空心莲子草根系分泌物对无机磷细菌的负化感效应*
王玉书 刘 海,2 袁 玲†
(1 西南大学资源环境学院,重庆 400716)
(2 贵州省农业科技信息研究所,贵阳 550006)
空心莲子草根系分泌物对无机磷细菌的负化感效应*
王玉书1刘 海1,2袁 玲1†
(1 西南大学资源环境学院,重庆 400716)
(2 贵州省农业科技信息研究所,贵阳 550006)
空心莲子草(Alternanthera philoxeroides Griseb)是全球难以根除的恶性杂草,能在有效磷较低的土壤中生长。了解空心莲子草对微生物转化土壤无机磷的影响,有益于揭示其入侵机制。利用固、液培养技术,以3株(B05、B07和B09)能溶解无机磷的伯克霍尔德氏菌(Burkholderia Yabunchi)为材料,研究了空心莲子草根系分泌物(Exudates from A. philoxeroides roots,EAR)对其生长繁殖和溶磷的化感作用。结果表明,在固体培养时,随EAR浓度提高,无机磷细菌(Phosphatesolubilizing bacteria,PSB)的菌落直径、溶磷圈直径和溶磷指数降低;在液体培养基中,EAR不同程度地抑制PSB繁殖生长,数量减少48.13%~73.03%。供试菌株均能分泌氢离子、草酸和柠檬酸,其中B05和B09还分别能分泌乙酸和苹果酸,B07能分泌乙酸和丁二酸,草酸和柠檬酸共占有机酸分泌总量的66.02%~74.72%。此外,有机酸分泌总量和氢离子分泌量分别与溶磷量呈显著正相关,相关系数依次为0.836和0.947(p<0.05,n=12)。EAR显著抑制PSB分泌有机酸和氢离子,与此同时溶磷量降低11.41%~47.32%。因此,EAR对PSB呈负化感效应,不同程度地抑制PSB繁殖生长、有机酸和氢离子分泌及无机磷溶解。
空心莲子草;化感效应;无机磷细菌
无机磷细菌(Phosphate-solubilizing bacteria,PSB)能溶解土壤中的难溶性磷酸盐,提高磷的生物有效性[1]。将PSB施入土壤,促进难溶性磷酸盐溶解,能使玉米和粟的植株干重增加32%~51%,并提高小麦、燕麦、水稻、玉米、花生、甘蓝和青菜等作物产量,改善水果品质[2-5]。伯克霍尔德氏菌(Burkholderia cepacia IS-16)属于PSB的一种,已应用于多种作物[6]。PSB能分泌氢离子和有机酸,降低局部土壤pH,溶解难溶性无机磷酸盐;有机酸还能络(鳌)合磷酸铁、铝、钙、镁中的金属离子,释放磷酸根或抑制磷酸根固定[7]。Rajikumar[8]和Wani[9]等发现,PSB显著降低土壤重金属活性,减少植物吸收。PSB还能抑制某些病原微生物和昆虫生长,可用于保鲜蔬果和防治植物病虫害[10]。此外,伯克霍尔德氏菌(Burkholderia glathei MB14)能除去湖泊或池塘沉积物中的磷,减轻水体富营养化[11]。
空心莲子草(Alternanthera philoxeroides Griseb)属苋科(Amaranthaceae)多年生草本植物,是全球公认的、难以根除的恶性杂草[12-13],遍及我国20多个省(直辖市)的陆地和水域[14]。在空心莲子草侵入过程中,通过茎叶淋溶、根系分泌和植物残体腐解等途径向土壤生态系统释放多种化感物质,对多种植物和土壤微生物具有毒性,抑制莴苣、小麦、油菜、生菜、萝卜、蚕豆和玉米等作物的种子发芽和植株生长[15-18],选择性地影响微生物的繁殖生长、新陈代谢、蛋白质和细胞结构、DNA复制与修复等[17,19]。PSB多为根际微生物,聚集在根系周围,可提高根际微区磷的生物有效性;另一方面,根系生命活动和分泌物显著影响它们的生长繁殖和生理生态功能[20-22]。空心莲子草在贫瘠土壤中也能较好地生长,排除其他植物,形成单优种群,并富集大量的磷[23-24],可能涉及PSB对土壤难溶性无机磷的转化。因此,研究空心莲子草对土壤PSB的影响很有必要。
本文以伯克霍尔德氏菌(Burkholderia Yabunchi)的3株PSB为供试菌株,通过固、液培养,研究空心莲子草根系分泌物对其生长繁殖、氢离子和有机酸分泌,以及溶磷作用的化感效应,为揭示其入侵机制和有效防除提供科学依据。
1 材料与方法
1.1 供试材料
供试菌株:伯克霍尔德氏菌(Burkholderia Yabunchi)的3株PSB(B05、B07和B09),从重庆市缙云山黄壤(106°20′E,29°49′N,pH 4.34)中分离获得,保存于西南大学资源环境学院微生物实验室。
固体培养基(g):琼脂20、葡萄糖10,磷酸三钙2.5、(NH4)2SO40.5、NaCl 0.2、MgSO4·7H2O 0.1、KCl 0.2、酵母膏0.5、MnSO4·4H2O和FeSO4·7H2O 各0.002,蒸馏水1 000 ml,pH 7.0~7.5,液体培养基不含琼脂。
供试植株:空心莲子草(Alternanthera philoxeroides Griseb),采自重庆市北碚区歇马镇(106°25′E,29°29′N)。
1.2 研究方法
考虑到空心莲子草内含多种化感物质,产生综合化感效应,故试验在固、液体培养基中,加入不同浓度的空心莲子草根系分泌物(Exudates from A. philoxeroides roots,EAR),研究PSB的生长状况和溶磷作用。采集空心莲子草植株(根系量约250 g),洗净,置于去离子水中培养48 h(25±2 ℃,光强15 000 Lex,昼夜交替12 h,根系鲜重∶去离子水体积约1∶1.5),取出植株,剪下根系称重,补充水分至根系鲜重:去离子水体积=1∶2,抽滤,制备出500 ml相当于每ml含0.5 g 鲜根重(用g fresh root weight ml-1表示,缩写为g ml-1)的母液,用细菌过滤器过滤后保存于4 ℃的冰箱中备用。
取保存菌株,用固体培养基接种活化,用液体培养基培养24 h,无菌水稀释至103cfu ml-1。蒸汽灭菌(121 ℃,1.5大气压,30 min,下同)固体培养基,冷却至45~50 ℃,加入EAR母液,使其浓度分别达到0.000(对照)、0.013(低)、0.025(中)和0.050(高)g ml-1,取10 ml倒入直径为6 cm培养皿,冷却后在培养皿中央分别接种0.1 ml B05、B07和B09菌液,避光培养7 d(25±1 ℃),用游标卡尺测量菌落和透明圈直径,计算溶磷指数[25]:

