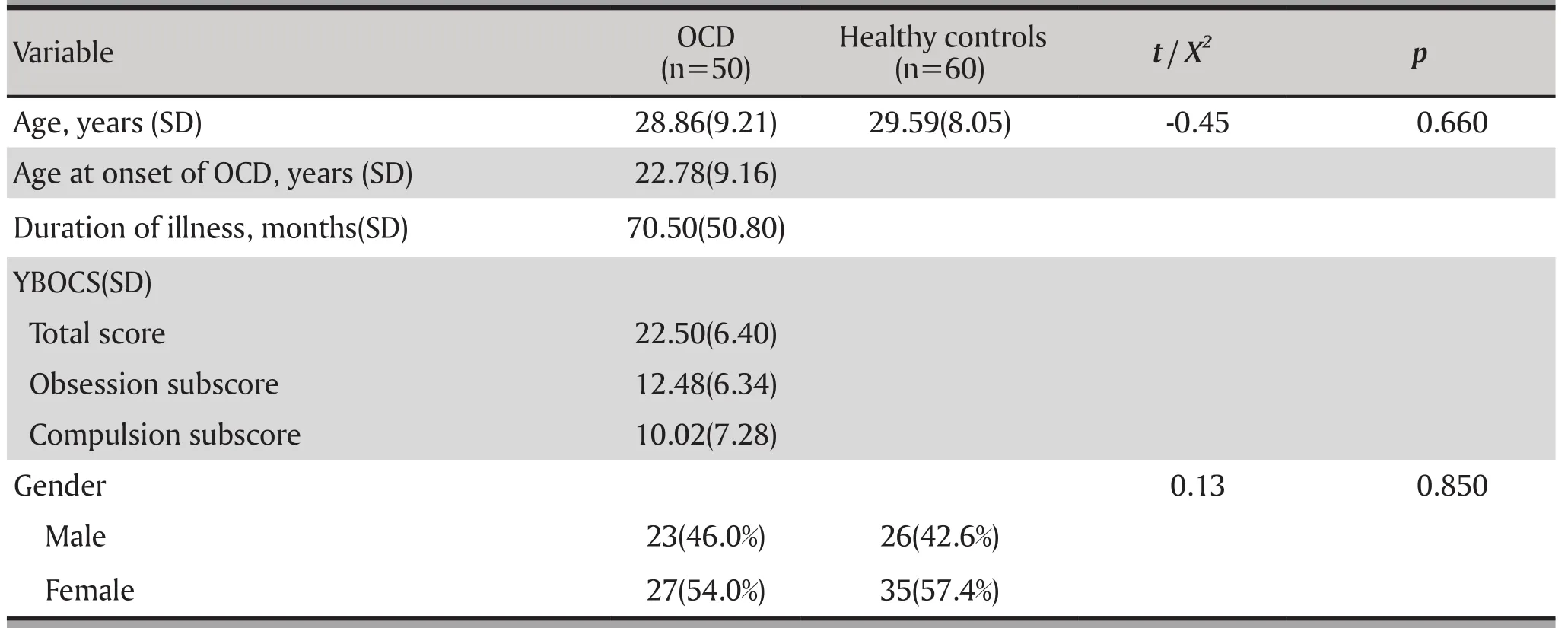
Table 1. Demographics characteristics of probands with OCD and healthy controls

Table 2. Demographic characteristics of probands with OCD and healthy controls.

Table 3. Demographic characteristics and gene expression levels of SLC6A4 between female and male patients with OCD.

Table 4. Correlation between of SLC6A4 gene expression levels and OCD.
Our current study was the first to examine gene expression of SLC6A4 in the peripheral blood of patients with OCD. We did not find a significant correlation between SLC6A4 expression levels and the severity and subtype of OCD. As shown in Figure2, the SLC6A4 gene expression of male and female subjects had a contrary trend in different groups, which may cover the significance between groups. These results were different from some studies about SLC6A4 polymorphisms and imaging studies of OCD. The discrepancy may be derived from differences in tissues and ethnicities. Ethnic differences were seen in allele frequencies between European and Japanese individuals. In cohorts of European ancestry, the S allele was carried by 42–57% of individuals,whereas in Asian cohorts, 82% of healthy Japanese subjects carried the S allele. It is also possible that SLC6A4 expression levels may result from a combination of several factors which we did not measure in this study,such as psychological stress and cognitive functioning.OCD may therefore be shaped by a combination of many genes with modest impact as well as susceptibility genes interacting with environmental factors. It is therefore critical to define factors such as age of onset and gender that influence genetic predisposition.
