Effects of Bupleurum extract on blood metabolism, antioxidant status and immune function in heat-stressed dairy cows
CHENG Jian-bo, FAN Cai-yun, SUN Xian-zhi, WANG Jia-qi, ZHENG Nan, ZHANG Xing-kai, QlN Jun-jie, WANG Xiu-min
1 College of Animal Science and Technology, Anhui Agricultural University, Hefei 230036, P.R.China
2 Laboratory of Quality and Safety Risk Assessment for Dairy Products of Ministry of Agriculture, Institute of Animal Science,Chinese Academy of Agricultural Sciences, Beijing 100193, P.R.China
3 State Key Laboratory of Animal Nutrition, Institute of Animal Science, Chinese Academy of Agricultural Sciences, Beijing 100193,P.R.China
4 Shanghai Bright Holstan Co., Ltd., Shanghai 200436, P.R.China
5 Beijing Centre Biology Co., Ltd., Beijing 102206, P.R.China
1.lntroduction
Heat stress not only reduces production performance(Collier et al.2006; Hammami et al.2012) but also has adverse effects on the health status of dairy cows.These adverse effects include an increased incidence of metabolic disorders (Wheelock et al.2010), in addition to increased oxidant stress (Lin et al.2006) and impaired immune function(Carroll et al.2012).Although many nutrition strategies,such as increasing the energy density of diets (Wang et al.2010) and rumen-protected niacin supplementation(Zimbelman et al.2013), have been used to alleviate heat stress, heat stress remains a costly issue for the global dairy industry (St-Pierre et al.2003).Consequently, new strategies need to be developed that can help maintain dairy cows’ health during hot summers.
The genus Bupleurum, a major ingredient of oriental folk medicine, has been widely used in Asia for the treatment of many diseases over the past 2 000 years (Ashour and Wink 2011).It contains many secondary metabolites,such as polysaccharides (Sun et al.1991), saikosaponins(Navarro et al.2001), and essential oil (Martine et al.1993).Saikosaponin constitutes the main class of secondary metabolites in the genus Bupleurum, amounting for up to 7% of roots (Ashour and Wink 2011).Plants belonging to the genus Bupleurum have various pharmacological functions, with studies reporting antipyretic properties(Huang 1993), anti-inflammatory activity in rats (Martin et al.1993), antioxidant activity in rats (Zhao et al.2012),and immunostimulant activity in mice (Sun 2006).Previous research on Bupleurum has focused on humans and mice,whereas there has been little research on the effect of Bupleurum supplementation on cows.Our previous study suggested that Bupleurum extract (BE) could mitigate negative effects of heat stress on production in lactating Holstein cows (Pan et al.2014), but the effects of direct supplementation with BE on blood metabolites, antioxidant status, and immune responses in heat-stressed lactating Holstein cows are unknown.Therefore, the objective of this study was to investigate the effects of oral administration of BE on blood metabolites, antioxidant status, and immune function of lactating Holstein cows subjected to heat stress.
2.Material and methods
2.1.Animals, diet and experimental design
All animals included in this study were maintained according to the principles of the Animal Care and Use Committee of the Chinese Academy of Agricultural Sciences.Forty lactating Holstein cows, (37.5±1.8) kg of milk d-1, (75±15) d in milk, and (1.7±0.4) parity, were categorized according to average daily milk yield, parity, and day in milk and randomly assigned to 1 of the following 4 treatments (n=10):0 (control), 0.25, 0.5, or 1.0 g of BE kg-1dry matter.A commercially available BE product was used (Beijing Centre Biology Co., Ltd., China) containing 35.0% BE (a mixture of 6.6% saikosaponins, 2.5% essential oil, and 25.9%polysaccharides) and 65.0% starch mix.In dairy cows, heat stress occurs when the temperature-humidity index (THI)exceeds 72 (Armstrong 1994).During the experiment, the mean THI in the barn in which the animals were housed was 78.2 (range: 71.9 to 80.8) at 06:00 h, 79.7 (range: 72.7 to 83.3) at 14:00 h, and 78.3 (range: 70.2 to 81.7) at 22:00 h(Pan et al.2014).
The basal diet (Table 1) was formulated to meet or exceed nutrient recommendations (NRC 2001).Feedstuffs were offered (roughage first and then concentrated feed) daily at 05:30, 13:00, and 20:00 h to ensure 5% refusals, and the BE supplements were top dressed in equal portions to concentrated feed in the morning feeding.All the cows were assigned to an individual feeding column cangue,with access to fresh water.The experimental period lasted 10 weeks.