取100 ml液体培养基于250 ml三角瓶中,蒸汽灭菌,冷却后加EAR母液形成浓度分别为0.000(对照)、0.013、0.025、0.050 g ml-1的处理,各接种1 ml B05、B07和B09菌液,摇瓶暗培养(25±1 ℃、75 r min-1)120 h,重复5次。
每隔6、12、18、36、72、120 h吸出5 ml液体培养基,用XSP-6C显微镜(上海迪诺力泰公司)观测计数培养液中的PSB数量。然后,用10 000 r min-1离心10 min,取上清液,用PHS-3C精密酸度计测定pH,钼蓝比色法测定无机磷含量[26],并计算溶磷量(培养液无机磷含量-对照培养液无机磷含量)×培养液体积[27],培养结束后,用0.1 mol L-1HCl酸化培养液,日本日立公司生产的D-7000高效液相色谱仪测定有机酸浓度。色谱条件为:L-7455二级管阵列检测器,Rezex Roa-Organic Acid 300离子交换柱(美国Phenomenex公司生产),进样量20 µl,流动相2.5 mmol L-1H2SO4,流速0.5 ml min-1,柱温35 ℃,压力 450 psi,Diode Array L-7455紫外检测器,检测波长210 nm。所检测的有机酸包括草酸、柠檬酸、苹果酸、丁二酸和乙酸,其出峰时间依次为11.60、13.73、16.05、19.47和23.92 min。
1.3 数据处理
分别用 Excel 2010、SPSS 17.0 和SigmaPlot软件对试验数据进行基本计算、统计分析和作图,采用LSD进行多重比较,显著水平为p<0.05。
2 结 果
2.1 EAR对PSB生长和溶磷的影响
由表1可知,在固体培养时,EAR显著降低B05和B09的溶磷圈直径、菌落直径和溶磷指数,浓度越高,降幅越大;对B07而言,低浓度的EAR提高上述生长和溶磷参数,但中、高浓度仍然使之降低。在高浓度的EAR培养基中,溶磷圈直径、菌落直径和溶磷指数的最大降幅依次为49.09%~67.98%、40.32%~50.00%和9.86%~27.38%。此外,EAR对B09溶磷能力的抑制作用最强,B05次之,B07最小。
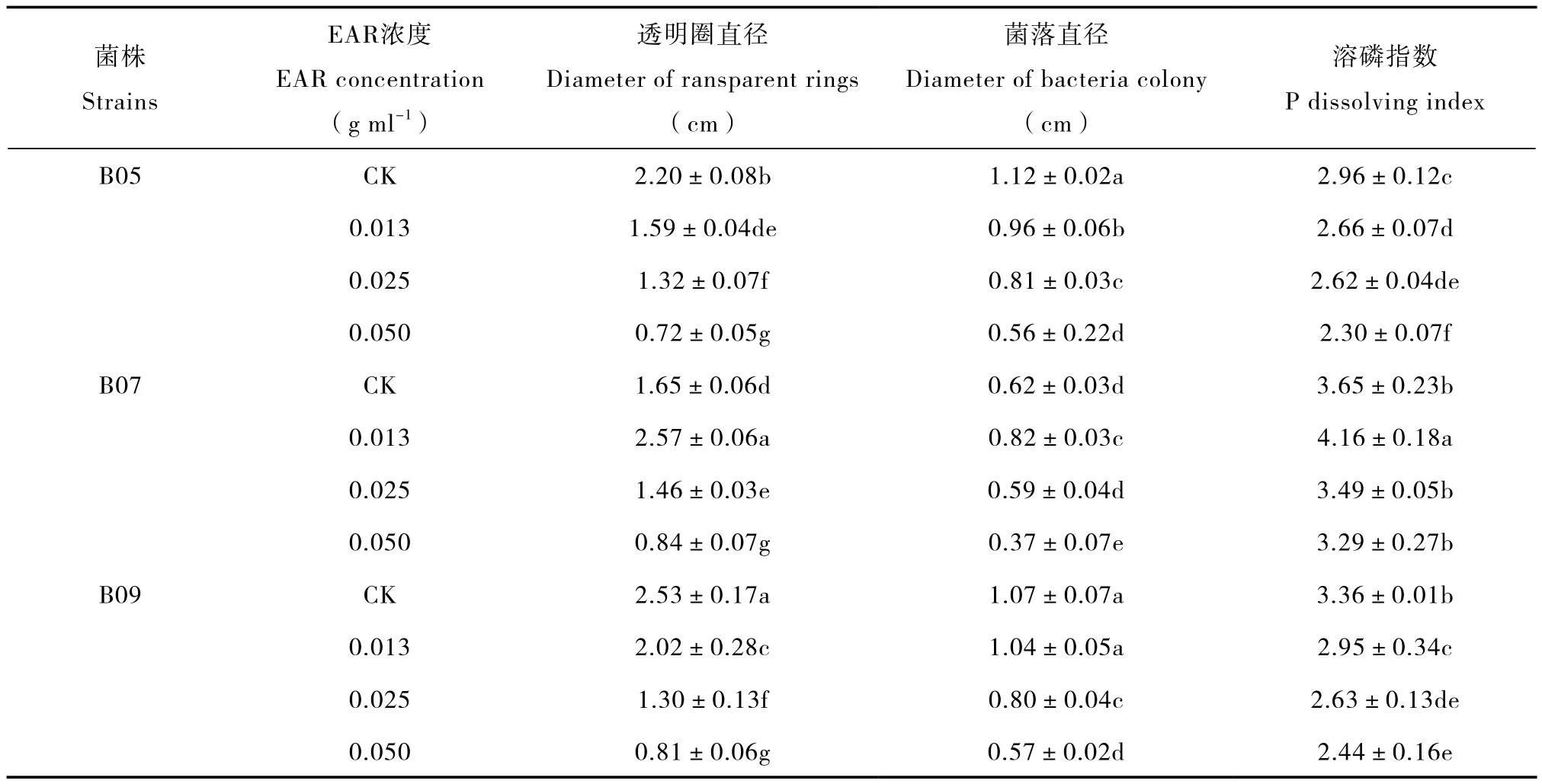
表1 固体培养时EAR对PSB生长和溶磷的影响Table 1 Effects of exudates from A. philoxeroides roots on growth of phosphate-solubilizing bacteria and phosphorus solubilization in solid culture media
在培养液中,PSB的数量因菌株和EAR浓度的不同而异,平均值变化于14.15×108ml-1(B05)~44.79×108ml-1(B07)(图1)。低浓度的EAR增加了B07数量,中、高浓度则相反;B05和B09的数量随EAR浓度提高而降低,最大降幅分别为48.13%和61.21%。
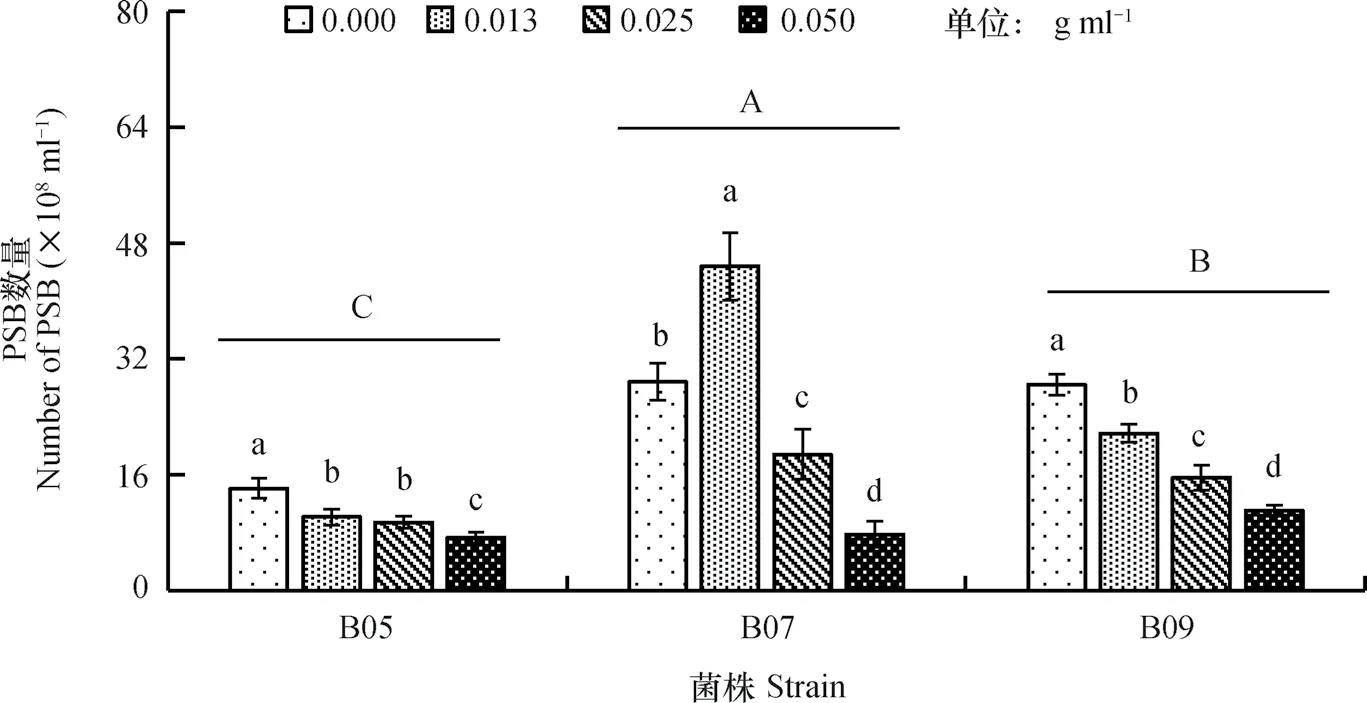
图1 不同浓度EAR培养液中PSB的数量变化Fig. 1 Variation of PSB in population in liquid culture media different in EAR concentration
2.2 EAR对PSB分泌有机酸和氢离子的影响
PSB株系和EAR浓度不同,氢离子和有机酸在液体培养基中的浓度也不同(表2)。
草酸:EAR对B07分泌草酸无显著影响;随EAR浓度提高,B05和B09分泌草酸的速率降低。此外,B05的草酸分泌量最低,在培养液中的平均浓度约为B07和B09的1/2。
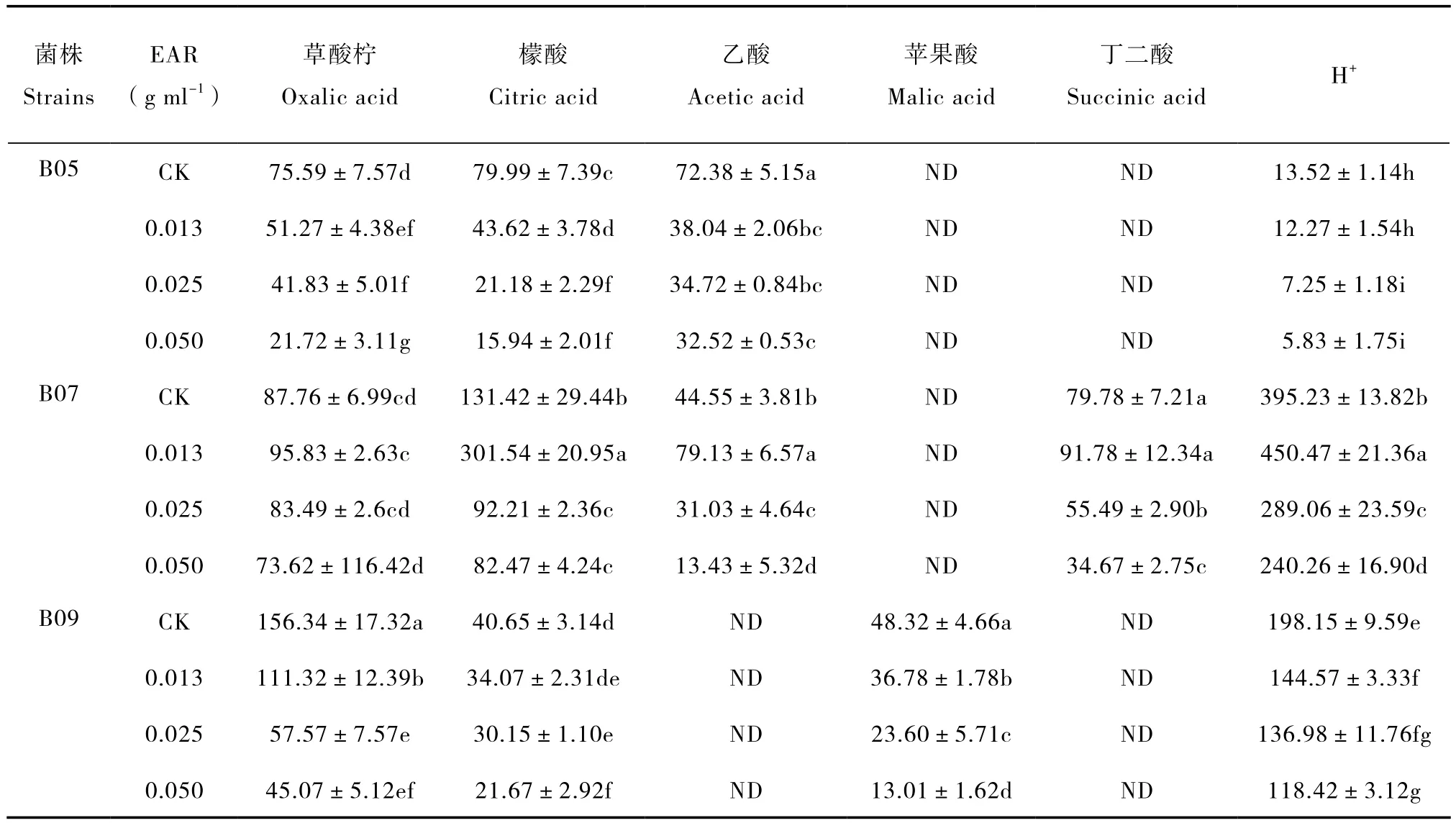
表2 PSB培养液中的有机酸和氢离子浓度Table 2 Concentrations of organic acids(mg L-1)and protons in PSB culture solutions(µg L-1)
柠檬酸:EAR浓度越高,B05和B09分泌柠檬酸的速率愈低;低浓度的EAR促进B07分泌柠檬酸,高浓度表现出抑制作用。此外,B07分泌柠檬酸最多,培养液中的平均浓度是B05和B09的3.78倍和4.80倍。
乙酸:在B09的培养液中,未检测出乙酸。随EAR浓度提高,B05分泌乙酸的速率降低,最大降幅高达55.07%;低浓度EAR促进B07分泌乙酸,高浓度表现出抑制作用。
苹果酸:B05和B07不分泌苹果酸。在B09的培养液中,EAR浓度越高,苹果酸含量越低,最大降幅较对照降低83.03%。
丁二酸:在供试菌株中,B05和B09不分泌丁二酸;中、高浓度的EAR抑制B07分泌丁二酸,其分泌量依次较对照降低30.45%和56.54%。
氢离子:培养液中的EAR浓度越高,B05和B09分泌氢离子的速率愈低;低浓度的EAR促进B07分泌氢离子,中、高浓度EAR产生抑制作用。此外,培养液中的氢离子浓度B07>B09>B05,其平均值依次为343.76、149.53、9.72 µg L-1。
2.3 EAR对PSB溶磷量的影响
图2可见,在培养液中,溶磷量B07>B09>B05。B05和B09的溶磷量(y)与培养时间(x)的关系可用y=x/(a+bx),B07则可用y=y0+a(1-e-bx)c描述。此外,EAR对PSB溶磷能力的影响类似于固体培养,即EAR显著降低B05和B09的溶磷量,浓度越高,降幅愈大;但对B07而言,低浓度的EAR提高溶磷量,中、高浓度仍然使之降低。在培养中加入EAR培养120 h时,溶磷量的最大降幅分别为47.32%(B05)、11.43%(B07)和36.00%(B09)。
2.4 有机酸和氢离子分泌与溶磷量的关系
