Myanmarorchestia victoria sp. nov. (Crustacea,Amphipoda, Talitridae), a new species of landhopper from the high altitude forests in Myanmar
Ya-Mi Zheng, Zhong-E Hou Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin Nay Pyi Taw 058, Myanmar
Myanmarorchestia victoria sp. nov. (Crustacea,Amphipoda, Talitridae), a new species of landhopper from the high altitude forests in Myanmar
Ya-Mi Zheng1,2, Zhong-E Hou1,*1Key Laboratory of Zoological Systematics and Evolution, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China2Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences, Yezin Nay Pyi Taw 05282, Myanmar
Myanmarorchestia victoria sp. nov. is described from high altitude habitats in Myanmar. The new species differs morphologically from its congeners by palp of maxilliped narrow; sexually dimorphic gnathopod II, propodus of male chelate and propodus of female mitten-shaped; and dimorphic uropod II, outer ramus of male with small teeth distally,outer ramus of female with three distal spines.Analysis of DNA barcode sequences and niche distinctiveness support recognition of the new species.
Taxonomy; Mt. Victoria; COI gene; Leaf litter; Morphology; New species
INTRODUCTION
The landhopper genus Myanmarorchestia Hou & Zhao (2017)currently includes two species, distributed in high altitude forests of Mt. Victoria, Myanmar. Myanmarorchestia species can be found in 3 000 m a.s.l. or higher, and show some vertical distribution patterns. For example, Myanmarorchestia peterjaegeri Hou & Zhao (2017) occurs above 2 000 m a.s.l., while M. seabri Hou & Zhao (2017) inhabits understorey leaf litter around 1 500 m a.s.l.. The genus Myanmarorchestia has the characteristic chelate, sexually dimorphic gnathopod II, simplidactylate pereopods and complexly lobed gills to adapt to terrestrial environments.
Mt. Victoria (Nat Ma Taung National Park) is situated between the Indian subcontinent and Asian continent, and harbours endemic montane species (Jäger, 2015; Jäger & Minn, 2015).From November 2016–April 2017, five field trips were organized by the Southeast Asia Biodiversity Research Institute(SABRI), Chinese Academy of Sciences (CAS), to explore the biodiversity of Myanmar. Following a detailed examination of the specimens, three Myanmarorchestia species were discovered from Mt. Victoria. Of the three Myanmarorchestia species, two species have been published (Hou & Zhao, 2017).In the current study, the third one, Myanmarorchestia victoria sp.nov., is described and illustrated. Moreover, DNA barcodes of the new species are obtained to confirm its distinctiveness.
MATERIALS AND METHODS
Sampling
Fieldworks were conducted in Mt. Victoria, Chin State,Myanmar (Figure 1) from November–December 2016 and from April–May 2017. The specimens were collected by sieving forest floor litter. Samples were preserved in 95% ethanol in the field, then deposited at –20 °C refrigerator for long preservation.Type specimens are lodged in the Institute of Zoology, Chinese Academy of Sciences (IZCAS), Beijing.
Morphology observation1
The body length was recorded by holding the specimen straight and measuring the distance along the dorsal side of the body from the base of the first antenna to the base of the telson. All dissected appendages were mounted on slides according to the methods described by Holsinger (1967), and were drawn using a Leica DM2500 compound microscope equipped with a drawing tube. Terminology and taxonomic descriptions follow Morino (2014). The holotype specimen was used for morphological observation, while one paratype specimen was used for both morphological and molecular parts.
DNA sequencing and COI genetic distance calculation
DNA barcode of the mitochondrial cytochrome oxidase subunite I (COI) was amplified and sequenced to obtain the geneticdistances between morphologically similar species and confirm identifications (Hou et al., 2009; Suzuki et al., 2017).The primers used are CRUSTF2 (5′-GGTTCTTCTCCACC AACCACAARGAYATHGG-3′) and HCO2198 (5′-TAAACTT CAGGGTGA CCAAAAAATCA-3′). Genomic DNA extraction,amplification and sequencing procedures were performed as in Hou et al. (2007). The new sequence was deposited in GenBank.