Gender differences have been observed in genetic association studies of OCD and HTR2A, COMT and MAOA[26,27], as well as in segregation studies.[28]Some positive reports have found a gender-based genetic effect of SLC64A4.[29]Lei and colleagues also found a significant gender main effect and genderspecific genetic effect of the SLC6A4 gene on OCD,and a conserved haplotype polymorphism (rs1042173 rs4325622| rs3794808| rs140701| rs4583306|rs2020942) covering from intron 3 to the 3’ UTR of the SLC6A4 gene showed male-specific genetic effects in normal Chinese college students.[30]But contrary to our hypothesis, we didn’t find significant genderdifferences of SLC6A4 gene expression between male and female OCD patients. In our sample, male OCD patients showed a tendency of low gene expression of SLC6A4 in peripheral blood. However, the small sample size may be a confounding reason responsible for the lack of significance. Thus, further examination of these loci on a larger sample set may also help rule out other confounding factors. In our study , there were group differences between male and female OCD patients for the age at onset of OCD, which may be explained as male patients having developed OCD at an earlier age. In particular, co-morbidities and type of obsessions present in females differ from males. For example, more females than males will present with co-morbid anxiety, eating disorders, and depression. Moreover, females are twice as likely to have contamination-centered obsessions(Labad et al.2008), whereas sexual obsessions are more associated with males. But we also need to consider that factors such as method of ascertainment, site of patient recruitment, mean age at the time of patient recruitment and mean age at the onset of OCD symptoms all may have had a contributory role. Therefore, future studies must take into account these factors when investigating genetic association.
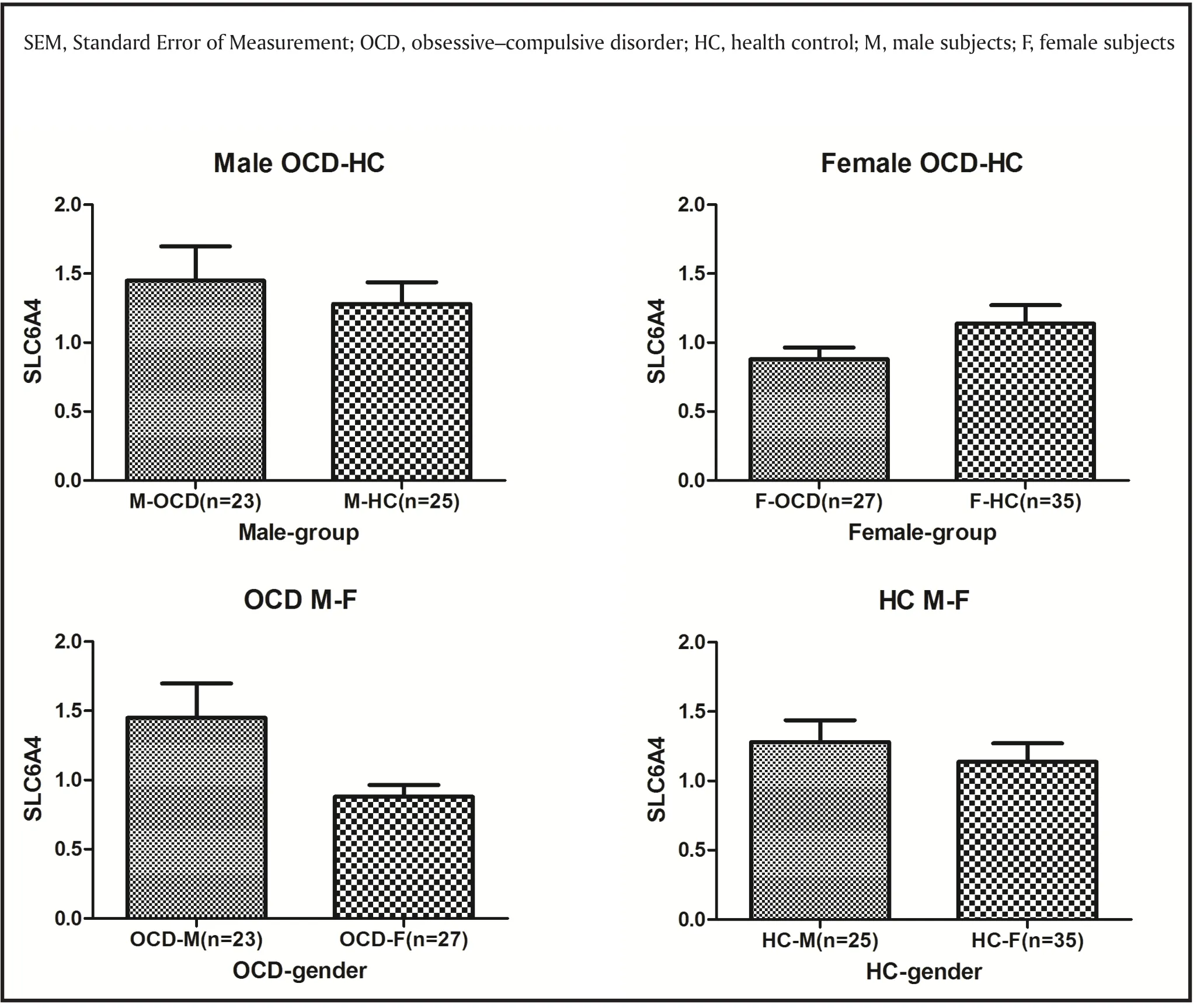
Figure 2. SLC6A4 gene expression in different genders and groups (mean with SEM).
We measured SLC6A4 gene expression peripherally in blood, since obtaining tissue from the relevant brain regions is essentially impossible for reasonably large and representative patient sample sets. Can the gene expression of peripheral blood, a more accessible tissue, reflect gene expression in the CNS? Middleton et al[31]first conducted a study on schizophrenia and bipolar disorder using quantitative real-time RTPCR, and found compelling evidence for the utility of analyzing peripheral blood leukocytes RNA as a marker for changes in expression in neuropsychiatric disorders. A study (Sullivan, Fan, & Perou, 2006)[32]used gene expression data from 79 diverse human tissues for 33,698 gene transcripts to address the broad question of whether peripheral blood lymphocyte gene expression data is a representative and reasonable surrogate for gene expression in the CNS. The results suggested that gene expression in lymphocytes is relatively strongly correlated with gene expression in the brain. These results indicate that peripheral blood markers reflect relevant expression and functional changes of SLC6A4 gene expression in the brain.