2.2.Sampling procedure
Blood samples were collected from the tail vein of each cow before the morning feed on days 0, 21, 42, and 63 of the experimental period.Blood samples were collected in EDTA-treated tubes for the analysis of CD4+and CD8+T lymphocytes.Additional blood samples were collected inserum tubes and centrifuged at 3 000×g for 10 min at 4°C.The serum samples were then stored at -40°C until analysis of the blood metabolites, antioxidant enzyme activities, and immune index.
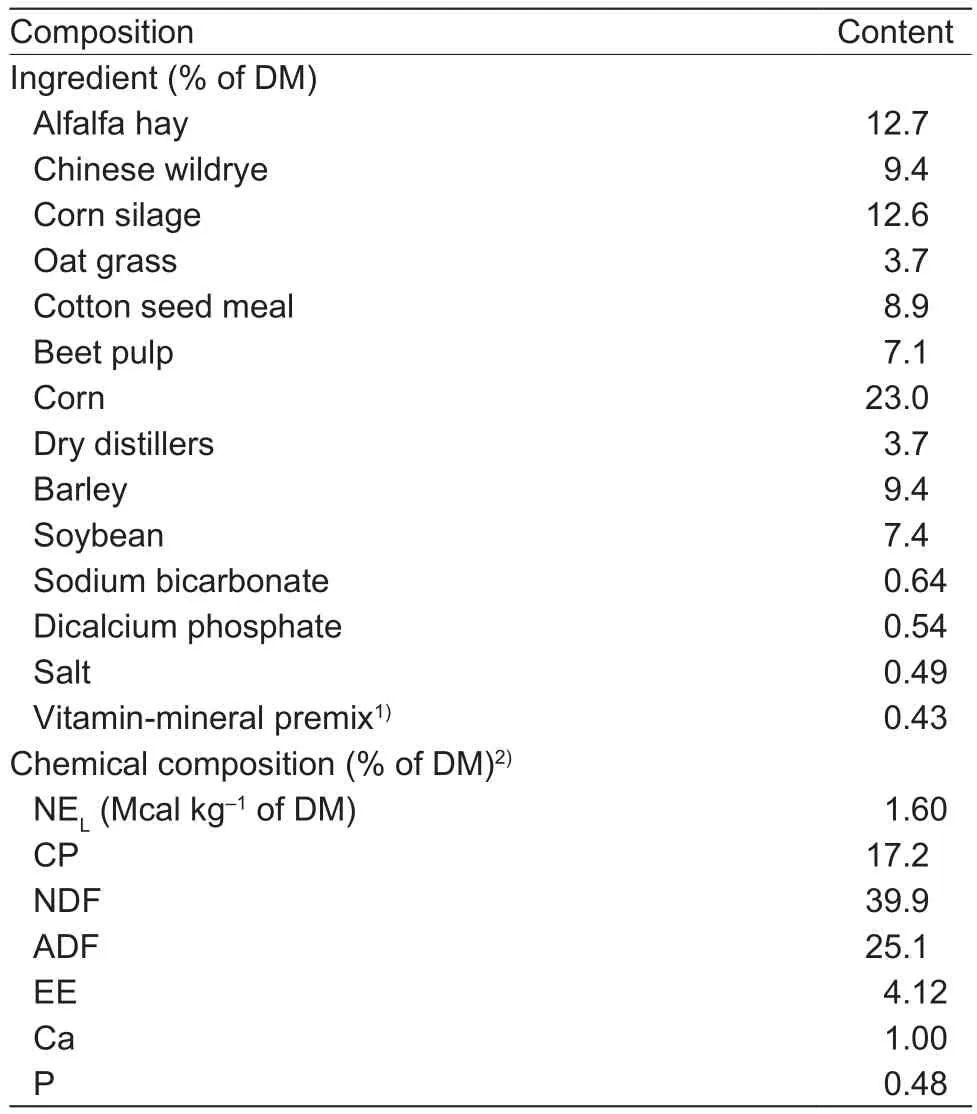
Table 1 Ingredients and chemical composition of basal diets(dry matter (DM) basis)
2.3.Analytical procedures
Blood metabolites were analyzed at 37°C using an automatic biochemistry analyzer (SYNCHRON CX5 PRO; Beckman Coulter, Fullerton, CA, USA).Glucose (GLU), triglyceride(TG), total cholesterol (TC), total protein (TP), albumin(ALB), blood urea nitrogen (BUN), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol(LDL-C) were analyzed using commercial kits (Instrumentation Laboratory, Lexington, MA, USA).Nonesterified fatty acid(NEFA) content was also analyzed using a commercial kit(Sekisui Medical Technology Ltd., Beijing, China).
Serum superoxide dismutase (SOD) activity, total antioxidant capacity (T-AOC), glutathione peroxidase(GSH-Px) activity, and malondialdehyde (MDA) content were measured using standard kits (Jiancheng Biology Co.,Nanjing, China) according to the manufacturer’s instructions.