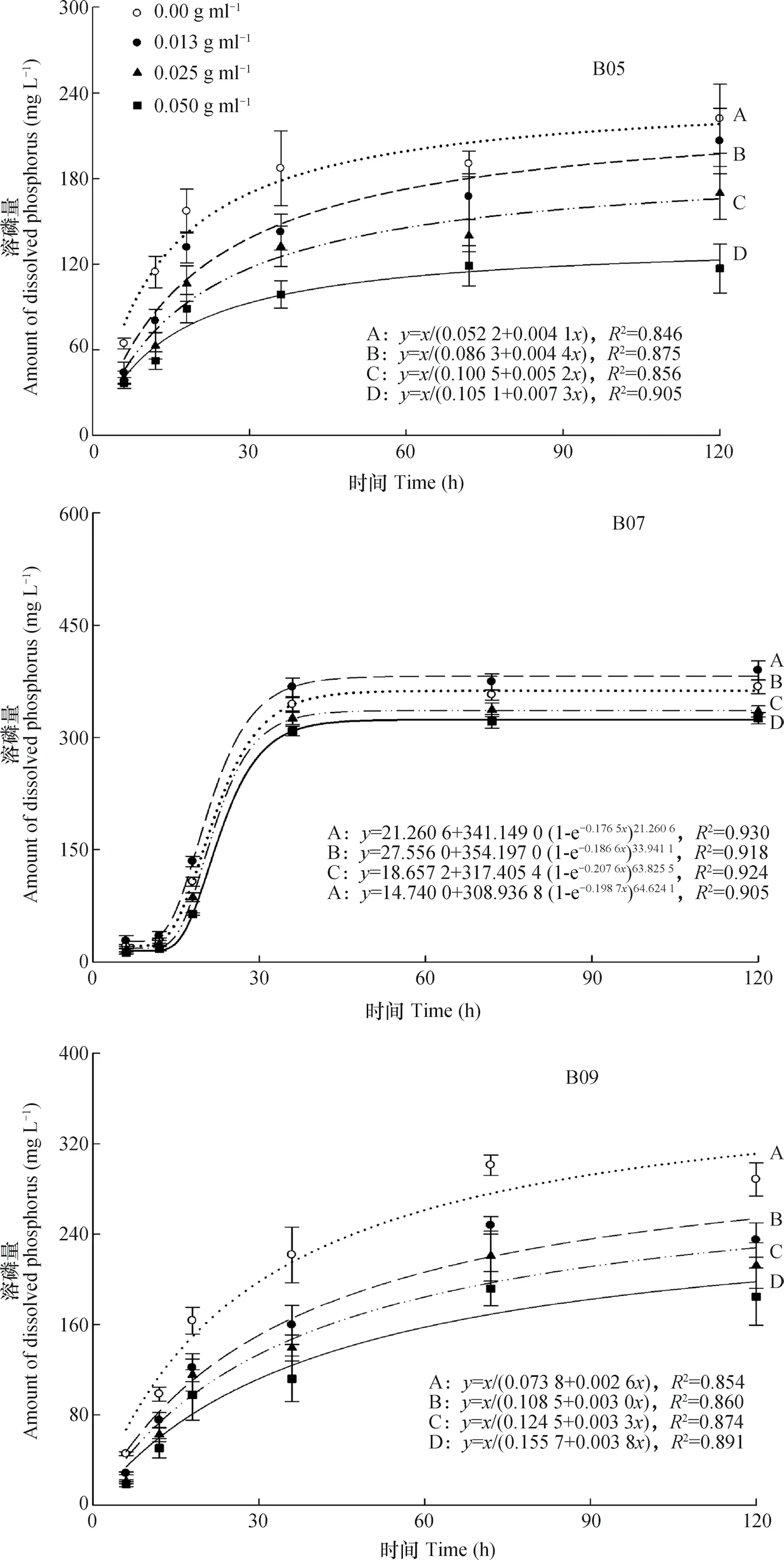
图2 不同浓度EAR培养液中PSB的溶磷量Fig. 2 Content of phosphorus dissolved by PSB in culture media different in EAR concentration
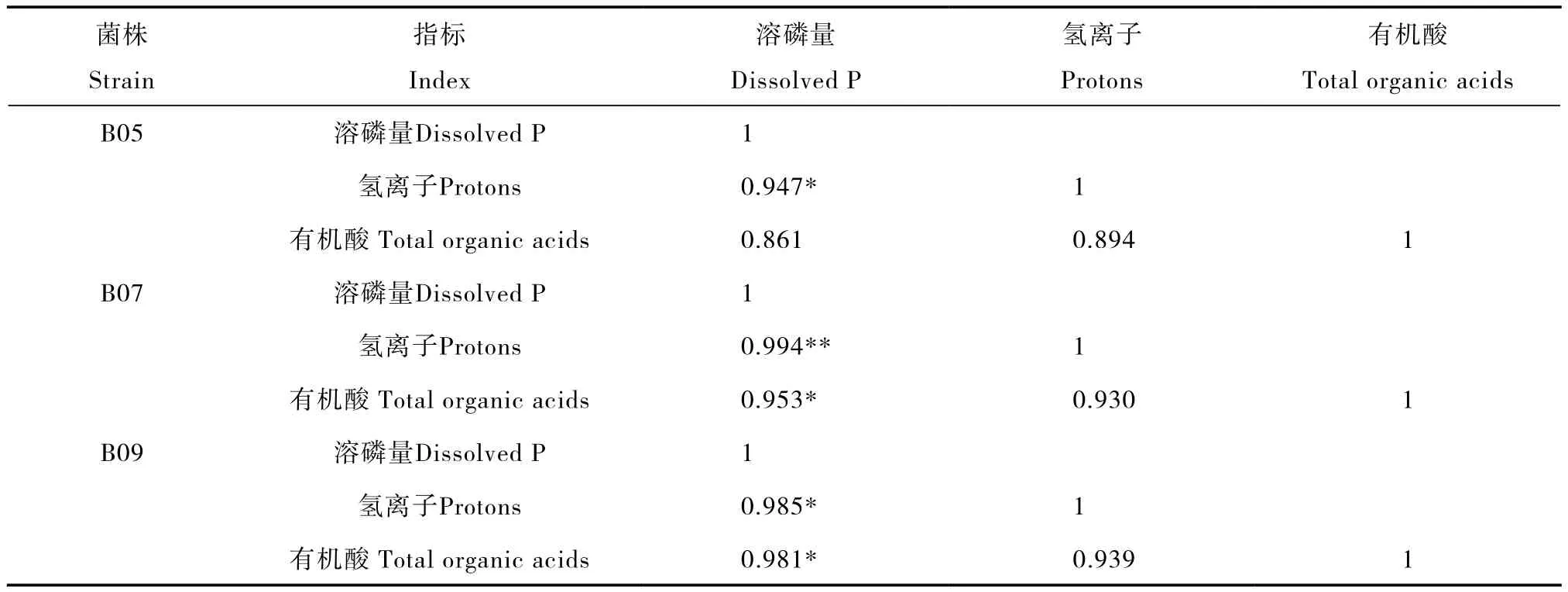
表3 有机酸和氢离子分泌与溶磷量之间的相关系数Table 3 Correlation coefficients of P dissolution with release of organic acids and protons
相关分析表明,在B07和B09培养液中,溶磷量与氢离子和有机酸分泌总量呈显著或极显著正相关;B05的溶磷量与氢离子呈显著正相关,与有机酸分泌总量的相关性未达到显著相关水平(表3)。但是,按三株PSB进行统计,PSB的溶磷量与氢离子和有机酸分泌总量呈显著正相关(r氢离子= 0.947*,r有机酸= 0.836*,n = 12)。此外,PSB的氢离子和有机酸分泌总量之间呈显著正相关,相关系数为 0.844*(n = 12)。
3 讨 论
在固、液培养基中,除低浓度的EAR对B07生长繁殖有一定的促进作用之外,EAR使PSB菌落直径减小,在培养液中的数量减少,说明EAR对PSB具有生物毒性,抑制其生长繁殖。前人研究表明,一定浓度的EAR对脑膜炎球菌(Meningococcus)、肺炎双球菌(Pneumococcus)、草绿色链球菌(Viridans streptococci)、酵母杆菌(Yeast bacillus)、枯草芽孢杆菌(Bacillus subtilis)、苏云金芽孢杆菌(Bvacillus thuringiensis)、金黄色葡萄球菌(Staphylococcus aureus)等也有抑制作用[28-29]。据报道,EAR降低微生物细胞中的Mg2+-ATPase活性,妨碍ATP合成与利用[30];阻止甲型H3N2流感病毒和柯萨奇病毒DNA复制;抑制哈维氏弧菌(Vibrio harveyi)基因表达和RNA合成[31-32],故很有必要继续研究EAR抑制PSB生长繁殖的有关机理。但是,低浓度的EAR促进B07生长繁殖,其原因有待进一步研究。
前人研究发现,在空心莲子草、菖蒲、慈姑、香蒲、狭叶泽泻和泽泻等6种水生植物中,空心莲子草根系与磷的亲和能力最强,吸收磷的速率最快,吸收最多[23]。空心莲子草吸收钾的Cmin(吸收养分要求的最低外源浓度)< 0.2 µmol L-1,Km值(养分吸收速率等于1/2最大吸收速率时的外液浓度)仅8.0~14.0 µmol L-1[24]。说明空心莲子草吸收磷、钾等养分的能力极强,可有效利用低浓度的外源养分,保持体内P2O5高达8.0 mg 100 g-1的积累量[33]。本项研究表明,在EAR抑制PSB繁殖生长的同时,不同程度地降低其溶磷指数和溶磷量。推测在空心莲子草侵入过程中,其根系分泌物也可能抑制PSB溶解土壤难溶性无机磷,不益于提高土壤难溶性无机磷的生物有效性,从而影响其他植物从土壤中获取磷素营养;而空心莲子草则可能依靠极强的养分吸收能力,从贫瘠土壤中摄取低浓度的磷,满足自身营养需要[23],从而扩大自己的种群数量,形成单优种群。
PSB主要依赖于分泌小分子有机酸和氢离子活化土壤难溶性磷酸盐[11]。洋葱伯克霍尔德氏菌(Burkholderia cepacia)分泌有机酸与溶解磷酸三钙、磷酸铁和磷酸铝等密切相关[34]。供试菌株均能分泌氢离子、草酸和柠檬酸,因菌株不同而分泌乙酸、苹果酸和丁二酸,溶磷指数和溶磷量也因菌株不同而异,说明PSB的溶磷机制和溶磷能力存在菌株间的差异。在分泌的有机酸中,柠檬酸和草酸合占有机酸分泌总量的66.02%~74.72%。根据化学原理,草酸和柠檬酸络合钙、铁、铝的能力最强,草酸与钙、铁、铝的络合/沉淀常数分别为3.00、20.20、16.30;柠檬酸与钙、铁、铝的络合/沉淀常数分别为3.42、25.00、20.00,远高于相应钙、铁、铝等离子与磷酸盐的络合/沉淀常数。因此,前人认为,PSB在活化土壤难溶性磷酸盐过程中,草酸和柠檬酸的络合/沉淀作用至关重要[35-36]。此外,草酸和乙酸的离解常数(pKa,25℃)分别为4.27和4.76,可电离出大量的氢离子,故有人发现氢离子能直接溶解难溶性无机磷[35]。在本项研究中,PSB的氢离子和有机酸分泌总量与溶磷量呈显著正相关(r有机酸= 0.836*,r氢离子= 0.947*,n = 12)。因此,有理由推测EAR抑制PSB分泌有机酸和氢离子可能是溶磷圈减小,溶磷指数和溶磷量降低的原因之一。
在PSB培养液中加入EAR,不同程度地降低溶液有机酸和氢离子浓度,说明它们的合成与分泌可能受到抑制。研究表明,PSB合成的有机酸主要源于三羧酸循环(TAC)和次生代谢,有机酸和氢离子分泌涉及细胞膜上的质子泵、载体蛋白和阴离子通道[37]。空心莲子草内含类黄酮、酚类和三萜皂甙类等多种化感物质,类黄酮可破坏微生物细胞膜结构,导致膜电子传递、营养吸收及ATP合成与分解等功能出现障碍[29,38];酚类使蛋白质凝固或变性,降低有机酸生物合成酶的活性[39];在细菌体内,EAR中的某些化学成分可抑制某些与物质代谢有关酶的基因表达和RNA合成[19]。因此,EAR中的化感成分也可能通过破坏PSB细胞膜结构,改变通道蛋白构象,抑制三羧酸循环和次生代谢相关酶的活性或基因表达等,妨碍PSB合成分泌有机酸和氢离子。从细胞和分子水平上进一步开展有关研究有益于揭示空心莲子草的侵入机制。
4 结 论
EAR对PSB呈负化感效应,不同程度地抑制PSB繁殖生长、有机酸和氢离子分泌及无机磷溶解。在空心莲子草侵入过程中,EAR抑制PSB溶解无机磷,无益于提高土壤难溶性无机磷的生物有效性,从而影响其他植物从土壤中获取磷素营养,空心莲子草则通过根系较强的亲和能力吸收磷而满足自身磷营养需要,从而扩大自己的种群数量,排除其他植物,形成单优种群。