4.2 Limitations
There are several limitations that should be considered.First, we only performed one method, real-time reverse transcription PCR, to assess SLC6A4 gene expression.Although it is often described as a ‘gold’ standard,future studies should consider examining regulatory RNAs, protein levels and protein activity. Second,although gender and age are matched, we cannot exclude the possibility that other confounding factors may have impacted our results such as life stresses and IQ. Thirdly, the main limitation of the present study is that the size of present study groups was relatively small. Given the role of SLC6A4 in the treatment of OCD, larger samples would also enable better delineation of subgroups of OCD for whom the SLC6A4 gene would be particularly relevant, such as individuals with childhood-onset symptoms.
4.3 Implications
This is the first study to date that examines gene expression of SLC6A4 in the peripheral blood of patients with OCD. We did not find significant differences in gene expression levels of SLC6A4 between the OCD patients and control subjects or a correlation between SLC6A4 expression levels and the severity and subtype of OCD.Future studies need to take into account the complexity and heterogeneous nature of this disorder in a larger sample size. We also need to investigate the relationships among the genetic, epigenetic, and expression changes of SLC6A4 in peripheral Leukocytes of OCD patients, in order to confirm the role of SLC6A4 in OCD in a Chinese population.
Acknowledgements
We thank Shanghai Mental Health Center and all psychiatrists, patients and volunteers who made this research possible.
Funding statement
This study was supported by grants from National Natural Science Foundation of China (81171280), and Biomedical Engineering Cross Research Foundation of Shanghai Jiao Tong University (YG2013MS65). Those institutions had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the paper; or in the decision to submit the paper for publication.
Ethical Approval
The institutional review board at Shanghai Mental Health Center(SMHC-IRB 2013-23) reviewed and approved our study. Written and verbal informed consent was obtained prior to participant enrollment,and all participants were informed that they could withdrawal from the study at any time.
Conflict of interest statement
The authors declare that they have no competing interests.