CD4+and CD8+T lymphocytes were measured using commercial kits (AbD Serotec, USA) according to the method described by Caraher et al.(2000), using a Cell Lab QuantaTMSC Flow Cytometer (Beckman Coulter, Fullerton,CA, USA).The levels of immunoglobulin (Ig) A, IgM, and IgG were determined using Cow IgA (catalogue no.E10-121), IgM (catalogue no.E10-101), and IgG (catalogue no.E11-118) Enzyme-Linked Immunosorbent Assay (ELISA)Kits (Bethyl Laboratories, Montgomery, TX, USA).Briefly,capture antibody was used to coat a 96-well microtiter plate (BioFil, Canada) for 1 h at room temperature, and then ELISA wash solution containing 0.05% Tween-20 and 50 mmol L-1of Tris buffered saline was added for 0.5 h to block free binding sites.Next, duplicate serum samples were diluted in ELISA wash solution and incubated for 1 h at room temperature.The plates were then incubated with IgA, IgM, and IgG horseradish peroxidase detection antibody for 1 h.After washing 5 times with ELISA wash solution, the plates were developed with tetramethylbenzidine for 15 min in the dark, and absorbance was then measured at 450 nm.Interleukin (IL)-2, IL-4, and IL-6 levels were determined using Cow ELISA Kits (Groundwork Biotechnology Diagnosticate,CA, USA).The tumor necrosis factor-α (TNF-α) level was measured using a Cow Radioimmunoassay Kit (North Institute of Biotechnology, Beijing, China) according to the manufacturer’s instruction.
2.4.Statistical analysis
Data on blood metabolic profiles, antioxidative enzyme activities, immunoglobulins, and cytokines were analyzed by repeated measures using the MIXED model procedure of SAS (2008).The statistical model contained treatment, time,and treatment×time interactions.Differences among the treatments were analyzed using Tukey’s multiple range test.Data on CD4+and CD8+T lymphocytes and the CD4+/CD8+ratio were analyzed using a GLM.Means are presented with the standard error of the mean in tabular form.A value of P<0.05 was considered statistically significant, P<0.01 was considered markedly significant, and P<0.1 was considered a tendency.
3.Results
3.1.Energy, lipid and protein metabolism in blood
The effects of BE supplementation on blood metabolites are shown in Table 2.Compared with the controls, cows supplemented with BE had higher (P<0.05) concentrations of TP, whereas there were no differences (P>0.05) in GLU,NEFA, TG, TC, LDL-C, and HDL-C among the treatments.Cows fed 0.25 g of BE kg-1had increased (P<0.05) ALB levels compared with control cows, and the ALB level also tended to be higher (P<0.10) in cows fed 0.5 g of BE kg-1.In addition, cows fed 0.25 and 0.5 g of BE kg-1had decreased (P<0.05) BUN concentrations, and a lower tendency (P<0.10) toward BUN concentrations was found in cows fed 1.0 g of BE kg-1.
3.2.Serum antioxidant enzyme activities
SOD activity was elevated (P<0.05) by BE supplementation.GSH-Px activity tended to be higher (P<0.10) in cows fed 0.25 g of BE kg-1compared with the controls.GSH-Px activity was also higher (P=0.05) in cows fed 0.5 and 1.0 g of BE kg-1.Supplementation with BE had no effect (P>0.05)on serum T-AOC and MDA content.GSH-Px, T-AOC, and MDA showed significant time-dependent effects (P<0.05),as shown in Table 3.
3.3.Serum CD4+ and CD8+ T lymphocyte levels and CD4+/CD8+ ratio
Compared with the controls, BE supplementation had no effect (P>0.05) on the proportion of CD4+and CD8+T lymphocytes or the CD4+/CD8+ratio (Table 4).