[1] 王春红. 大豆根际土壤溶无机磷细菌的溶磷特性研究.长春:吉林农业大学,2015 Wang C H. Solubilizing properties of the inorganic phosphate-solubilizing bacteria isolated from soybean rhizosphere soil(In Chinese). Changchun:Jilin Agricultural University,2015
[2] 唐勇,陆玲,杨启银. 解磷微生物及其应用的研究进展. 天津农业科学,2001,7(2):1—4 Tang Y,Lu L,Yang Q Y. Application and research progress of phosphate-solubilizing microorganisms(In Chinese). Tianjin Agricultural Sciences,2001,7(2):1—4
[3] Yanes M L,De La Fuente L,Altier N,et al.Characterization of native fluorescent Pseuudomonas isolates associated with alfalfa roots in Uruguayan agroecosystems. Biological Control,2012,63(3):287—295
[4] Kumar A,Devi S,Patil S,et al. Isolation,screening and characterization of bacteria fromrhizospheric soils for different plant growth promotion(PGP)activities:An in vitro study. Recent Research in Science and Technology,2012,4(1):1—5
[5] Pastor N,Rosas S,Luna V,et al. Inoculation with Pseudomonas putida PC12,a phosphate solubilizing rhuzobacterium,stimulates,the growth of tomato plants. Symbiosis,2014,62(3):157—167
[6] Rodríguez H,Goire I,Rodríguez M. Caracterización de cepas de Pseudomonas solubilizadoras de fósforo.Rev ICIDCA,1996,30:47—54
[7] 任嘉红,刘辉,吴晓蕙,等. 南方红豆杉根际溶无机磷细菌的筛选、鉴定及其促生效果. 微生物学报,2012,52(3):295—303 Ren J H,Liu H,Wu X H,et al. Screening,identification and promoting effect of phosphatesolubilizing bacteria in rhizosphere of Taxuschinensis var. mairei(In Chinese). Acta Microbiologica Sinica,2012,52(3):295—303
[8] Rajkumar M,Nagendran R,Lee K J,et al. Influence of plant growth promoting bacteria and Cr6+on the growth of Indian mustard. Chemosphere,2006,62(5):741—748
[9] Wani P A,Khan M S,Zaidi A. Chromium reduction,plant growth-promoting potentials and metal solubilizatrion by Bacillus sp. isolated from Alluvial soil. Current Microbiology,2007,54(3):237—243
[10] 林燕青,吴承祯,洪伟,等. 解磷菌的研究进展. 武夷科学,2015,31:161—169 Lin Y Q,Wu C Z,Hong W,et al. Research progress of phosphate-solubilizing microorganisms(In Chinese).Wuyi Science Journal,2015,31:161—169
[11] Kimy H,Bae B,Choung Y K. Optimization of biological phosphorus removal from contaminated sediments with phosphate-solubilizing microorganisms.Journal of Bioscience and Bioengineering,2005,99(1):23—29
[12] Liu C H,Yu D. Invade mechanism and controls of Alternanthera philoxeroides// Xu R M,Ye W H. The theorics and practice of biological invasion. Beijing:Science Press,2004:219—235
[13] Garbari F,Pedulla M L. Alternanthera philoxeroides(Mart.)Griseb.(Amaranthacease),a new species for the exotic flora of Italy. Webbia,2001,56:139—143