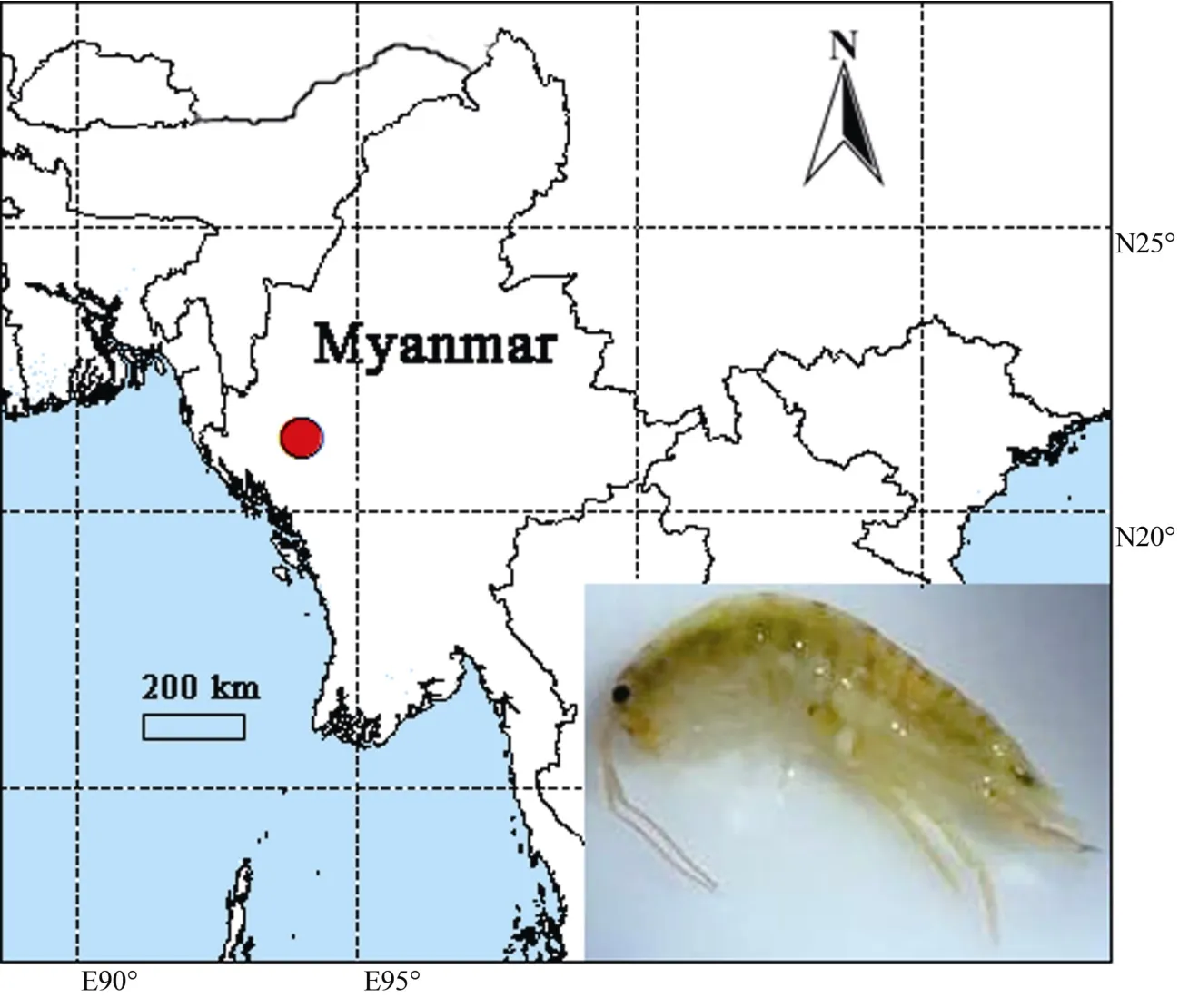
Figure 1 Collection locality of Myanmarorchestia victoria sp. nov. from Myanmar (red circle=type locality) (photo by Jiang-Lang Wu)
The COI gene sequences were manually aligned, because no indels were observed. Genetic uncorrected p-distances among the known Myanmarorchestia taxa were calculated using MEGA7.0.16 (Kumar et al., 2016).
TAXONOMY
Family Talitridae Rafinesque (1815)
Genus Myanmarorchestia Hou & Zhao (2017)
Myanmarorchestia victoria Hou sp. nov. (Figures 1–7)
Material examined: Holotype: male (IZCAS-I-A2087-1), 14 mm,near 17.5 km of the roadside between Kanpetlet to Nat Ma Taung National Park, Chin State, Myanmar (E93.94°, N21.22°),altitude 2 654 m a.s.l., collected by J. Wu and Z. Chen on April 30, 2017. Paratype: female (IZCAS-I-A2087-2), 11 mm, same data as holotype, GenBank accession No. MF969263;paratypes, two males and one female (IZCAS-I-A2087-3).