3.4.Serum antibody and cytokine levels
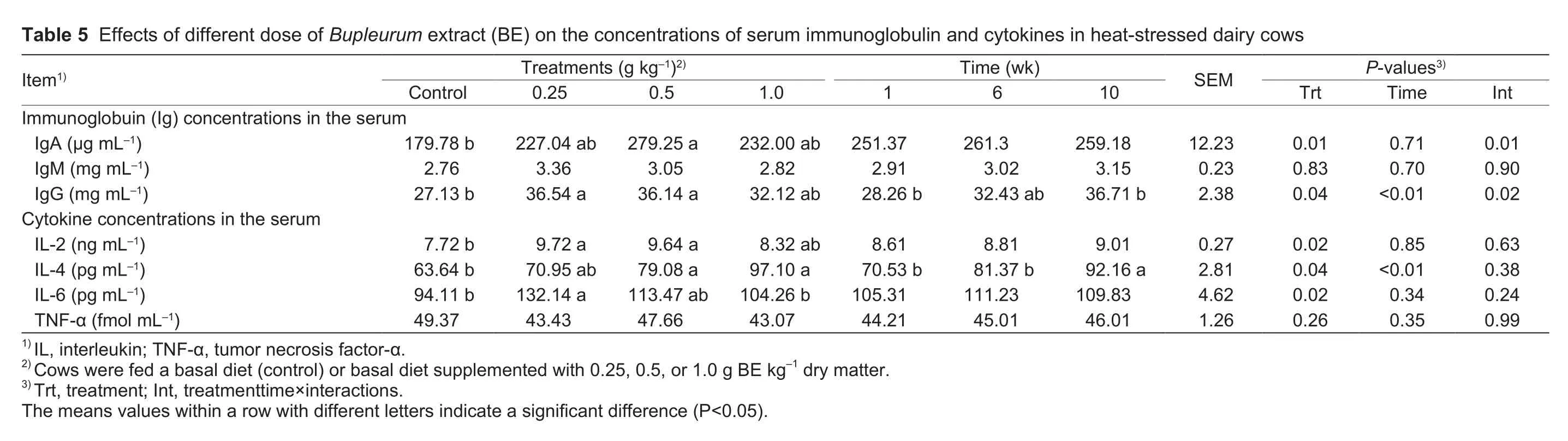
The serum Ig and cytokine concentrations are shown in Table 5.Supplementation with BE had no effect (P>0.05)on the IgM concentration.However, IgA levels tendedto be higher (P<0.10) in cows fed 0.25 and 1.0 g of BE kg-1compared with the controls, and they were increased(P=0.01) in cows supplemented with 0.5 g of BE kg-1.Compared with the controls, concentrations of IgG and IL-2 were increased (P<0.05) in cows supplemented with 0.25 and 0.5 g of BE kg-1, and they tended to be higher (P<0.10)in cows fed 1.0 g of BE kg-1.Cows fed 0.5 and 1.0 g of BE kg-1had increased (P<0.05) IL-4 concentrations.IL-4 levels also tended to be higher (P<0.10) in cows fed 0.25 g of BE kg-1.Cows fed 0.25 g of BE kg-1had increased (P<0.05)IL-6 concentrations.A tendency (P<0.10) toward higher IL-6 concentrations was also found in cows fed 0.5 g of BE kg-1, whereas BE supplementation had no effect (P>0.05)on TNF-α levels.There were significant time-dependent changes (P<0.01) in IgG and IL-4 levels.
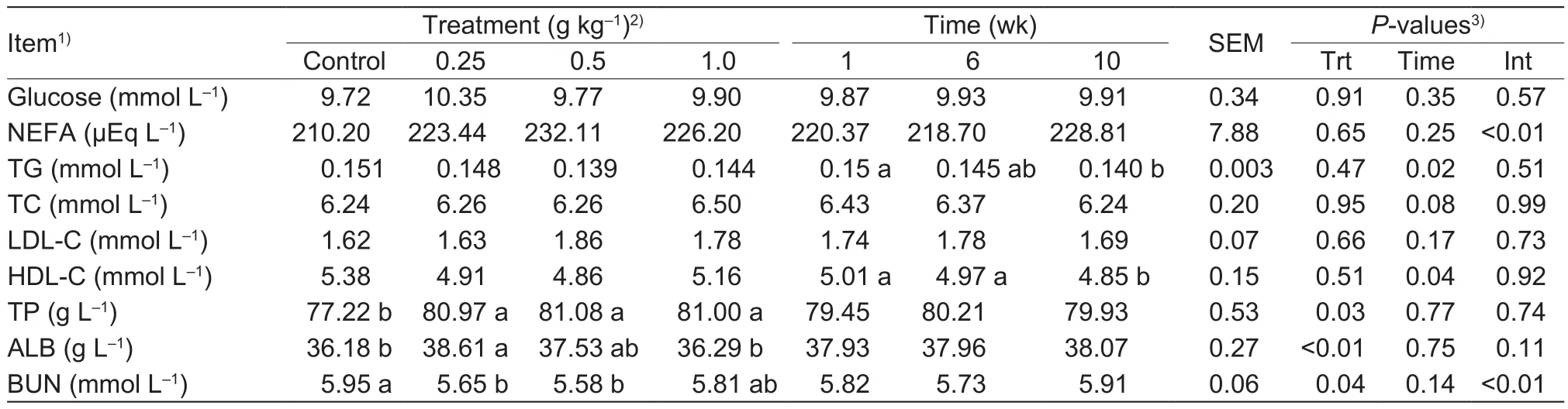
Table 2 Effects of different dose of Bupleurum extract (BE) on blood metabolites related to energy, lipid and protein metabolism in heat-stressed dairy cows
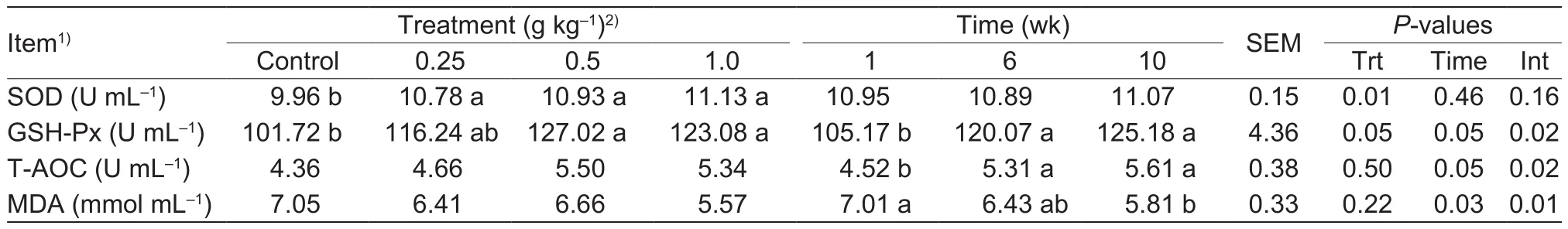
Table 3 Effects of different dose of Bupleurum extract (BE) on the activities of serum antioxidant enzymes in heat-stressed dairy cows
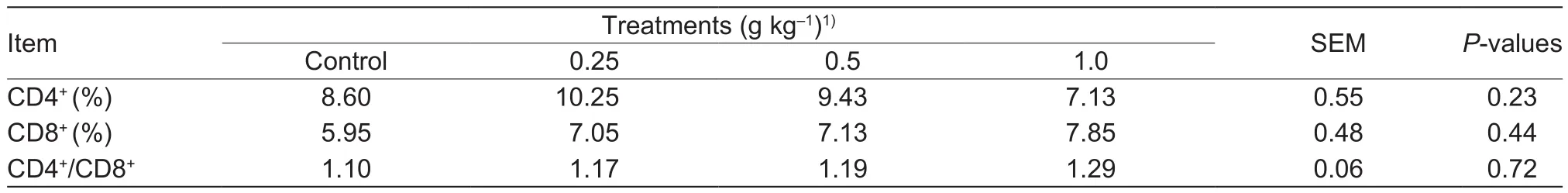
Table 4 Effects of different dose of Bupleurum extract (BE) on the proportion of T lymphocyte subsets and the CD4+/CD8+ ratio in heat-stressed dairy cows
4.Discussion
Several studies demonstrated that heat stress reduced plasma GLU, cholesterol, TG, NEFA, and TP (Abeni et al.2007; Wheelock et al.2010), as well as causing oxidative stress (Bernabucci et al.2002) and reducing plasma antioxidant activity in dairy cows (Harmon et al.1997).Our previous study suggested that BE supplementation appeared to mitigate heat stress in lactating Holstein cows(Pan et al.2014).There have been no previous studies of the effect of BE