Authors’ contributions
Xuemei Wang carried out the molecular genetic studies.
Xuemei Wang and Qing Zhao participated in sample collection and laboratory work, and drafted the manuscript.
Zeping Xiao and Zhen Wang participated in the design of the study and coordination and helped to draft the manuscript.
Qing Zhao, Shunying Yu and Wen Chen performed the statistical analysis. All authors read and approved the final manuscript.
1. Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, et al. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder.Am J Hum Genet. 2006; 78(5): 815-826. doi: http://doi.org/10.1086/503850
2. Mataix-Cols D, Boman M, Monzani B, Ruck C, Serlachius E, Langstrom N, et al. Population-based, multigenerational family clustering study of obsessive-compulsive disorder.JAMA Psychiatry. 2013; 70(7): 709-717. doi: http://doi.org/10.1001/jamapsychiatry.2013.3
3. Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, et al. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003; 8(11): 933-936. doi: http://doi.org/10.1038/sj.mp.4001365
4.Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry. 2013; 18(7): 799-805.doi: http://doi.org/10.1038/mp.2012.76
5.McDougle CJ, Epperson CN, Price LH, Gelernter J. Evidence for linkage disequilibrium between serotonin transporter protein gene (SLC6A4) and obsessive compulsive disorder.Mol Psychiatry. 1998; 3(3): 270-273
6.Voyiaziakis E, Evgrafov O, Li D, Yoon HJ, Tabares P, Samuels J, et al. Association of SLC6A4 variants with obsessivecompulsive disorder in a large multicenter US family study. Mol Psychiatry. 2011; 16(1): 108-120. doi: http://doi.org/10.1038/mp.2009.100
7. Dickel DE, Veenstra-VanderWeele J, Bivens NC, Wu X,Fischer DJ, Van Etten-Lee M, et al. Association studies of serotonin system candidate genes in early-onset obsessivecompulsive disorder. Biol Psychiatry. 2007; 61(3): 322-329.doi: http://doi.org/10.1016/j.biopsych.2006.09.030
8. Lin PY. Meta-analysis of the association of serotonin transporter gene polymorphism with obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry.2007; 31(3): 683-689. doi: http://doi.org/10.1016/j.pnpbp.2006.12.024
9. Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, et al. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008; 147b(6): 850-858. doi: http://doi.org/10.1002/ajmg.b.30699
10. Tibrewal P, Kumar HB, Shubha GN, Subhashree D,Purushottam M, Thennarasu K, et al. Association of serotonin transporter gene polymorphisms with obsessivecompulsive disorder (OCD) in a south Indian population.Indian J Med Res. 2010; 132: 690-695
11. Xu Y, Zhang H. [The reliability and validity of the Chinese version of Yale-Brown obsessive-compulsive scale]. Shanghai Arch Psychiatry. 2006; 18: 321-323. Chinese
12. Denys D, Van Nieuwerburgh F, Deforce D, Westenberg HG. Association between serotonergic candidate genes and specific phenotypes of obsessive compulsive disorder.J Affect Disord. 2006; 91(1): 39-44. doi: http://doi.org/10.1016/j.jad.2005.12.011
13. Mak L, Streiner DL, Steiner M. Is serotonin transporter polymorphism (5-HTTLPR) allele status a predictor for obsessive-compulsive disorder? A meta-analysis. Arch Womens Ment Health. 2015; 18(3): 435-445. doi: http://doi.org/10.1007/s00737-015-0526-z
14. Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J,Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998; 59(Suppl 20): 22-33; 34-57
15. Si TM, Shu L, Dang WM, Su YA, Chen JX, et al. [Evaluation of the reliability and validity of Chinese version of the Mini-International Neuropsychiatric Interview in patients with mental disorders]. Zhongguo Xin Li Wei Sheng Za Zhi. 2009;23(7): 493-497. Chinese. doi: http://dx.chinadoi.cn/10.3969/j.issn.1000-6729.2009.07.011
16. Kiejna A, Grzesiak M, Malyszczak K. [The Yale-Brown Scale: instrument for assessing the severity of obsessivecompulsive disorders]. Psychiatria Polska. 1998; 32(1): 69-76. Polish
17. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif). 2001; 25(4): 402-408. doi: http://doi.org/10.1006/meth.2001.1262
