The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep)
GAO Ling-ling, WANG Zhen-yu, LI Zheng, ZHANG Cai-xia, ZHANG De-quan
Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences/Key Laboratory of Agro-products Processing, Ministry of Agriculture, Beijing 100193, P.R.China
1.Introduction
Bones are the major by-products after sheep are slaughtered.With the rapidly increasing production of sheep in recent years, a large sum of ovine bones are being produced in China (Zhou et al.2012; Toldrá et al.2016).It was estimated that over 400 000 tons ovine bones were produced in China annually.However, only a small fraction is used as animal feed with low-market-value, but the rest majority ends up in landfills, resulting in wasting of resources and causing environmental problems (Toldrá et al.2016).Ovine bones are rich in collagen, which has been widely used in food, pharmaceuticals, cosmetic and tissue engineering(Veeruraj et al.2015).Therefore, the research and utilization of collagen from ovine bones not only improve environmental quality, but also enhance additional value and economical benefit of bones.
Collagen, as the most abundant structural protein in living bodies, constitutes approximately 30% of the total proteins(Liu et al.2015).Currently, at least 29 collagen types have been identified, varying considerably in their amino acid composition, amino acid sequence, spatial structures and function (Liu et al.2012; Li et al.2013; Chen et al.2016).Among all these different types, type I collagen is the most common in mammals and fish, widely distributed in bones,skins and tendons.Type I collagen is composed of two identical α1 chains and one α2 chain in the molecular form of[α1(I)]2α2(I).Three chains, existing in left-handed helix form by itself, stagger one another to develop a triple right-handed superhelical structure.The triple helical structure is flanked by two short N- and C- peptides, called telopeptides, which determine the intermolecular interactions and cross-link.-Gly-Xaa-Yaa- repeating triplets are the main feature of the helix domain, and the Xaa and Yaa positions are predominant proline and hydroxyproline residues, respectively(Engel and Bächinger 2005; Shoulders and Raines 2009).Traditionally, collagen mainly came from cows and pigs,which were suffered from religious restrictions.In recent years, collagen from fish by-products also studied extensively (Karayannakidis et al.2014; Liu et al.2014; Chen et al.2015; Kittiphattanabawon et al.2015; Veeruraj et al.2015), but the practical application of fish collagen was limited because of its low thermal stability.Ovine collagen is religiously and traditionally allowed to be used in almost all regions and by multifarious religionary groups compared to collagen from pigs.In addition, ovine bones collagen is expected to be type I collagen and has higher thermal stability compared to fish collagen as sheep are mammals.However, the structure characteristics and properties of collagen from ovine bones are not well characterized so far.
Pepsin is usually used to facilitate extraction of collagen by cleaving the telopeptides and doesn’t destroy the triple helical structure.Therefore, for better application of ovine bones collagen, the present study extracted acid soluble collagens (ASC) and pepsin soluble collagens (PSC) from ovine bones.Structures and properties of ovine bones collagen were analyzed by protein identification, amino acid composition, secondary structure analysis and determination of denaturation temperature, etc., and further provided a synchronous comparison of two bones collagens due to different extraction methods.
2.Materials and methods
2.1.Preparation of ovine bones
The fresh ovine bones (Ujumuqin sheep aged 8-month-old)were obtained from a slaughterhouse in Xilingol League,Inner Mongolia Autonomous Region of China.Bones were transported to laboratory on ice.The two terminals of bones were cut off using a saw and bone marrows were removed.Bones were then broken into small pieces (0.5 cm in length)and shattered using an ultra-high speed grinder (FW100,Taisite, Tianjin, China).The shattered bones were freezedried and stored at -20°C until used.
Ovine bones were pretreated at 4°C.The prepared bones were first soaked in 0.1 mol L-1NaOH with a sample/alkali solution ratio of 1:10 (w/v) for 48 h to remove non-collagen proteins, and NaOH solution was changed every 12 h.Then 10 volumes of 10% (v/v) butyl alcohol was used for 72 h to remove fat, which the butyl alcohol solution was replaced every 12 h.After being washed with distilled water, the defatted bones were decalcified with 10 volumes of 0.5 mol L-1EDTA-2Na solution (pH=7.5) for 5 days, and the solvent replaced every 12 h.Bones were freeze-dried and ready for collagen extraction.
2.2.Collagen extraction
A total of 0.5 mol L-1acetic acid was used to extract ASC from sheep bones with a solid/solvent ratio of 1:10 (w/v) for 3 days.The extracting solution was centrifuged at 10 000×g for 30 min at 4°C, and the pellet was extracted again under the same conditions.The two filtrates were combined together.NaCl with a concentration of 2.0 mol L-1was used to salt out collagen.The sequent precipitate was centrifuged at 10 000×g for 30 min.The sediment was redissolved in 0.5 mol L-1acetic acid, then salted out and centrifuged again.The sediment was suspended in 0.5 mol L-1acetic acid and dialysed against 0.1 mol L-1acetic acid for 1 day and ultrapure water for 2 days with dialysate changed every 12 h and lyophilize.