[14] 王桂芹,高瑞如,王玉良,等. 异质生境空心莲子草的结构基础与生态适应性. 草业学报,2011,20(4):143—152 Wang G Q,Gao R R,Wang Y L,et al. Structure foundation and ecological adaptability of Alternathera philoxeroides in heterogeneous habitats(In Chinese).Acta Prataculturae Sinica,2011,20(4):143—152
[15] 余柳青,Yoshiharu Fujii,周勇军,等. 外来入侵杂草空心莲子草与本土杂草莲子草的化感作用潜力比较. 中国水稻科学,2007,21(1):84—89 Yu L Q,Fujii Y,Zhou Y J,et al. Comparison of allelopathy potential between an exotic invasive weed Alternanthera philoxeroides and a local weed A. sessilis.Chinese Journal of Rice Science,2007,21(1):84—89
[16] 熊勇,屈睿,王红斌,等. 空心莲子草不同部位水浸提液对蚕豆、玉米化感作用机制的研究. 中国农学通报,2011,27(18):158—163 Xiong Y,Qu R,Wang H B,et al. The study on allelopathy mechanism of aqueous extracts from the different organizations of Alternanthera philoxeroides Griseb on Viciafaba and Zea mays(In Chinese).Chinese Agricultural Science Bulletin,2011,27(18):158—163
[17] 张志忠,石秋香,孙志浩,等. 入侵植物空心莲子草对生菜和萝卜的化感效应. 草业学报,2013,22(1):288—293 Zhang Z Z,Shi Q X,Sun Z H,et al. Alleloapathy of the invasive plant Alternanther philoxeroides to radish and lettuce(In Chinese). Acta Prataculturae Sinica,2013,22(1):288—293
[18] Yang H U,Wang Y,Wen G,et al. The interactive effects of different sterilization levels and aqueous extracts from Alternanthera philoxeroides on the seed germination and seedling growth of Trifoliumrepens.Ecological Science,2015,528:230-237
[19] 李洁,雷波. 空心莲子草水浸提液对蚕豆根尖细胞有丝分裂的影响. 南方农业学报,2013,44(12):1972—1976 Li J,Lei B. Effects of Alternanthera philoxeroides Griseb. aqueous extract on mitosis of Viciafaba root tip cell(In Chinese). Journal of Southern Agriculture,2013,44(12):1972—1976
[20] Illmer P,Schinner F. Solubilization of inorganic phosphates by micro-organisms isolated from forestsoils.Soil Biology amp; Biochemistry,1992,24(2):389—395
[21] Gaind S,Gaur A C. Thermotolerant phosphate solubilization micro-organism and their interaction with mung bean. Plant and Soil,1991,133:141—149
[22] Chabot R,Antoun H,Cescas M P. Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarum biovar. phaseoli. Plant and Soil,1996,184:311-321
[23] 单丹,罗安程. 不同水生植物对磷的吸收特性. 浙江农业学报,2008,20(2):135—138 Shan D,Luo A C. Phosphorus uptake characteristics of six aquatic plants(In Chinese). Acta Agriculturae Zhejiangensis,2008,20(2):135—138
[24] 李钟宝,蔡晨露,刘秀梅. 邻苯二甲酸酯类增塑剂合成与应用研究进展. 塑料助剂,2010(4):8—15 Li Z B,Cai C L,Liu X M. Research progress in synthesis and application of phthalate plasticizers(In Chinese). Plastic Additives,2010(4):8—15
[25] 罗世琼. 黄花蒿土壤微生物与抗疟相关成分的关联性研究. 重庆:西南大学,2010 Luo S Q. Study on correlation between soil microorganism and anti-malaria-related compounds of Artemisia annua L.(In Chinese). Chongqing:Southwest University,2010