Etymology: The species name is derived from the type locality,noun in apposition.
Diagnosis: The new species assigns to Myanmarorchestia on the basis of the following morphological characteristics: (1) simple gnathopod I in both sexes; (2) sexually dimorphic gnathopod II,propodus of male chelate and produced on ventral margin,propodus of female mitten-shaped; (3) simplidactylate pereopods III–VII; and (4) complexly lobed coxal gills. The new species is characterized by a combination of the following morphological characteristics: (1) mandible spine row with four plumose setae; (2) maxilliped palp article 2 narrow, article 3 not lobate, article 4 distinct; (3) gnathopod I coxal plate not produced on anterior margin; (4) gnathopod II strongly dimorphic, propodus of male produced, triangle-shaped,propodus of female mitten-shaped; (5) uropod II sexually dimorphic, outer ramus of male weak, with small teeth distally;and (6) uropod III peduncle with one strong posterodistal spine.
Description of holotype male (IZCAS-I-A2087-1), 14 mm
Head: eyes rounded, medium in size, about 35% of head length (Figure 2A).
Antenna I (Figure 2B): reaching 36% of antenna II, peduncle articles 1–3 in length ratio 1.0: 0.8: 1.2; flagellum with seven articles (six large ones and one tiny distal one), a little shorter than peduncle, each article with short distal setae.
Antenna II (Figure 2C): peduncle articles 3–5 in length ratio 1.0: 1.7: 2.5, with setae on anterior and posterior margins;flagellum with 15 articles, each article with setae on dorsal and ventral margins.
Upper lip (Figure 2D): ventral margin rounded, bearing minute setae.
Mandible (Figures 2F, G): incisor of left mandible with five teeth;lacinia mobilis with four teeth; spine row with four plumose setae;molar with a plumose seta; incisor of right mandible with four teeth, lacinia mobilis bifurcate, with small teeth.

Figure 2 Myanmarorchestia victoria sp. nov., male holotype (IZCAS-I-A2087-1)
Lower lip (Figure 2E): inner lobes indistinct, outer lobes covered with thin setae.
Maxilla I (Figure 2H): inner plate with two terminal strong setae, outer plate with nine apical spines (three of them bifid),palp with one article.
Maxilla II (Figure 2I): inner plate narrower and shorter than outer plate, with one plumose seta and numerous simple setae on medial margin, outer plate with two rows of apical spines.
Maxilliped (Figure 2J): inner plate, with one stout apical spine and 12 plumose setae; outer plate bearing eight simple setae and two plumose setae apically; palp with four articles,articles 1–2 not broad; articles 1–3 subequal in length ratio;articles 2–3 with fine setae; article 3 with two spines on interior margin, two setae on exterior margin and two setae on ventral surface; article 4 small but distinct, with two simple setae apically.
Pereon
Gnathopod I (Figures 3A, B): coxal plate broad, bearing seven setae on ventral margin; basis with short setae on anterior and posterior margins; merus, carpus, and propodus in length ratio 1.0: 1.5: 1.1; merus bearing setae on posterior margin; carpus with setae on anterior and posterior margins; propodus simple,with setae on anterior margin and five spines accompanied by setae on posterior margin; dactylus with two spines on posterior margin and three setae at hinge of unguis.
Gnathopod II (Figures 3C, D): coxal plate ventral margin with ten setae, posterior process prominent; basis with a fine seta on posterior margin; merus protuberant on posterior margin;carpus 1.7 times as long as wide, with tumescent hump at posterodistal corner; propodus with tumescence, subtriangular,with setae on surface, palm margin anteriorly slant, forming chela, with two rows of spines (a lateral and a medial one);dactylus as long as palm, with setae on posterior margin.
Pereopod III (Figures 3E, I): coxal plate with posterior cusp,bearing eight setae on ventral margin; basis longest, with spines on anterior and posterior margins; merus, carpus, and propodus in length ratio 1.0: 0.8: 1.0; carpus and propodus with spines on posterior margins; dactylus with two spines at hinge of unguis. Pereopods III–VII simplidactylate.