For the extraction of PSC, sheep bones were soaked in 0.5 mol L-1acetic acid containing porcine pepsin (Amresco,USA) (20 U g-1bones) with a solid/solvent ratio of 1:10 (w/v)at 4°C for 3 days with stirring.The extract was then treated as stated above for the extraction of ASC to obtain PSC.
2.3.UV absorption spectrum
Samples were redissolved in 0.5 mol L-1acetic acid and subjected to a wavelength scan from 200 to 350 nm using a spectrophotometer (UV-1700, Shimadzu, Kyoto, Japan)at room temperature.The scan speed was 50 nm per min.
2.4.Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE analysis of proteins was performed as previously described (Laemmli 1970).Samples were dissolved in 5%SDS.Protein concentration was determined by the BCA assay (Pierce Chemical Co., Rockford, IL, USA).A volume of collagen solution was added with a same volume of 2×loading buffer and boiled for 5 min.Electrophoresis was performed using 7.5% resolving gel (Mini-PROTEAN Tetra electrophoresis system; Bio-Rad, Hercules, CA, USA).High molecular weight marker was obtained from Thermo Fisher Scientific (Waltham, USA, #26630).After electrophoresis,Coomassie Brilliant Blue R-250 (Amresco, USA) were used to stain overnight, and then destained.The gels were imaged using the ChemiDOCTMMP Imaging System (Bio-Rad,California, USA).
2.5.Protein identification by liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Protein identification was performed as previously described(Chen L et al.2016) with some modifications.The NCBI Mascot database was used to analyze all MS/MS data (Matrix Science, London, UK; PLGS v2.3).Protein identification was deemed to be valid by confidence level of >95%.
2.6.Amino acid composition of sheep bone collagen
The amino acid composition was analyzed on the basis of the method of Li et al.(2013) with some modifications.Sheep bone collagens were hydrolyzed with 10 mL of 6 mol L-1HCl at 110°C for 24 h.The hydrolysates were diluted to 50 mL, then 1 mL solution was evaporated to dryness at 25°C and then diluted to 5 mL with 0.02 mol L-1HCl.The hydrolysates were determined using an amino acid analyser (Hitachi L-8900, Shimadzu Seisakusho Co., Ltd.,Kyoto, Japan).
2.7.Attenuate total reflection-fourier transform infrared spectroscopy (ATR-FTIR)
Secondary structure of both ASC and PSC were obtained by ATR-FTIR spectra as described by Li et al.(2013) at 25°C using a FTIR spectrometer (Tensor 27, Bruker, Ettlingen,Germany).
2.8.Differential scanning calorimetry (DSC)
The thermal denaturation temperature (Td) of samples were measured according to the method of Kittiphattanabawon et al.(2015) with some modifications.Samples were redissolved in 0.05 mol L-1acetic acid and were accurately weighted.Samples were heated from 20 to 60°C at a scanning rate of 1°C min-1using the Q-200 Series DSC (TA Instruments, Inc., New Castle, DE, USA).
2.9.Zeta potential analysis
Samples were re-dissolved completely in 0.5 mol L-1acetic acid to obtain a final concentration of 0.05% (w/v).Zeta potential were measured using Zeta Potential Analyzer(Zetasizer Nano ZS, Malvern, UK) according to Kittiphattanabawon et al.(2010) with some modification.
2.10.Statistical analysis
Statistical analysis was carried out by SPSS version 21.0(SPSS Inc., Chicago, IL, USA).Significant differences(P<0.05) were identified by multiple comparisons of the means, using the LSMEANS.All tests were performed in triplicate.Data were shown as means±standard deviation.
3.Results and discussion
3.1.UV absorption spectra
The UV absorption spectra of ovine bones collagen at the wavelengths 210-300 nm were presented in Fig.1.ASC and PSC from sheep bone displayed a maximum absorption at 231.3 and 231.8 nm, respectively, which were in agreement with those of collagens from brownbanded bamboo shark (Kittiphattanabawon et al.2010) and squid (Veeruraj et al.2015).It turned out that the functional groups of C=O,-COO and CONH2existing in polypeptides chains of collagen lead to the absorbance peak at about 230 nm (Edwards et al.1997).Aromatic residues, including tryptophan, tyrosine and phenylalanine, are responsible for the ultraviolet absorption at approximate 280 nm.In the present study,sheep bones collagen did not exhibit a distinct absorption peak at about 280 nm.This was in accordance with amino acid composition of sheep bones collagen, which contained low amount of aromatic residues.The high purity of collagen extracted from ovine bones was also proved by no absorption peak at 280 nm (Kittiphattanabawon et al.2010;Jeevithan et al.2014).