[26] 鲁如坤. 土壤农业化学分析方法. 北京:中国农业科技出版. 2000:76—79 Lu R K. Analytical methods for soil and agro-chemistry(In Chinese). Beijing:China Agricultural Science and Technology Press,2000:76—79
[27] 李娟,王文丽,卢秉林,等.甘肃省河西高钙土溶磷菌筛选及其溶磷能力初步研究. 干旱地区农业研究,2008,26(2):8—9 Li J,Wang W L,Lu B L,et al. Selection and measurement of phosphate-solubilizing micro-organisms and their phosphate-dissolving ability in high calcium soil of Hexi corridor in Gansu Province(In Chinese).Agricultural Research in the Arid Areas,2008,26(2):8—9
[28] 范铮,李丹青,张国亮. 空心莲子草不同溶剂提取液的抑菌活性研究. 湖北农业科学,2013,52(6):1317—1319 Fan Z,Li D Q,Zhang G L. Study on the antibacterial activity of extracts from Alternanthera philoxeroides(Mart.)Griseb in different solvents(In Chinese).Hubei Agricultural Sciences,2013,52(6):1317—1319
[29] 程超,李伟,汪兴平,等. 空心莲子草不同提取物抑菌作用及有效成分分析. 食品科学,2007,28(9):52—56 Chen C,Li W,Wang X P,et al. Study on antisepitic effect of Altemanthe raphiloxeroides(Mart.)Griseb extracts and analysis of functional components(In Chinese). Food Science,2007,28(9):52—56
[30] 谭苹,张学俊,杨建明,等. 空心莲子草对钉螺酶组织化学的影响. 中国寄生虫学与寄生虫病杂志,2009,27(1):11—16 Tan P,Zhang X J,Yang J M,et al. Effect of Alternanther aphiloxeroides on enzymichis to chemistry of Oncomelania hupensis(In Chinese). Chinese Journal of Parasitology and Parasitic Diseases,2009,27(1):11—16
[31] 李爽,彭晓芝,廖鹏程,等. 空心莲子草有效部位提取物抗甲型H3N2流感病毒作用. 天津中医药,2012,29(5):478—480 Li S,Peng X Z,Liao P C,et al. Antiviral activity of Alternanther aphiloxeroides effective fraction extracts against virus H3N2(In Chinese). Tianjin Journal of Traditional Chinese Medicine,2012,29(5):478—480
[32] 王薇. 空心莲子草微乳体外抗柯萨奇病毒B3的实验研究. 武汉大学学报医学版,2005,26(6):700—703 Wang W. Antiviral effect of alternanthera philoxeroides microemulsion on coxsackie virus group B3in Vitro(In Chinese). Medical Journal of Wuhan University,2005,26(6):700—703
[33] 王志勇. 空心莲子草入侵对土壤微生物群落结构和土壤酶活的影响. 武汉:华中农业大学,2010 Wang Z Y. Effects of Alternanthera philoxeroides(Mart.)Griseb invasion on soil mierobial community structure and enzyme aetivity(In Chinese). Wuhan:Huazhong Agricultural University,2010
[34] 刘文干,何园球,张坤,等. 一株红壤溶磷菌的分离、鉴定及溶磷特性. 微生物学报,2012,52(3):326—333 Liu W G,He Y Q,Zhang K,et al. Isolation,identification and characterization of a strain of phosphate-solubilizing bacteria from red soil(In Chinese). Acta Microbiologica Sinica,2012,52(3):326—333
[35] 陈时洪. 分析化学. 北京:中国农业出版社,2013 Chen S H. Analytical chemistry(In Chinese).Beijing:China Agriculture Press,2013
[36] 房福力,李玉中,李巧珍,等. 柠檬酸与土壤磷相互作用的研究进展. 中国农学通报,2012,28(18):26—30 Fang F L,Li Y Z,Li Q Z,et al. Research advance in the interaction of citric acid and phosphorous in soils(In Chinese). Chinese Agricultural Science Bulletin,2012,28(18):26—30