18. Cui DH, Jiang KD, Jiang SD, Xu YF, Yao H. The tumor suppressor adenomatous polyposis coli gene is associated with susceptibility to schizophrenia. Mol Psychiatry. 2005;10(7): 669-677. doi: http://doi.org/10.1038/sj.mp.4001653
19. Zhu G, Lipsky RH, Xu K, Ali S, Hyde T, Kleinman J, et al.Differential expression of human COMT alleles in brain and lymphoblasts detected by RT-coupled 5’ nuclease assay.Psychopharmacology. 2004; 177(1-2): 178-184. doi: http://doi.org/10.1007/s00213-004-1938-z
20. Pizarro M, Fontenelle LF, Paravidino DC, Yucel M, Miguel EC, de Menezes GB. An updated review of antidepressants with marked serotonergic effects in obsessive-compulsive disorder. Expert Opin Pharmacother. 2014; 15(10): 1391-1401. doi: http://doi.org/10.1517/14656566.2014.914493
21. Zai G, Brandl EJ, Muller DJ, Richter MA, Kennedy JL.Pharmacogenetics of antidepressant treatment in obsessivecompulsive disorder: an update and implications for clinicians. Pharmacogenomics. 2014; 15(8): 1147-1157. doi:http://doi.org/10.2217/pgs.14.83
22. Hasselbalch SG, Hansen ES, Jakobsen TB, Pinborg LH,Lonborg JH, Bolwig TG. Reduced midbrain-pons serotonin transporter binding in patients with obsessive-compulsive disorder. Acta Psychiatr Scand. 2007; 115(5): 388-394. doi:http://doi.org/10.1111/j.1600-0447.2006.00940.x
23. Hesse S, Muller U, Lincke T, Barthel H, Villmann T,Angermeyer MC, et al. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder.Psychiatry Res.2005; 140(1): 63-72. doi: http://doi.org/10.1016/j.pscychresns.2005.07.002
24. Stengler-Wenzke K, Muller U, Angermeyer MC, Sabri O,Hesse S. Reduced serotonin transporter-availability in obsessive-compulsive disorder (OCD). Eur Arch Psychiatry Clin Neurosci. 2004; 254(4): 252-255. doi: http://doi.org/10.1007/s00406-004-0489-y
25. Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, et al. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems.Neuropharmacology. 2008; 55(6): 932-960. doi: http://doi.org/10.1016/j.neuropharm.2008.08.034
26. Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, et al. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry. 1999; 45(9): 1178-1189
27. Enoch MA, Greenberg BD, Murphy DL, Goldman D. Sexually dimorphic relationship of a 5-HT2A promoter polymorphism with obsessive-compulsive disorder. Biol Psychiatry. 2001;49(4): 385-388
28. Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, Liang KY, et al. Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. Am J Hum Genet.2000; 67(6): 1611-1616. doi: http://doi.org/10.1086/316898
29. Chabane N, Millet B, Delorme R, Lichtermann D, Mathieu F,Laplanche JL, et al. Lack of evidence for association between serotonin transporter gene (5-HTTLPR) and obsessivecompulsive disorder by case control and family association study in humans. Neurosci Lett. 2004; 363(2): 154-156. doi:http://doi.org/10.1016/j.neulet.2004.03.065
30. Lei X, Chen C, He Q, Chen C, Moyzis RK, Xue G, et al. Sex determines which section of the SLC6A4 gene is linked to obsessive-compulsive symptoms in normal Chinese college students. J Psychiatr Res. 2012; 46(9): 1153-1160. doi: http://doi.org/10.1016/j.jpsychires.2012.05.002
31. Middleton FA, Pato CN, Gentile KL, McGann L, Brown AM, Trauzzi M, et al. Gene expression analysis of peripheral blood leukocytes from discordant sib-pairs with schizophrenia and bipolar disorder reveals points of convergence between genetic and functional genomic approaches. Am J Med Genet B Neuropsychiatr Genet. 2005;136B(1): 12-25. doi: http://doi.org/10.1002/ajmg.b.30171
32. Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006; 141B(3): 261–268. doi: http://doi.org/10.1002/ajmg.b.30272
中国汉族人群强迫症的外周SLC6A4基因表达
王雪梅, 赵青, 陈文, 禹顺英, 王振, 肖泽萍
强迫症, SLC6A4, 基因表达,逆转录聚合酶链反应, Yale–Brown强迫症量表(Y-BOCS )
Background: Serotonergic system dysfunction has been implicated in obsessive-compulsive disorder (OCD).This study examined peripheral SLC6A4 gene expression in OCD patients and healthy controls to explore the relationship between SLC6A4 and OCD.