3.2.SDS-PAGE patterns and protein identification of ovine bones collagen
The SDS-PAGE analysis of ASC and PSC from sheep bones were shown in Fig.2.Both ASC and PSC from sheep bones had similar electrophoretic patterns but some differences in molecular weight when compared with type I collagen.Sheep bones collagen was composed of two different α-chains (α1 and α2).The band intensity of α1-chain was approximately double heavier than that of α2-chain for both ASC and PSC.β- and γ-bands, indicating dimer and trimer of α-chains, respectively, which belong to high molecular weight components, were also observed.The electrophoretic patterns of sheep bones collagen resemble type I collagen from other sources (Nam et al.2008; Liu et al.2012; Li et al.2013).Thus, it was logical to infer that sheep bone collagen was mainly type I collagen.According to the results of Quantity One, the molecular weights of α1 and α2 chains of ASC were 110.18 and 126.87 kDa,respectively, and those of PSC were 108.58 and 123.92 kDa,respectively.The molecular weights of both α1 and α2 chains of PSC were lower compared with ASC.Type I collagen contains a triple helical structure, namely collagen domain, and two telopeptides (N- and C-), namely non-collagenous domains (Shoulders et al.2009).The results demonstrated that pepsin, as a limited proteolysis,were able to cleave telopeptides.
The results of protein identification were shown in Table 1.Proteins were identified based on scores, the number of matched peptides and coverage of protein sequences (%).Peptides sequences detected by LC-MS/MS were matched with ovine proteins.The chain α1 was identified to be uncharacterized protein (gene name: COL1A1, collagen α1(I)(Ovis aries (sheep)), gene ID: 443483) in the NCBI, and the chain α2 was also uncharacterized protein (gene name:LOC443512, collagen α2 (I) (Ovis aries (sheep)), gene ID:443512).The characteristic -Gly-Xaa-Yaa- repeating triplets were identified in the matched peptides sequences.Thus,the ASC and PSC from ovine bones were type I collagen.It was also certain that W5P481 (accession no.) was collagen α1(I) (Ovis aries (sheep)) even though W5P481 was not found in gene of NCBI.
3.3.Amino acid composition of sheep bones collagen
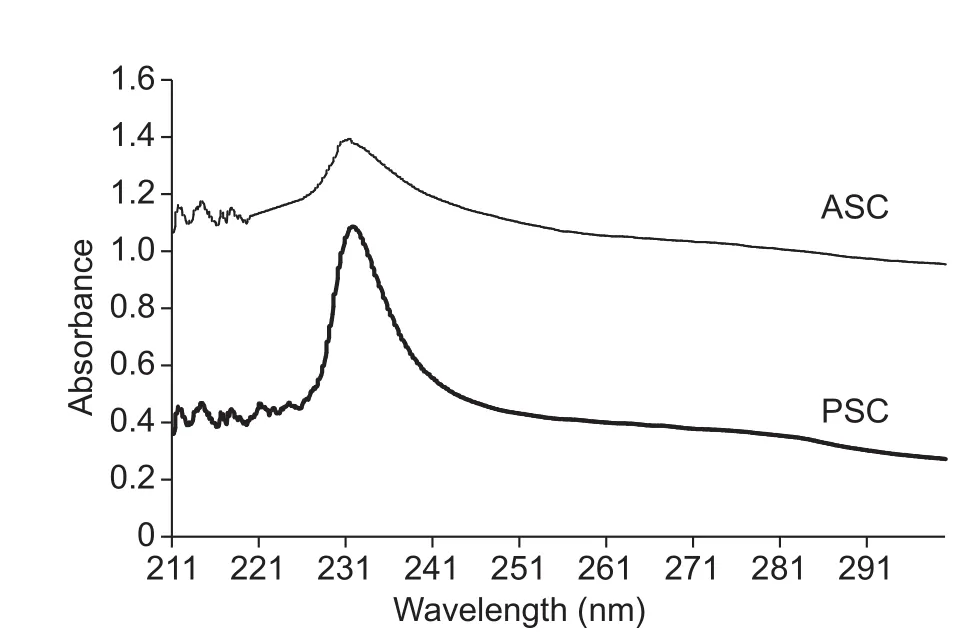
Fig.1 UV absorption spectra of acid soluble collagens (ASC)and pepsin soluble collagens (PSC) from sheep bones.
As presented in Table 2, both ASC and PSC from sheep bones exhibited semblable amino acid compositions pattern.Glycine, as the most abundant amino acid, accounted for approximately one-third of total amino acids.The followings were proline, alanine and hydroxyproline.The collagen is characterized by three helix domains with the-Gly-Xaa-Yaa- repeating triplets, other than 24 amino acid residues at the NC-terminus (Foegeding et al.1996).The contents of methionine, histidine, cysteine, hydroxylysine and tyrosine were very low in sheep bones collagen, which is corresponding with other bones collagens (Duan et al.2009; Liu et al.2012; Li et al.2013).
The content of imino acid, including proline and hydroxyproline, was higher in sheep bones collagen than those of fish bone collagens ranging from 174 to 200 per 1 000 residues (Liu et al.2012; Li et al.2013), but almost equal to those of collagens from pig skin (220.0 residues per 1 000 residues), calf skin (215-216.6 residues per 1 000 residues)(Li et al.2013) and bovine tendon collagen (213 residues per 1 000 residues) (Nam et al.2008).The result in this study agreed with Foegeding et al.(1996): The content of imino acid of mammalian collagen was higher than equivalent of fish collagen.
