基于D(-)/L(+)-对羟基苯甘氨酸配体的两个铜配合物的合成、结构和电化学性质
刘曼玉 史亚静 王兰芝 宋会花
(河北师范大学化学与材料科学学院,石家庄 050024)
0 Introduction
Currently,there has been increasing interest in creating of chiral compounds due to their potential applications,such as gas adsorption,catalysis,drug delivery,separation,fluorescence,non-linear optics[1-10].Chiral coordination polymers endued with plentiful structure,ultrahigh surface area and tunable ability should be regarded as promising potential chiral sensors[11-15]. Cyclic voltammogram(CV)technique provides highly selective,and fast speed operation,which be regarded as promising tool for exploring redox properties and chiral recognition[16-18].Generally,the predictable syntheses of chiral coordination frameworks have been accomplished in the following three ways:わusing the chiral ligands as a linker to connect metal ions[19];ぷusing achiral ligands under spontaneous resolution induced by chiral auxiliary[20];ぺusing the racemic organic ligands to self-assemble with metal ions in spontaneous resolution[21].However,the relative ease of formation by self-assembly usually be effected by many factors such as solvent system,ligand-to-metal ratios,temperature,metal ions,pH value of solution[22-27].Hence,the controllable synthesis of stable chiral coordination compounds becomes one of the most burdensome challenges to chemists.
In the above methods,using the chiral ligands as a linker to construct chiral coordination compounds is the most effective method.Some optically active amino acid molecules with chiral centers such as L-glutamic acid,L-tartric acid,L-lactic acid,and organic ligands containing chiral polypyridyl or polycarboxylate groups,have already been widely used to obtain artificial chiral compounds[28-34].D(-)/L(+)-4-Hydroxyphenylglycine(D/L-Hhpg)have both amino and carboxyl functional groups,and as ligands they can coordinate metal ions by monodentate,bidentate,chelating and bridging mode.A literature survey revealed that examples of complexes based on D/L-Hhgp are sparse.Only a few examples of chiral cyclic assemblies have been reported to date.Ivan Bernal et al.[35]have reported[Cu(4HPG)(bpy)]·2H2O compounds(4HPG=D-4-hydroxyphenylglycinato,bpy=2,2′-bipyridine),and in 2015 the related compound[Cu(hpg)2(H2O)]nwas synthesized by the group of Yao[36].Our laboratory have reported three enantiomeric pairs of chiral coordination compounds,namely{[Zn(D-hpg)(4,4′-bipy)(H2O)]NO3}n,{[Zn(L-hpg)(4,4′-bipy)(H2O)]NO3}n,{[Zn(D-hpg)(4,4′-bipy)(H2O)]ClO4}n,{[Zn(L-hpg)(4,4′-bipy)(H2O)]ClO4}n,[Zn(D-hpg)2(4,4′-bipy)]·2(4,4′-bipy)·H2O,[Zn(L-hpg)2(4,4′-bipy)]·2(4,4′-bipy)·H2O(4,4′-bipy=4,4′-bipyridine),and discussed how the metal salts and pH value affect the structures and properties of the compounds[37].
In this work two chiral coordination compounds assembled from copper salts and D/L-Hhpg,{[Cu(D-hpg)(phen)(NO3)]·1.5H2O}n(1)and{[Cu(L-hpg)(phen)(NO3)]·2H2O}n(2)(phen=1,10-phen-anthroline),were successfully synthesized and struc-turally characterized.Their crystal structures,TG-DTA analysis and cyclic voltammetry are discussed in detail.