supplementation on blood metabolism of heat-stressed cows.The present experiment showed that GLU, NEFA, TG, TC, LDL-C, and HDL-C did not differ among the treatments, suggesting that feeding BE had no effect on GLU and lipid metabolism.However, in the experiment,cows fed BE, especially 0.25 or 0.5 g kg-1had increased TP and ALB levels and decreased BUN concentrations.Thus, supplementation with BE appeared to improve nitrogen metabolism in heat-stressed dairy cows.In general, most plasma protein is synthesized by the liver, and the protein content is decreased when liver cells are damaged.The elevated blood protein levels found in the present study may be due to BE supplementation alleviating liver cell damage (Zhao et al.2012).Heat stress is known to increase BUN levels in dairy cows (Kamiya et al.2006).In the present study, the decrease in BUN levels induced by BE supplementation might be due to the alleviation of heat stress in dairy cows (Pan et al.2014).
?
In this study, BE supplementation significantly increased the activities of SOD and GSH-Px, providing convincing evidence that BE can improve the antioxidant status of lactating cows under heat stress.A previous study reported that BE contained a high total phenol content, which inhibited lipid peroxidation and removed free radicals (Wang et al.2005).Zhao et al.(2012) found that SOD activity was elevated following treatment with a soluble polysaccharide fraction from Bupleurum chinese in a rat model of hepatic injury caused by D-galactosamine.Furthermore,purified polysaccharide isolated from Bupleurum chinense showed significant scavenging activity against free radicals (Sun et al.2010).In the present study, BE supplementation had no effect on the serum T-AOC and MDA content.This may be explained by the study animal or the BE supplemental level.In this study, activities of GSH-Px and T-AOC were higher in weeks 6 and 10 than in week 1, and MDA actavity was lower in week 10 than in week 1.The increase in antioxidant levels over time may explained by a decrease in the degree of heat stress in the dairy cows (Pan et al.2014).