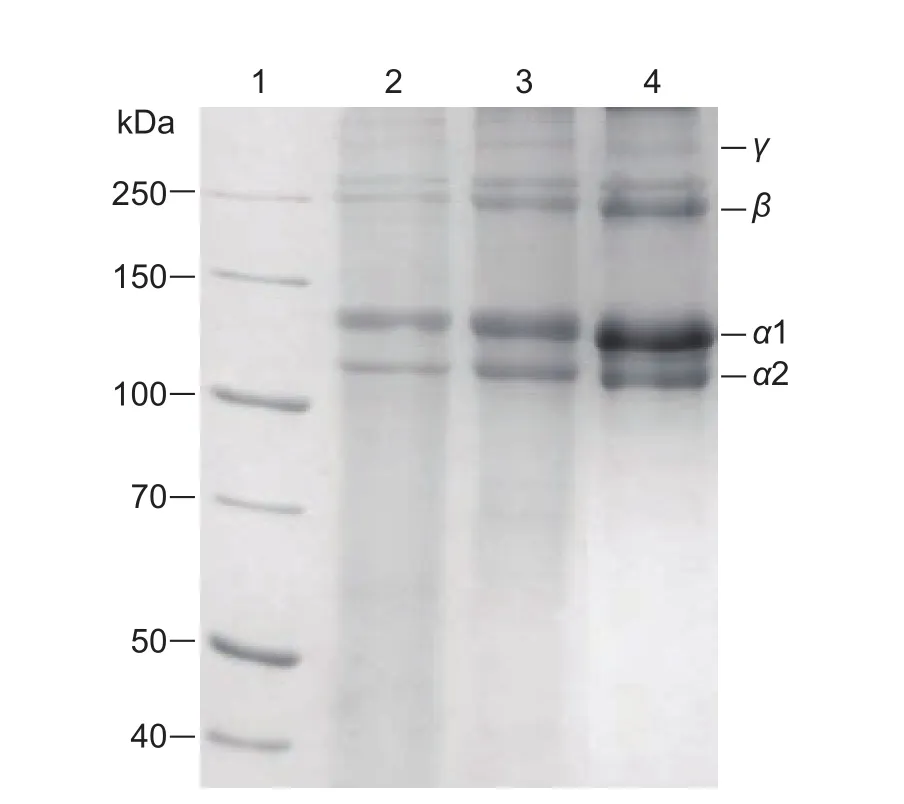
Fig.2 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of collagen from sheep bones.Lane 1, marker; lane 2, bovine tendon collagen; lane 3, acid soluble collagens; lane 4, pepsin soluble collagens.
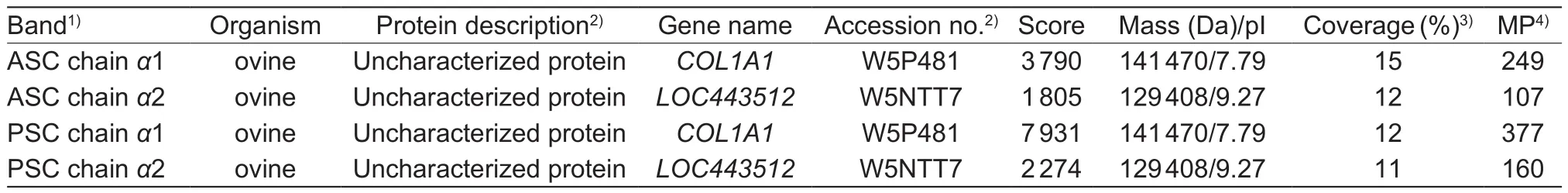
Table 1 LC-MS/MS identification of α1 and α2 chains
The levels of hydroxylation of proline of sheep bones collagen were 45.29 and 45.91% for ASC and PSC, respectively, and thoes of lysine were 20.57 and 18.27%, respectively.The level of hydroxylation of proline was slightly higher than that of calf skin (43.91%) and fish collagen ranging from 38.33 to 40.10% (Li et al.2013), but lower than bovine tendon collagen (47.42%) (Nam et al.2008).The level of hydroxylation of lysine was higher than that of fish bones collagen ranging from 15.34 to 17.76%, and lower than calf skin collagen (22.51%) (Liu et al.2012; Li et al.2013).Thermostability of collagen was able to be enhanced byhydroxylation of proline and lysine because hydroxyproline was a key factor to stabilize the triple superhelix structure and hydroxylysine as well as promote the formation and stabilization of cross-links (Li et al.2013).