Pereopod IV (Figures 3F, J): similar but shorter than pereopod III; coxal plate with posterior cusp, bearing nine setae on ventral margin; merus, carpus, and propodus in length ratio 1.0: 0.8: 1.1, dactylus weakly pinched.
Pereopod V (Figures 3G, K): coxal plate bilobed, anterior lobe larger than posterior lobe, bearing five setae and two setae on anterior and posterior lobes, respectively; basis suboval, with four spines on anterior margin and eight setae on posterior margin, anterodistal corner with two spines; merus, carpus, and propodus in length ratio 1.0: 1.1: 1.5, with spines on both margins; dactylus with two spines at hinge of unguis.
Pereopod VI (Figures 3H, L): coxal plate bilobed, anterior lobe much smaller than posterior lobe, bearing one seta on anterior lobe and two setae on posterior lobe; basis suboval,with six spines on anterior margin and seven setae on posterior margin, anterodistal corner with two spines; merus, carpus, and propodus in length ratio 1.0: 1.3: 1.7, with spines on both margins; propodus and dactylus slender, dactylus with two spines at hinge of unguis.
Pereopod VII (Figures 4A, B): coxal plate unilobate, shallow,with five setae on posterodistal margin; basis oval, with five setae on anterior margin and 12 setae on posterior margin,anterodistal corner with two spines; merus, carpus, and propodus in length ratio 1.0: 1.3: 1.6, with spines on both margins; propodus and dactylus slender, dactylus with two spines at hinge of unguis.
Coxal gills (Figures 3C, E–H): present on gnathopod II and pereopods III–VI, complexly lobed and convoluted; gill of gnathopod II broad, with ridged margin; gills of pereopods III–VI sac-shaped.
Pleon
Epimeral plates (Figures 4C–E): acuminate posterodistally,distal margins without armature; plate I with four fine setae on posterior margin; plate II with two fine setae on posterior margin;plate III with two fine setae on posterior margin.
Pleopods I–III (Figures 4F–H): similar, peduncle with two retinacula on interior margin, exterior margin with dense plumose setae; outer ramus about 85% of peduncle, outer ramus about 70% of inner ramus, both inner and outer rami fringed with plumose setae.
Urosome
Uropods I–III (Figures 4I–K): uropod I peduncle longer than rami, with three spines on interior margin and three spines on exterior margin, distolateral spine longer than subdistal one;inner ramus with four spines on interior side and four terminal spines; outer ramus marginally bare, with three terminal spines.Uropod II short, peduncle bearing one spine on interior margin and six spines on exterior margin; inner ramus with three spines on interior side and five terminal spines; outer ramus weak,shorter than inner ramus, with one spine on interior side and some small teeth distally (we have examined all three males to confirm this unique state). Uropod III peduncle expanded, with one seta on dorsal margin and one strong posterodistal spine;ramus short, about 0.5 times as long as peduncle, with one long slender spine and one short spine apically.
Telson (Figure 4L): apically notched, about 7% of depth; each lobe with one apical spine.
Description of paratype female (IZCAS-I-A2087-2), 11 mm Head (Figures 5A–I): similar to that of male except Antenna II peduncle articles 3–5 in length ratio 1.0: 2.0: 3.2; Maxilliped inner plate with three or four apical spines.
Pereon
Gnathopod I (Figures 6A, B): propodus with interlocking setae for dactylus.
Gnathopod II (Figures 6C, D): coxal plate ventral margin with seven setae; basis slender; merus protuberant on posterior margin; carpus with tumescent hump at posterodistal comer,with two setae on anterior margin; propodus mitten-shaped,with tumescence, with setae on surface and palm margin;dactylus shorter than palm margin.