Methods: Participants included 50 first episode OCD patients and 60 age and gender-matched healthy controls. Relative SLC6A4 gene expression were examined by real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) in peripheral leukocytes of all the subjects. The Yale-Brown Obsessive Compulsive Scale (Y-BOCS) was used to assess the severity and subtype of OCD.
Results: SLC6A4 gene expression, normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH), were not significantly different between the OCD patients and healthy controls(z=-0.79, p=0.428). Male OCD patients showed a tendency of low gene expression of SLC6A4 in peripheral blood (z=-1.66, p=0.096). We did not find a significant correlation between SLC6A4 expression and the severity and subtype of OCD.
Conclusion: There is no correlation between SLC6A4 expression levels and the severity and subtype of OCD, but male OCD patients showed a tendency of low gene expression of SLC6A4 in peripheral blood.These results suggest that gene expression of SLC6A4 in peripheral blood may not be a useful biomarker of OCD in the Han Chinese population.
[Shanghai Arch Psychiatry. 2017; 29(3): 146-153.
http://dx.doi.org/10.11919/j.issn.1002-0829.216105]
1The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu Province, China
2Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
3Shanghai Key Laboratory of Psychotic Disorders, Shanghai, China
4Shanghai Jiao Tong University School of Medicine, 227 Chong Qing Nan Rd, Shanghai, China
#The first two authors contributed equally to this study and share first authorship
*correspondence: Dr. Zhen Wang, Professor Zeping Xiao. Mailing address: 600 South Wanping RD, Shanghai, China. Postcode: 200030; E-Mail:xiaozeping88@163.com; wangzhen@smhc.org.cn
背景:有研究提示5 -羟色胺系统的功能障碍与强迫症(obsessive-compulsive disorder, OCD)有关。
目标:本研究拟定量OCD患者的外周SLC6A4基因表达,以探讨SLC6A4和OCD之间的关系。
方法:受试为50例首发OCD患者和60例年龄和性别匹配的健康对照,通过实时定量逆转录聚合酶链反应(real-time quantitative reverse transcription polymerase chain reaction, RT-PCR)定量外周白细胞SLC6A4基因表达。采用Yale-Brown Obsessive Compulsive Scale (Y-BOCS)评估OCD的严重性和亚型。
结果:以甘油醛- 3 -磷酸脱氢酶(GAPDH)标准化定量外周SLC6A4基因表达量,在OCD组和健康对照组(z= 0.79, p = 0.428)之间没有显著差异。男性OCD患者显示外周SLC6A4低基因表达的倾向(z =-1.66, p =0.096)。研究也未能发现SLC6A4基因表达量与OCD的严重性和亚型之间有显著相关性。
结论:SLC6A4基因表达量与OCD的严重程度和亚型之间没有相关性,但男性OCD患者呈外周SLC6A4低基因表达倾向。本研究表明,中国汉族人群的外周SLC6A4基因表达可能并非有效的生物标记物。

Dr. Xuemei Wang obtained her bachelor's degree from Xuzhou Medical University in 2007, and a PhD from Shanghai Jiao Tong University School of Medicine in 2012. She has been working in the department of psychology at the First Affliated Hospital of Soochow University since 2014. She is currently working in a unit specializing in the treatment of Obsessive Compulsive Disorder. Her research interests include etiology and clinical treatments for Obsessive Compulsive Disorder.

Qing Zhao obtained bachelor degree from Shanxi Medical University in 2009, and is currently a master student in Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine.Her research interest is resting state functional Magnetic Resonance Imaging(fMRI) of obsessive compulsive disorder.
- 上海精神医学的其它文章
- Adjunctive melatonin for tardive dyskinesia in patients with schizophrenia: a meta-analysis
- Changes in cognitive function in patients with primary insomnia
- Dysfunction of cognition patterns measured by MATRICS Consensus Cognitive Battery (MCCB) among first episode schizophrenia patients and their biological parents
- The fantasmatic and imaginary child of the pregnant woman
- Is depression the result of immune system abnormalities?
- Autoimmune thyroiditis presenting as psychosis