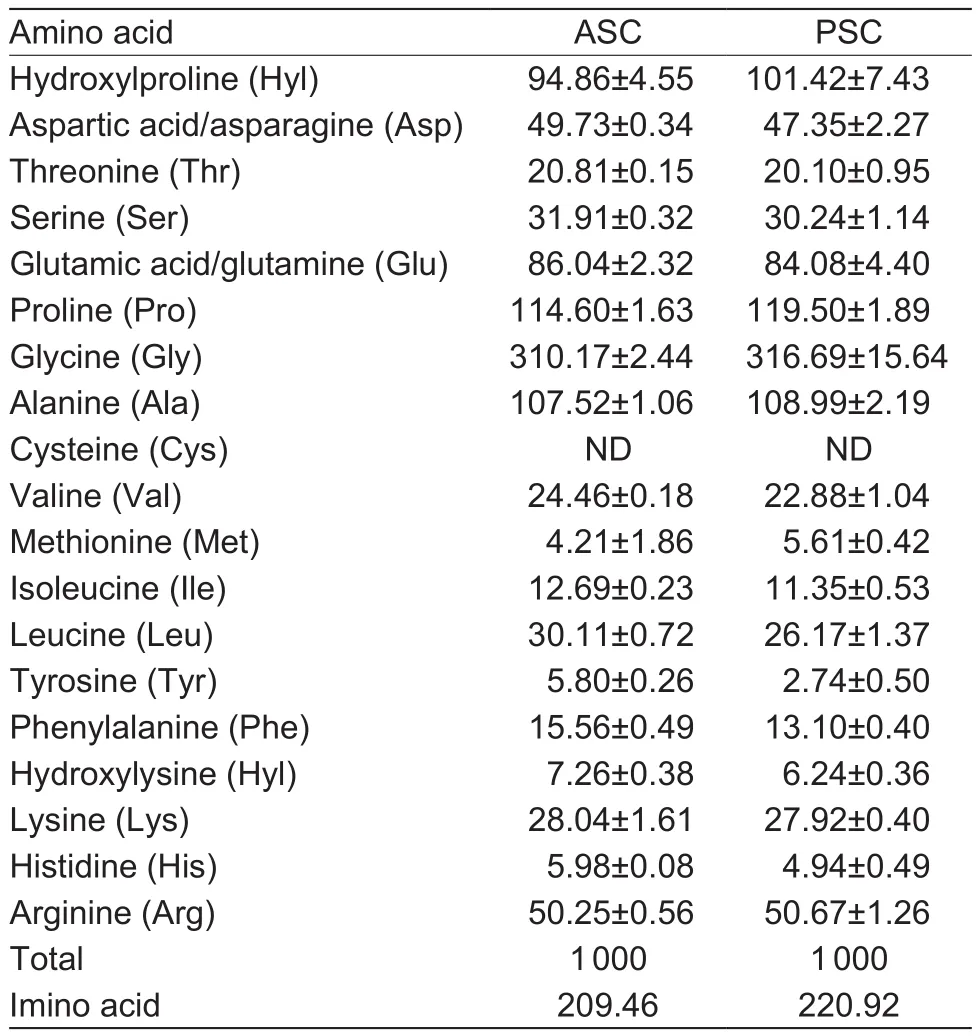
Table 2 Amino acid compositions of acid soluble collagens(ASC) and pepsin soluble collagens (PSC) from sheep bones(expressed as residues per 1 000 residues)
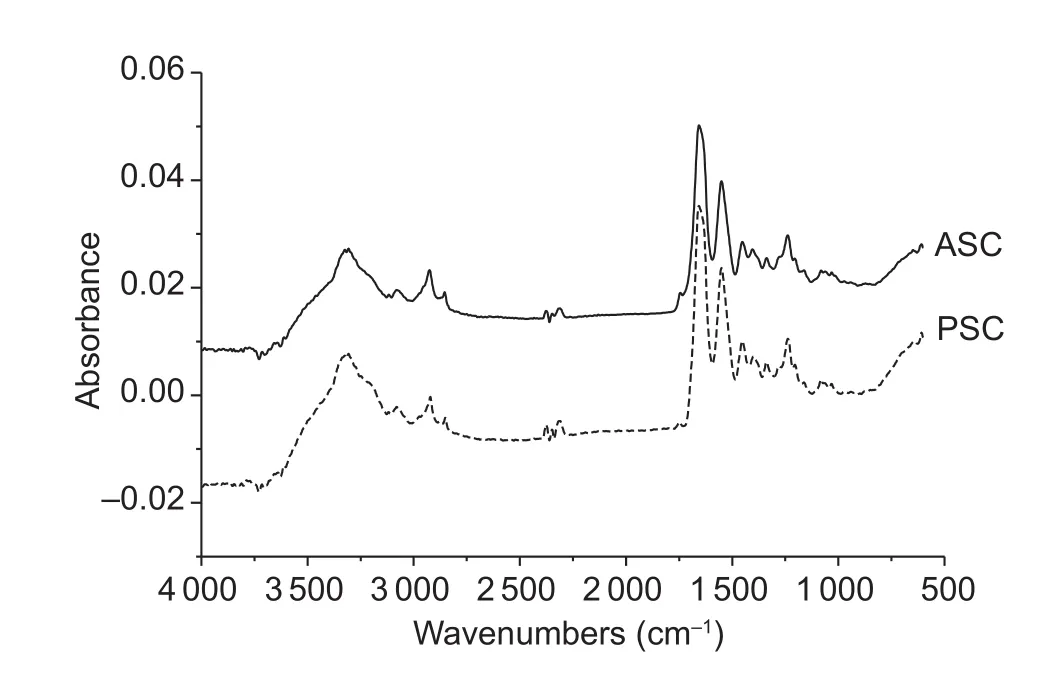
Fig.3 Fourier transforms infrared spectra of acid soluble collagens (ASC) and pepsin soluble collagens (PSC).