[37] 聂磊,全国明,陈路书. 铅胁迫下景天属和花生属植物根系有机酸分泌特性分析. 广东林业科技,2013,29(6):14—20 Nie L,Quan G M,Chen L S. Influence of lead stress on root organic acid exudates of sedum and arachis plants(In Chinese). Guangdong Forestry Science and Technology,2013,29(6):14—20
[38] 游庭活,刘凡,温露,等. 黄酮类化合物抑菌作用研究进展. 中国中药杂志,2013,38(21):3645—3650 You T H,Liu F,Wen L,et al. Advance in studies on antibacterial effect of flavonoids(In Chinese). China Journal of Chinese Materia Medica,2013,38(21):3645—3650
[39] 范金波,周素珍,郑立红,等. 果蔬中酚类成分及其与蛋白质相互作用的研究进展. 食品工业科技,2013,34(19):352—357 Fan J B,Zhou S Z,Zheng L H,et al. Advances on phenol in fruits and vegetables and its interaction with protein(In Chinese). Science and Technology of Food Industry,2013,34(19):352—357
(责任编辑:卢 萍)
Negative Allelopathic Effects of Root Exudate of Alternanthera Philoxeroides on Growth and Phosphate Dissolution of Inorganic Phosphorus Bacteria
WANG Yushu1LIU Hai1,2YUAN Ling1†
(1 College of Resources and Environment,Southwest University,Chongqing 400716,China)
(2 Guizhou Institute of Agricultural Science and Technology Information,Guiyang 550006,China)
【Objective】Alternanthera philoxeroides Griseb,a kind of ill weed hard to eliminate in the world,is able to grow in both soils and waters low in available phosphorus(P)and now widely distributed in over twenty provinces(regions or municipalities)in this country,causing enormous hazard to agriculture,forestry,animal husbandry and aquiculture. It is,therefore,essential to get to know how the weed affects microbes transforming inorganic P in soil. Hopefully,the knowledge will help understand its invasive mechanism,and serve as a scientific basis for effective control of the weed.【Method】Plants of A. philoxeroides were gathered and cultured in deionized water(fresh root weight :deionized water volume = 1∶1.5)for 48 hours formed of two cycles of day and night,12 hours each shift,at 25 ℃±2℃with light intensity being 15 000 Lex in the day shift. Then the plants were removed,leaving the water as root exudate solution of the plant for the follow-on experiment,which was designed to have two types of culture media,liquid and solid,inoculated with three P-dissolving strains of Burkholderia Yabunchi(B05,B07 and B09)and then amended with the root exudate solution at exudates of A. philoxeroides roots(EAR)0.000(CK),0.013,0.025,and 0.050 g ml-1,separately. Bacterial colonies on the solid media were measured for diameters and diameters of their P-dissolving rings and then P-dissolving indexes worked out. And the liquid media were analyzed for proton,organic acids,and dissolved phosphorus with a pH meter,a high performance liquid chromatography(HPLC),and the molybdenum blue colorimetric method,separately.