Previous research reported that heat stress inhibited immune function in cows (Caroprese et al.2009).Orally administered Bupleurum markedly promoted lymphocyte proliferation, enhanced the antibody titer (Wang et al.2005; Sun et al.2006), and increased cytokine and Ig secretion (Guo et al.2000).In the present study, serum IgA and IgG concentrations were statistically increased in cows fed 0.25 or 0.5 g of BE kg-1compared with the controls, suggesting that BE acts as an immune stimulant and enhances humoral immunity in heat-stressed dairy cows.This finding was consistent with that of Matsumoto et al.(2010), who observed that Hochuekkito, a Kampo (traditional Japanese herbal) medicine, enhanced mucosal IgA antibody responses in mice.Moreover, Yen et al.(1995) reported that serum levels of IgA, IgG,and IgM increased following treatment with a Bupleurum-derived saikosaponin in mice.However, supplementation with BE had no effect on IgM levels in the present study, which was not consistent with the findings of a previous study of mice (Guo et al.2000).The difference may be attributed to differences in breeds.
CD4+and CD8+T lymphocytes are surface markers of T lymphocytes,which reflect the body’s cellular immune state (Park et al.1993).Currently,there is no direct evidence for effects of BE on CD4+T lymphocytes.However, one in vitro study showed that Sho-saiko-to (a Chinese herbal decoction containing BE, known as So-shi-ho-tang in Japanese and Xiao Chai-hu-tang in Chinese) had no effect on CD4+T lymphocyte counts in cultured splenocytes and hepatic mononuclear cells (Ohtake et al.2005),which was similar to that found in the present study.In our study, BE supplementation had no effect on the proportion of CD8+T lymphocytes, indicating that BE did not exert cytotoxic effects on cellular immunity.
In the present study, BE supplementation increased the level of IL-2, which was in accordance with that found in a previous study by Liu et al.(1997), who observed in vitro that Xiao Chai-hu-tang, a BE water soluble substance,enhanced IL-2 secretion.The increased IL-2 levels in the BE-supplemented groups may be caused by T-lymphocyte proliferation (Malek 2003).Studies also reported that saikosaponin from Bupleurum augmented antibody formation (Yamaguchi et al.1985) and that it significantly increased IL-2 levels in mice (Yen et al.1995).An in vivo study showed that Sho-saiko-to significantly increased the IL-4 content in splenocytes of mice (Kang et al.2009),which was similar to the findings in our study.The increased IL-4 content found in the present study indicated that BE enhanced humoral and cellular immune responses in heatstressed dairy cows.Previous research reported that IL-6 contributed to the enhancement of nonspecific Ig secretion from B cells (Sakurai et al.1999).Guo et al.(2000) reported that bupleuran 2IIc (a pectic polysaccharide from the roots of Bupleurum falcatum) induced IL-6 secretion in normal murine B cells and in some specific B-cell lines in mice.In the present study, the IL-6 content was significantly increased in cows fed 0.25 g of BE kg-1, suggesting the BE promoted the differentiation of B lymphocytes.
Overall, the results of the present study indicated that BE supplementation stimulated the secretion of serum immunoglobulins and cytokines, including IgA, IgM, IgG,IL-2, IL-4, and IL-6, thereby helping to enhance immune function.The enhanced immune status and function of dairy cows supplemented with BE in this study might be due to the alleviation of heat stress (Pan et al.2014), as well as that some active ingredients in Bupleurum transport to lymphoid tissue and subsequent interactions with lymphocytes (Yen et al.1995).Alternatively, polysaccharides from Bupleurum may have enhanced binding of immune complexes to peritoneal macrophages through Fc receptor expression(Matsumoto et al.1993).
5.Conclusion
The present study demonstrated that feeding BE to heatstressed dairy cows improved protein metabolism by increasing blood TP and ALB levels.It increased antioxidant activity by upregulating the activities of SOD and GSH-Px and enhanced immune function by increasing IgA, IgG,IL-2, and IL-4 concentrations.This study points to a new strategy to help mitigate negative effects of heat stress in lactating Holstein cows.
Acknowledgements
This study was supported financially by the National Key Research and Development Program of China(2016YFD0500503), the Agricultural Science and Technology Innovation Program, China (ASTIP-IAS12) and the Anhui International Science and Technology Cooperation Plan Program, China (1503062019).
Abeni F, Calamari L, Stefanini L.2007.Metabolic conditions of lactating Friesian cows during the hot season in the Po valley.1.Blood indicators of heat stress.International Journal of Biometeorology, 52, 87-96.