3.4.ATR-FTIR spectroscopy analysis of collagen
The FTIR spectrums of sheep bone collagens in the range of 600-4 000 cm-1were shown in Fig.3.The absorption characteristic of amide A is assigned to N-H stretching vibration.Generally, absorption in the wavenumber range of 3 400-3 440 cm-1means a free N-H group stretching vibration.However, the band shifts to lower wavenumber,usually around 3 300 cm-1, once the N-H functional group is coupled with hydrogen bonds (Doyle et al.1975).Amide A bands of ASC and PSC from sheep bones were found at wavenumber of 3 307 and 3 305 cm-1, respectively,which suggested that N-H groups of sheep bones collagen contained abundant hydrogen bonds.The amide B band indicated the asymmetrical stretch of CH2, and the amide B bands of sheep bones collagen were at wavenumber of 2 925 and 2 922 cm-1for ASC and PSC, respectively (Abe and Krimm 1972).
The amide I band, the characteristic frequencies ranging from 1 600 to 1 700 cm-1, was mainly associated with the stretching vibrations of the carbonyl group (C=O bond) along the polypeptide backbone (Payne and Veis 1988).Amide I band is mainly applied to analyze the secondary structure of proteins because it is scarcely changed by the conformation of the side-chain (Barth and Zscherp 2002).The maximum absorbance in the amide I band of different secondary structures in the order: α-helix, 1 645-1 659 cm-1; β-sheet or nonstranded extended structure, 1 620-1 640 cm-1;β-turn, 1 660-1 700 cm-1; irregular structure, 1 640-1 644 cm-1(Farrell et al.2001).Both ASC and PSC from sheep bones were found at the wavenumber of 1 656 cm-1, which suggested that the secondary structure of ovine bones collagen was α-helix.The characteristic frequencies ranging from 1 550-1 600 cm-1, named amide II band, represented N-H bending vibrations (40-60%) coupled to C-N stretching vibrations (18-40%) (Jeevithan et al.2014).The maximum absorbance of amide II bands of sheep bones collagen was obtained at the wavenumber of 1 550 cm-1.The amide II band is also scarcely affected by the conformation of the side-chain as the amide I band, however, the correlation between wavenumber and secondary structure is less directly compared with the amide I band (Barth et al.2002).The characteristic frequencies ranging from 1 200 to 1 400 cm-1,named amide III band, were mainly associated with intermolecular interactions.The amide III band indicates C-N stretching vibrations and N-H distortion from amide linkages along with vibrations caused by wagging of CH2from the glycine backbone and proline side-chains, which signifies complicated the combination peaks (Jackson et al.1995).Both of ASC and PSC from sheep bones were at the wavenumber of 1 238 cm-1.The ratios of absorption peak between 1 240 cm-1(amide III) and 1 452 cm-1of ASC and PSC were 1.1 and 1.0, respectively.The ratios verified both ASC and PSC preserved the triple helical structures since the ratios were close to 1.0 (Guzzi Plepis et al.1996).Further, it turned out that pepsin didn’t destroy the triple helical structure.
3.5.Td of collagen
DSC thermograms of ASC and PSC from sheep bones were shown in Table 3.The result suggested that slightly higher Tdwas determined for ASC when compared to that of PSC.ASC and PSC from spotted golden goatfish (Matmaroh et al.2011) and Spanish mackerel (Li et al.2013) showed similar results.It was inferred that collagen telopeptides,which determines the intermolecular interactions, exerted an effect on stabilizing triple helix structure of nature collagen.The cleavage of telopeptides maybe accelerate thermal denaturation of PSC.
On account of species, living conditions and age, etc., Tdof nature collagen were different in the previously studies(Barzideh et al.2014).The present study detected that Tdof ASC and PSC from sheep bones was a little higher than that of collagen equivalent from fish ranging from 15 to 39°C(Li et al.2013; Liu et al.2014; Lee et al.2016).In addition,Tdof sheep bones collagen was slightly higher copmpared with porcine skin collagen (37.0°C) (Nagai and Suzuki 2000),calf skin collagen (40.8°C) (Duan et al.2009) and bovine tendon collagen (36.0°C) (Nam et al.2008).Interchain hydrogen bonding between N-H (glycine) and C=O (Xaa)per triplet is the key intramolecular force to stabilize the collagen structure (Dai and Etzkorn 2009; Shoulders and Raines 2009).Furthermore, the higher imino acid content increases thermal stability of collagen.The triple helical structure is mainly governed by the steric conformational restrictions compelled by the pyrrolidine rings of imino acids and as well as preserved partly by hydrogen bonds between the α-chains developed the hydroxyl group of hydroxyproline(Nagai et al.2008).