【Result】Bacterial colonies,P-dissolving rings and P-dissolving indexes in the solid culture media decreased with increasing EAR concentration. The effect on was found the highest with Strain B09,which was followed by Strain B05 and B07. In the solid culture media amended with EAR 0.050 g ml-1of the root exudate,bacterial colonies decreased by 40.32%~50.00% in diameter,by 49.09%~67.98%in diameter of P-dissolving rings,and by 9.86%~27.38% in P-dissolving index. Growth of the PSB in the liquid culture media was also inhibited by EAR in varying degrees,and hence their populations reduced by 48.13%~73.03%. In the liquid culture media amended with EAR 0.050 g ml-1,the amount of P dissolved by phosphate-solubilizing bacteria(PSB)decreased by 47.32%(B05),11.43%(B07)and 36.00%(B09)as compared with the control. All the three tested strains of bacteria released protons,oxalate and citrate into culture solutions. Besides these,acetate was also found in the culture solution of B05,malate of B09,and acetate and succinate of B07. Oxalate and citrate together accounted for 66.02%~74.72% of total organic acids released from PSB. In addition,the content of inorganic P dissolved by the bacteria was positively related to the efflux of proton(r = 0.836,p< 0.05,n = 12)and total organic acids(r = 0.947,p<0.05,n = 12). EAR inhibited significantly the release of protons and organic acids from PSB and hence the dissolution of P remarkably.【Conclusion】Obviously EAR has some negative allelopathic effects on PSB,which are reflected in inhibiting reproduction and growth of the bacteria,their release of protons and organic acids,and P-solubilization in varying degrees. In the invasive process of A. philoxeroides,EAR might inhibit PSB solubilizing inorganic P in the soil,thus hindering P mobilization in the soil and reducing P supply to other plants. In contrast,A. philoxeroides is able to absorb P efficiently through its root high in affinity with P to satisfy its own P requirement,which favors multiplication of its own population,competition for P with other plants and formation of pure A. philoxeroides communities.
Alternanthera philoxeroides;Allelopathy;Phosphate-solubilizing bacteria
S144
A
10.11766/trxb201705180092
* 国家重点基础研究发展计划(973计划)项目(2013CB127405)资助 Supported by the National Basic Research Program of China(973 Program)(No.2013CB127405)
† 通讯作者 Corresponding author,E-mail:lingyuanh@aliyun.com
王玉书(1991—),男,山西人,硕士研究生,主要从事土壤微生物研究。Email:969014327@qq.com
2017-05-18;
2017-07-10;优先数字出版日期(www.cnki.net):2017-09-04