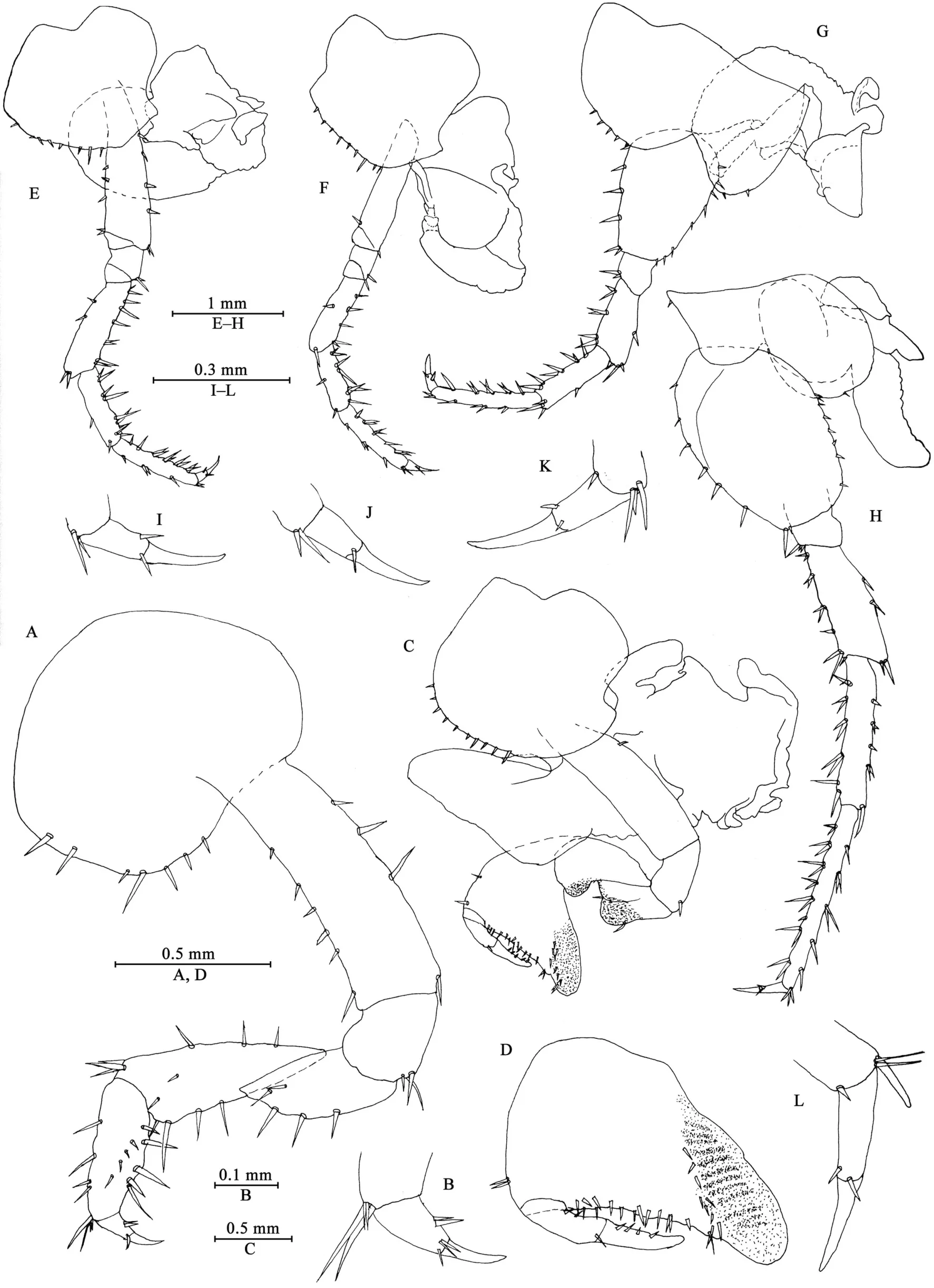
Figure 3 Myanmarorchestia victoria sp. nov., male holotype (IZCAS-I-A2087-1)

Figure 4 Myanmarorchestia victoria sp. nov., male holotype (IZCAS-I-A2087-1)
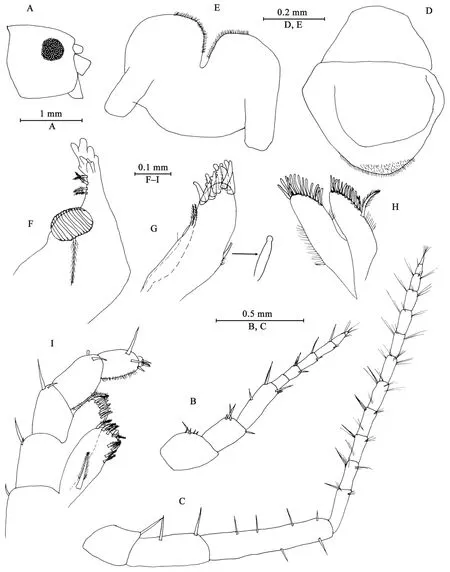
Figure 5 Myanmarorchestia victoria sp. nov., female paratype (IZCAS-I-A2087-2)
Pereopods III–VII (Figures 6E–L, 7A, B): similar to those of male.