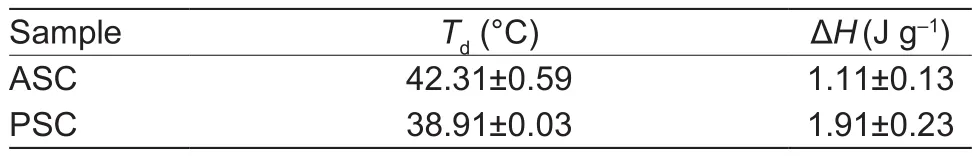
Table 3 The denaturation temperature (Td) of acid soluble collagens (ASC) and pepsin soluble collagens (PSC) from sheep bones
3.6.Zeta potential of sheep bones collagen
The zeta potentials of sheep bones collagen were shown in Fig.4.Both ASC and PSC had positive charge when pH increased from 2 to 4.5, while electric potential became negative in pH range from 6 to 11.When potential of ASC and PSC was zero, the pH values were 4.95 and 5.76,respectively, which were estimated to be their isoelectric points (pI).When potential of collagen molecules was zero, net charge of collagen and hydrophobic interactions between collagen molecules increased, afterwards leading to protein precipitation and aggregation.On the contrary,once pH value was above or below isoelectric points, net charge of collagen and the repulsion increased, leading to higher solubility of collagen (Singh et al.2011).
It turned out that collagen for ASC and PSC from various species exhibited different pI values ranging from pH 4 to 7 (Kittiphattanabawon et al.2010; Matmaroh et al.2011).The composition of amino acid might be responsible for the differences in pI.In addition, the variety of distribution of surface amino acid residues is also an important factor.In addition, ASC have a lower pI than PSC.Protonation and deprotonation of amino acid residues at different pHs regulated the charge of collagen.Hence, the cleavage of telopeptide regions by pepsin made an obvious distinction in amino acid composition in α-chain (as shown in Table 2).The higher content of acidic amino acid is also an important reason for decreased pIs of collagens.
4.Conclusion
Both ASC and PSC from ovine bones were type I collagens with some differences at terminals.FTIR analysis testified that both ASC and PSC preserved triple helical structures.Higher content of imino acid was determined in ovine bones collagen, which contributed to the higher thermostability of ovine bones collagen.Ovine bones are the potential source of collagen.
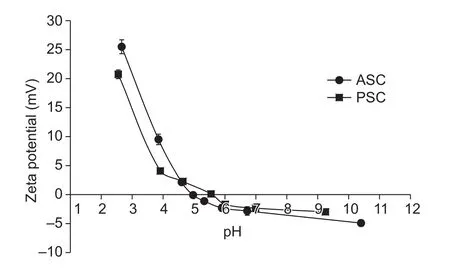
Fig.4 Zeta potential of acid soluble collagens (ASC) and pepsin soluble collagens (PSC) from sheep bones.Bars are SD.
Acknowledgements
This work has been funded by the emarked fund for China Agriculture Research System (CARS-39), the National Agricultural Science and Technology Innovation Program.The authors thank Inner Mongolia Grassland Hongbao Sheep Co., Ltd.for their helpful support in sampling.
Abe Y, Krimm S.1972.Normal vibrations of crystalline polyglycine I.Biopolymers, 11, 1817-1839.
Barth A, Zscherp C.2002.What vibrations tell about proteins.Quarterly Reviews of Biophysics, 35, 369-430.
Barzideh Z, Latiff A A, Gan C Y, Benjakul S, Karim A A.2014.Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.).International Journal of Food Science & Technology, 49, 1490-1499.
Chen J, Li L, Yi R, Xu N, Gao R, Hong B.2016.Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus).LWT-Food Science and Technology, 66, 453-459.
Chen L, Li X, Ni N, Liu Y, Chen L, Wang Z, Shen Q W, Zhang D.2016.Phosphorylation of myofibrillar proteins in postmortem ovine muscle with different tenderness.Journal of the Science of Food and Agriculture, 96, 1474-1483.
Chen Y, Ye R, Wang Y.2015.Acid-soluble and pepsin-soluble collagens from grass carp (Ctenopharyngodon idella)skin: A comparative study on physicochemical properties.International Journal of Food Science & Technology, 50,186-193.
Dai N, Etzkorn F A.2009.cis-Trans proline isomerization effects on collagen triple-helix stability are limited.Journal of the American Chemical Society, 131, 13728-13732.
Doyle B B, Bendit E G, Blout E R.1975.Infrared spectroscopy of collagen and collagen-like polypeptides.Biopolymers,14, 937-957.
Duan R, Zhang J, Du X, Yao X, Konno K.2009.Properties of collagen from skin, scale and bone of carp (Cyprinus carpio).Food Chemistry, 112, 702-706.
Edwards H G M, Farwell D W, Holder J M, Lawson E E.1997.Fourier-transform Raman spectroscopy of ivory:II.Spectroscopic analysis and assignments.Journal of Molecular Structure, 435, 49-58.
Engel J, Bächinger H P.2005.Structure, stability and folding of the collagen triple helix.Topics in Current Chemistry,247, 7-33.
Farrell Jr H M, Wickham E D, Unruh J J, Qi P X, Hoagland P D.2001.Secondary structural studies of bovine caseins:Temperature dependence of β-casein structure as analyzed by circular dichroism and FTIR spectroscopy and correlation with micellization.Food Hydrocolloids, 15, 341-354.