Armstrong D V.1994.Heat stress interaction with shade and cooling.Journal of Dairy Science, 77, 2044-2050.
Ashour M L, Wink M.2011.Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action.Journal of Pharmacy and Pharmacology, 63, 305-321.
Bernabucci U, Ronchi B, Lacetera N, Nardone A.2002.Markers of oxidative status in plasma and erythrocytes of transition dairy cows during hot season.Journal of Dairy Science,85, 2173-2179.
Caraher E M, Parenteau M, Gruber H, Scott F W.2000.Flow cytometric analysis of intracellular IFN-γ, IL-4 and IL-10 in CD3+4+T-cells from rat spleen.Journal of Immunological Methods, 244, 29-40.
Caroprese M, Marzano A, Entrican G, Wattegedera S,Albenzio M, Sevi A.2009.Immune response of cows fed polyunsaturated fatty acids under high ambient temperatures.Journal of Dairy Science, 96, 2796-2803.
Carroll J A, Burdick N C, Chase J C C, Coleman S W, Spiers D E.2012.Influence of environmental temperature on the physiological, endocrine, and immune responses in livestock exposed to aprovocative immune challenge.Domestic Animal Endocrinology, 43, 146-153.
Collier R J, Dahl G E, VanBaale M J.2006.Major advances associated with environmental effects on dairy cattle.Journal of Dairy Science, 89, 1244-1253.
Guo Y J, Matsumoto T, Kikuchi Y J, Ikejima T, Wang B X,Yamada H.2000.Effects of a pectic polysaccharide from a medicinal herb, the roots of Bupleurum falcatum L.on interleukin 6 production of murine B cells and B cell lines.Immunopharmacology, 49, 307-316.
Hammami H, Bormann J, M’hamdi N, Montaldo H H, Gengler N.2012.Evaluation of heat stress effects on production traits and somatic cell score of Holsteins in a temperate environment.Journal of Dairy Science, 96, 1-12.
Harmon R J, Lu M, Trammel D S, Smith B A.1997.Influence of heat stress and calving on antioxidant activity in bovine blood.Journal of Dairy Science, 80(Suppl.1), 264.
Huang K.1993.Antipyretic herbs.In: The Pharmacology of Chinese Herbs.CRC Press, Tokyo.pp.151-152.
Kamiya M, Kamiya Y, Tanaka M, Oki T, Nishiba Y, Shioya S.2006.Effects of high ambient temperature and restricted feed intake on urinary and plasma 3-methylhistidine in lactating Holstein cows.Animal Science Journal, 77,201-207.
Kang H, Choib T W, Ahnb K S, Leec J Y, Hamc I H, Choic H Y,Shim E S, Sohn N W.2009.Upregulation of interferon-γ and interleukin-4, Th cell-derived cytokines by So-Shi-Ho-Tang(Sho-Saiko-To) occurs at the level of antigen presenting cells, but not CD4+T cells.Journal of Ethnopharmacology,123, 6-14.
Lin H, Decuypere E, Buyse J.2006.Acute heat stress induces oxidative stress in broiler chickens.Comparative Biochemistry and Physiology (Part A), 144, 11-17.
Liu J J, Xue H, Chen Z F.1997.The effects of water soluble substance extracted from Xiao Chai-hu-tang on IL-2 production and CD antigen change of PHA-stimulated PBMC.Journal of Luzhou Medical College, 20, 331-334.(in Chinese)
Malek T R.2003.The main function of IL-2 is to promote the development of T regulatory cells.Journal of Leukocyte Biology, 74, 961-965.
Martin S, Padilla E, Ocete M A, Galvez J, Jiménez J, Zarzuelo A.1993.Anti-inflammatory activity of the essential oil of Bupleurum fruticescens.Planta Medica, 59, 533-536.
Matsumoto T, Cyong J C, Kiyohara H, Matsui H, Abe A,Hirano M, Danbara H, Yamada H.1993.The pectic polysaccharides from Bupleurum falcatum L.enhances immune-complexes binding to peritoneal macrophages through Fc receptor expression.International Journal of Immunopharmacology, 15, 683-693.
Matsumoto T, Noguchi M, Hayashi O, Makino K, Yamada H.2010.Hochuekkito, a kampo (traditional Japanese herbal)medicine, enhances mucosal IgA antibody response in mice immunized with antigen-entrapped biodegradable microparticles.Evidence-Based Comple and Alternative Medicine, 7, 69-77.
Navarro P, Giner R M, Recio M C, Máñez S, Cerdá-Nicolás M,Ríos J L.2001.In vivo anti-inflammatory activity of saponins from Bupleurum rotundifolium.Life Science, 68, 1199-1206.