Coxal gills (Figures 6C, E–H): present on gnathopod II and pereopods III–VI, complexly lobed and convoluted; gill of gnathopod II broad, with marginal filamentous projections; gill of pereopod III and IV similar, lobed and convoluted, with weakly ridged margins; gill of pereopod V with ridged margin; gill of pereopod VI smallest.
Oostegites (Figures 6C, E, F): present on gnathopod II and pereopods III–IV, slender, with setae on apical margins;oostegite of pereopod V missing.
Pleon
Epimeral plates (Figures 7C–E): acuminate posterodistally,ventral margins without armature; posterior margins with two fine setae.
Pleopods I–III (Figures 7F–H): similar, peduncle with two retinacula on interior margin, exterior margin with dense plumose setae; outer ramus about 86% of peduncle, outer ramus about 76% of inner ramus, both inner and outer rami fringed with plumose setae.

Figure 6 Myanmarorchestia victoria sp. nov., female paratype (IZCAS-I-A2087-2)

Figure 7 Myanmarorchestia victoria sp. nov., female paratype (IZCAS-I-A2087-2)
Urosome
Uropods I–III (Figures 7I–K): uropod I (Figure 7I) peduncle longer than rami, with four spines on interior margin and five spines on exterior margin, distolateral spine distinct, longer than subdistal one; inner ramus with four spines on interior side and five terminal spines; outer ramus marginally bare, with four terminal spines. Uropod II (Figure 7J) short, peduncle bearing one spine on interior margin and six spines on exterior margin;inner ramus with four spines on interior side and five terminal spines; outer ramus shorter than inner ramus, with three terminal spines. Uropod III (Figure 7K) peduncle expanded, with one simple spine on dorsal margin and one strong spine on posterodistal corner; ramus short, about 0.3 times as long as peduncle, with one long slender spine and one short spine apically.Telson (Figure 7L): apically notched, about 5% of depth; each lobe with one apical spine.
Habitat: This species was collected from a disturbed primary forest, with bamboo and understorey leaf litter, with altitude 2 654 m a.s.l. in Mt. Victoria.
Remarks: Myanmarorchestia victoria sp. nov. is most similar to M. seabri in maxilla I palp with one article, coxal gills convoluted,uropod II sexually dimorphic, and telson bare on surface. The new species can be distinguished from M. seabri by the following characters (M. seabri in parentheses): (1) maxilliped palp article 2 narrow (broad); (2) gnathopod I coxal plate not produced on anterior margin (produced proximally); (3) coxal gills of pereopods IV–V lobed and convoluted, with no filamentous projections (with ridged margins and filamentous projections); and (4) uropod III peduncle with one strong posterodistal spine (two posterodistal spines).
Myanmarorchestia victoria sp. nov. can be distinguished from M. peterjaegeri by the following characters (M.peterjaegeri in parentheses): (1) palp of maxilla I with one article (with two small articles); (2) maxilliped palp article 2 narrow (broad); (3) coxal gills lobed and convoluted, with no filamentous projections (with more filamentous projections); and(4) uropod II sexually dimorphic, outer ramus of male weak,with small teeth distally (similar for male and female, with three or four terminal spines). Distinguishing features of Myanmarorchestia species can be found in the key below.
The uncorrected p-distance among the three Myanmarorchestia species ranged from 14.8%–18.8% for COI gene. The new species differed from M. peterjaegeri and M. seabri by 17.5%and 14.8%, respectively. High genetic diversity between the new species and the other species suggests it could be a new species, in comparison with previous molecular threshold (16%)used for crustacean species delimitation (Hou & Li, 2010;Lefébure et al., 2006).
In addition, the new species of M. victoria is located higher elevation at 2 654 m a.s.l. than M. peterjaegeri at 2 150 m a.s.l.and M. seabri at 1 585 m a.s.l., with up to 500 a.s.l.m elevation difference. According to their weak dispersal potential, the vertical barrier may have promoted the speciation events of the genus Myanmarorchestia.
Numerous differences in morphology, barcode sequences and niches give support to recognizing the new species.Accordingly, the exploration of biodiversity of Myanmar is necessary in the future.