Foegeding E, Lanier T, Hultin H.1996.Characteristics of edible muscle tissues.Food Chemistry, 3, 879-942.
Guzzi Plepis A M D, Goissis G, Das-Gupta D K.1996.Dielectric and pyroelectric characterization of anionic and native collagen.Polymer Engineering and Science, 36,2932-2938.
Jackson M, Choo L P I, Watson P H, Halliday W C, Mantsch H H.1995.Beware of connective tissue proteins: Assignment and implications of collagen absorptions in infrared spectra of human tissues.Biochimica et Biophysica Acta-Molecular Basis of Disease, 1270, 1-6.
Jeevithan E, Wu W H, Wang N P, Lan H, Bao B.2014.Isolation,purification and characterization of pepsin soluble collagen isolated from silvertip shark (Carcharhinus albimarginatus)skeletal and head bone.Process Biochemistry, 49,1767-1777.
Karayannakidis P D, Chatziantoniou S E, Zotos A.2014.Effects of selected process parameters on physical and sensorial properties of yellowfin tuna (Thunnus albacares) skin gelatin.Journal of Food Process Engineering, 37, 461-473.
Kittiphattanabawon P, Benjakul S, Sinthusamran S, Kishimura H.2015.Characteristics of collagen from the skin of clown featherback (Chitala ornata).International Journal of Food Science & Technology, 50, 1972-1978.
Kittiphattanabawon P, Benjakul S, Visessanguan W, Kishimura H, Shahidi F.2010.Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum).Food Chemistry, 119, 1519-1526.
Laemmli U K.1970.Cleavage of structural proteins during the assembly of the head of bacteriophage T4.Nature, 227,680-685.
Lee J K, Kang S I, Kim Y.J, Kim M J, Heu M S, Choi B D,Kim J S.2016.Comparison of collagen characteristics of sea- and freshwater-rainbow trout skin.Food Science and Biotechnology, 25, 131-136.
Liu D, Liang L, Regenstein J M, Zhou P.2012.Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis).Food Chemistry, 133,1441-1448.
Liu D, Nikoo M, Boran G, Zhou P, Regenstein J M.2015.Collagen and gelatin.Annual Review of Food Science and Technology, 6, 527-557.
Liu D, Zhou P, Li T, Regenstein J M.2014.Comparison of acid-soluble collagens from the skins and scales of four carp species.Food Hydrocolloids, 41, 290-297.
Li Z, Wang B, Chi C, Zhang Q, Gong Y, Tang J, Luo H, Ding G.2013.Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius).Food Hydrocolloids, 31, 103-113.
Matmaroh K, Benjakul S, Prodpran T, Encarnacion A B,Kishimura H.2011.Characteristics of acid soluble collagen and pepsin soluble collagen from scale of spotted golden goatfish (Parupeneus heptacanthus).Food Chemistry,129, 1179-1186.
Nagai T, Suzuki N.2000.Isolation of collagen from fish waste material-skin, bone and fins.Food Chemistry, 68, 277-281.
Nagai T, Suzuki N, Nagashima T.2008.Collagen from common minke whale (Balaenoptera acutorostrata) unesu.Food Chemistry, 111, 296-301.
Nam K A, You S G, Kim S M.2008.Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates.Journal of Food Science, 73, C249-C255.
Payne K J, Veis A.1988.Fourier transform IR spectroscopy of collagen and gelatin solutions: Deconvolution of the amide I band for conformational studies.Biopolymers, 27,1749-1760.
Shoulders M D, Raines R T.2009.Collagen structure and stability.Annual Review of Biochemistry, 78, 929-958.
Singh P, Benjakul S, Maqsood S, Kishimura H.2011.Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus).Food Chemistry, 124, 97-105.
Toldrá F, Mora L, Reig M.2016.New insights into meat byproduct utilization.Meat Science, 120, 54-59.
Veeruraj A, Arumugam M, Ajithkumar T, Balasubramanian T.2015.Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis).Food Hydrocolloids, 43, 708-716.
Zhou G, Zhang W, Xu X.2012.China’s meat industry revolution:Challenges and opportunities for the future.Meat Science,92, 188-196.
 Journal of Integrative Agriculture2018年3期
Journal of Integrative Agriculture2018年3期
- Journal of Integrative Agriculture的其它文章
- Characteristic analysis of tetra-resistant genetically modified rice
- A wheat gene TaSAP17-D encoding an AN1/AN1 zinc finger protein improves salt stress tolerance in transgenic Arabidopsis
- Characterization of GhSERK2 and its expression associated with somatic embryogenesis and hormones level in Upland cotton
- GmNAC15 overexpression in hairy roots enhances salt tolerance in soybean
- Molecular cloning and functional characterization of a soybean GmGMP1 gene reveals its involvement in ascorbic acid biosynthesis and multiple abiotic stress tolerance in transgenic plants
- Responses of the antioxidant system to fluroxypyr in foxtail millet(Setaria italica L.) at the seedling stage