NRC (National Research Council).2001.Nutrient Requirements of Dairy Cattle.7th ed.National Academy Press, Washington,D.C.
Ohtake N, Yamamoto M, Takeda S C, Aburada M, Ishige A, Watanabe K, Inoue M.2005.The herbal medicine Sho-saiko-to selectively inhibits CD8+T-cell proliferation.European Journal of Pharmacology, 507, 301-310.
Pan L, Bu D P, Wang J Q, Cheng J B, Sun X Z, Zhou L Y, Qin J J, Zhang X K, Yuan Y M.2014.Effects of Radix Bupleuri extract supplementation on lactation performance and rumen fermentation in heat-stressed lactating Holstein cows.Animal Feed Science and Technology, 187, 1-8.
Park Y H, Fox L K, Hamilton M J, Davis W C.1993.Suppression of proliferative response of BoCD4+T lymphocytes by activated BoCD8+T lymphocytes in the mammary gland of cows with Staphylococcus aureus mastitis.Veterinary Immunology and Immunopathology, 36, 137-151.
Sakurai M H, Matsumoto T, Kiyohara H, Yamada H.1999.B cell proliferation activity of pectic polysaccharide from amedicinal herb, the roots of Bupleurum falcatum L.and its structural requirement.Immunology, 97, 540-547.
SAS (Statistical Analysis System).2008.User’s Guide:Statistics.Version 9.2 edition.SAS Institute, Cary, NC.
St-Pierre N R, Cobanov B, Schnitkey G.2003.Economic losses from heat stress by stressby US livestock industries.Journal of Dairy Science, 86(Suppl.), E52-E77.
Sun H X.2006.Haemolytic activities and adjuvant effect of Bupleurum chinense saponins on the immune responses to ovalbumin in mice.Vaccine, 24, 1324-1331.
Sun L W, Feng K, Jiang R, Chen J Q, Zhao Y, Ma R, Tong H B.2010.Water-soluble polysaccharide from Bupleurum chinense DC: Isolation, structural features and antioxidant activity.Carbohydrate Polymers, 79, 180-183.
Sun X B, Matsumoto T, Yamada H.1991.Effects of a polysaccharide fraction from the roots of Bupleurum falcatum L.on experimental gastric ulcer models in rats and mice.Journal of Pharmacy and Pharmacology, 43, 699-704.
Wang B J, Liu C T, Tseng C Y, Yu Z R.2005.Antioxidant activity of Bupleurum kaoi Liu (Chao et Chuang) fractions fractionated by supercritical CO2.LWT-Food Science and Technology, 38, 281-287.
Wang J P, Bu D P, Wang J Q, Huo X K, Guo T J, Wei H Y,Zhou LY, Rastani R R, Baumgard L H, Li F D.2010.Effect of saturated fatty acid supplemen-tation on production and metabolism indices in heat-stressed mid-lactation dairy cows.Journal of Dairy Science, 93, 4121-4127.
Wheelock J B, Rhoads R P, VanBaale M J, Sanders S R,Baumgard L H.2010.Effects of heat stress on energetic metabolism in lactating Holstein cows.Journal of Dairy Science, 93, 644-655.
Yamaguchi N, Kohno H, Tawara M, Odashima S.1985.Effect of saikosaponin derivatives upon the immune response against T-dependent and T-independent antigens in mice.International Journal of Immunopharmacology, 7, 827-832.
Yen M H, Lin C C, Yen C M.1995.The immunomodulatory effect of saikosaponin derivatives and the root extract of Bupleurumkaoi in mice.Phytotherapy Research, 9, 351-358.
Zhao W, Li J J, Yue S Q, Zhang L Y, Dou K F.2012.Antioxidant activity and hepatoprotective effect of a polysaccharide from Bei Chaihu (Bupleurum chinense DC).Carbohydrate Polymers, 89, 448-452.
Zimbelman R B, Collier R J, Bilby T R.2013.Effects of utilizing rumen protected niacin on core body temperature as well as milk production and composition in lactating dairy cows during heat stress.Animal Feed Science and Technology,180, 26-33.
 Journal of Integrative Agriculture2018年3期
Journal of Integrative Agriculture2018年3期
- Journal of Integrative Agriculture的其它文章
- Characteristic analysis of tetra-resistant genetically modified rice
- A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis
- Characterization of GhSERK2 and its expression associated with somatic embryogenesis and hormones level in Upland cotton
- GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean
- Molecular cloning and functional characterization of a soybean GmGMP1 gene reveals its involvement in ascorbic acid biosynthesis and multiple abiotic stress tolerance in transgenic plants
- Responses of the antioxidant system to fluroxypyr in foxtail millet(Setaria italica L.) at the seedling stage