Key to the species of Myanmarorchestia
1 Coxal gills with filamentous projections, uropod II similar in both sexes........................................M. peterjaegeri Hou, 2017
– Coxal gills with few filamentous projections, uropod II sexually dimorphic....................................................................2
2 Maxilliped palp article 2 broad..................M. seabri Hou, 2017
– Maxilliped palp article 2 narrow..................M. victoria sp. nov.
ACKNOWLEDGEMENTS
We are grateful to Jiang-Lang Wu and Zhi-Gang Chen for their assistance in the field. We thank Prof. Hiroshi Morino, Prof. Alan Myers, Prof. Cédric d'Udekem d'Acoz, and five anonymous reviewers for their constructive comments.
Holsinger JR. 1967. Systematics, speciation, and distribution of the subterranean amphipod genus Stygonectes (Gammaridae). Bulletin of the United States National Museum, 259: 1-176.
Hou Z, Zhao S. 2017. A new terrestrial talitrid genus, Myanmarorchestia,with two new species from Myanmar (Crustacea, Amphipoda, Talitridae).ZooKeys, 705: 15-39.
Hou Z, Li Z, Li S. 2009. Identifying Chinese species of Gammarus(Crustacea: Amphipoda) using DNA barcoding. Current Zoology, 55(2): 158-164.Hou Z, Li S. 2010. Intraspecific or interspecific variation: delimitation of species boundaries within the genus Gammarus (Crustacea, Amphipoda,Gammaridae), with description of four new species. Zoological Journal of the Linnean Society, 160(2): 215-253.
Hou Z, Fu J, Li S. 2007. A molecular phylogeny of the genus Gammarus(Crustacea: Amphipoda) based on mitochondrial and nuclear gene sequences. Molecular Phylogenetics and Evolution, 45(2): 596-611.
Jäger P. 2015. Conductor-less and vertically niched: new species of the genus Pseudopoda (Araneae: Sparassidae: Heteropodinae) from Myanmar.Arachnology, 16(9): 333-350.
Jäger P, Minn MZ. 2015. New species in the family Ctenidae Keyserling,1877 from high altitude habitats in Myanmar, with the first case of penetration of the female's cuticle by a male in the RTA-clade (Arachnida:Araneae: Ctenidae). Zootaxa, 3994(2): 235-252.
Kumar S, Stecher G, Tamura K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33(7): 1870-1874.
Lefébure T, Douady CJ, Gouy M, Gibert J. 2006. Relationship between morphological taxonomy and molecular divergence within Crustacea:proposal of a molecular threshold to help species delimitation. Molecular Phylogenetics and Evolution, 40(2): 435-447.
Morino H. 2014. A new land-hopper genus, Mizuhorchestia, from Japan(Crustacea, Amphipoda, Talitridae). Bulletin of the National Museum of Nature and Science, Series A, 40(3): 117-127.
Rafinesque CS. 1815. Analyse de la Nature ou Tableau de L’univers et des Corps Organisés. Palermo: l’Imprimerie de Jean Barravecchia, 1-224.
Suzuki Y, Nakano T, Nguyen ST, Nguyen ATT, Morino H, Tomikawa K. 2017.A new landhopper genus and species (Crustacea: Amphipoda: Talitridae)from Annamite Range, Vietnam. Raffles Bulletin of Zoology, 65: 304-315.
05 July 2017; Accepted: 22 August 2017
s: This study was supported by the Southeast Asia Biodiversity Research Institute, Chinese Academy of Sciences(Y4ZK111B01), the National Natural Sciences Foundation of China(NSFC-31422048/31372156), and a grant for Science and Technology Basic Research (2014FY210700)
*Corresponding author, E-mail: houze@ioz.ac.cn
10.24272/j.issn.2095-8137.2017.067
- Zoological Research的其它文章
- Nine new species of the spider genus Stedocys(Araneae, Scytodidae) from China and Thailand
- A new species of rain-pool frog (Dicroglossidae:Fejervarya) from western Thailand
- A new species of the Southeast Asian genus Opisthotropis (Serpentes: Colubridae: Natricinae) from western Hunan, China
- Bird diversity in northern Myanmar and conservation implications
- A new species of sisorid catfish of the genus Exostoma from the Salween drainage, Yunnan, China
- Five newly recorded Cyprinid fish (Teleostei:Cypriniformes) in Myanmar