1 Experimental
1.1 Materials and methods
All reagents and solvents for syntheses were purchased from commercial sources and were used as received without further purification.Element analyses(C,H and N)were performed on an Elemental Vario EL elemental analyzer.Infrared (IR)spectra were measured on a FTIR-8900 spectrometer from 4 000 to 400 cm-1(KBr pellets).Thermogravimetry-differential scanning calorimetry (TG-DSC)experiments were carried out on a simultaneous STA 449F3/TENSOR 27 thermal analyzer under a static N2atmosphere with a heating rate 10℃·min-1from room temperature to 850℃.Powder X-ray diffraction(PXRD)patterns were collected on a Bruker D8-Advance X-ray diffractometer using Cu Kα radiation(λ=0.154 2 nm,U=40 kV,I=40 mA)in 2θrange of 5°~50°at room temperature.The solid state circular dichroism (CD)spectra were recorded on a JASCOJ-810 spectropolari-meter with KCl pellets.Cyclic voltammetry measurements were carried out on a CHI 660 electrochemical workstation at room temperature.Platinum gauze was used as a counter electrode,and a saturated calomel electrode(SCE)was used as reference electrode.Chemically bulk-modified carbon paste electrodes (CPEs)were used as the working electrodes.The compound(1 and 2)modified CPEs(1/2-CPE)was fabricated as follows:0.1 g of graphite powder and 0.01 g of(1 and 2)were mixed and ground together by an agate mortar and pestle to achieve a uniform mixture and then 0.1 mL paraffin oil was added with stirring.The homogenized mixture was packed into a plastic tube with a 2 mm inner diameter.Electrical contact was established with a copper rod through the back of electrode.
1.2 Synthesis
1.2.1 {[Cu(D-hpg)(phen)(NO3)]·1.5H2O}n(1)
Cu(NO3)2·3H2O(0.024 1 g,0.1 mmol),D-Hhpg(0.016 7 g,0.1 mmol)was stirred into a 10 mL aqueous solution.A solution of phen(0.019 8 g,0.1 mmol)in EtOH(6 mL)was slowly added.The resulting solution was stirred for 20 minutes,The pH value of solution was adjusted to 4.8 with 1 mol·L-1NaOH solution,and the blue block-shaped transparent crystals 1 suitable for X-ray analysis were obtained with 66%yield based on Cu.Anal.Calcd.For C20H19N4O7.5Cu(%):C,48.14;H,3.83;N,11.22.Found(%):C,48.03;H,3.91;N,11.00.IR (KBr,cm-1):3 417 (w),3 286(s),3 232(m),1 635(s),1 519(m),1 465(w),1 427(m),1 388(s),1 327(s),1 249(m),1 141(m),1 103(w),1 018(m),848(m),833(m),794(w),725(m),609(w),570(w)。
1.2.2 {[Cu(L-hpg)(phen)(NO3)]·2H2O}n(2)
Compound 2 was synthesized in a procedure similar to that for 1 except that L-Hhpg(0.016 7 g,0.1 mmol)was used instead of D-Hhpg(0.016 7 g,0.1 mmol).The blue block-shaped transparent crystals of 2 were obtained with 62%yield based on Cu.Anal.Calcd.for C20H20N4O8Cu(%):C,47.29;H,3.96;N,11.03.Found(%):C,47.73;H,3.91;N,11.29.IR(KBr,cm-1):3 417(w),3 286(s),3 232(m),1 635(s),1 519(m),1 465(w),1 427(m),1 388(s),1 327(s),1 249(m),1 141(m),1 103(w),1 018(m),848(m),833(m),794(w),725(m),609(w),570(w).
1.3 X-ray crystallography
Single-crystal X-ray crystals for title compounds were selected for single-crystal diffraction analyses(Crystal size:0.31 mm×0.20 mm×0.15 mm for 1;0.29 mm×0.18 mm×0.12 mm for 2).The data for 1 and 2 were collected on a Bruker SMART-CCD diffractometer by φ-ω scan mode.The structure was solved through direct methods using SHELXS-97 and all non-hydrogen atoms were refined anisotropically by full-matrix leastsquares on F2using SHELXL-97[38].Further crystallographic data and experimental details for structural analyses of compounds 1 and 2 are summarized in Table 1.
CCDC:1440300,1;1505353,2.
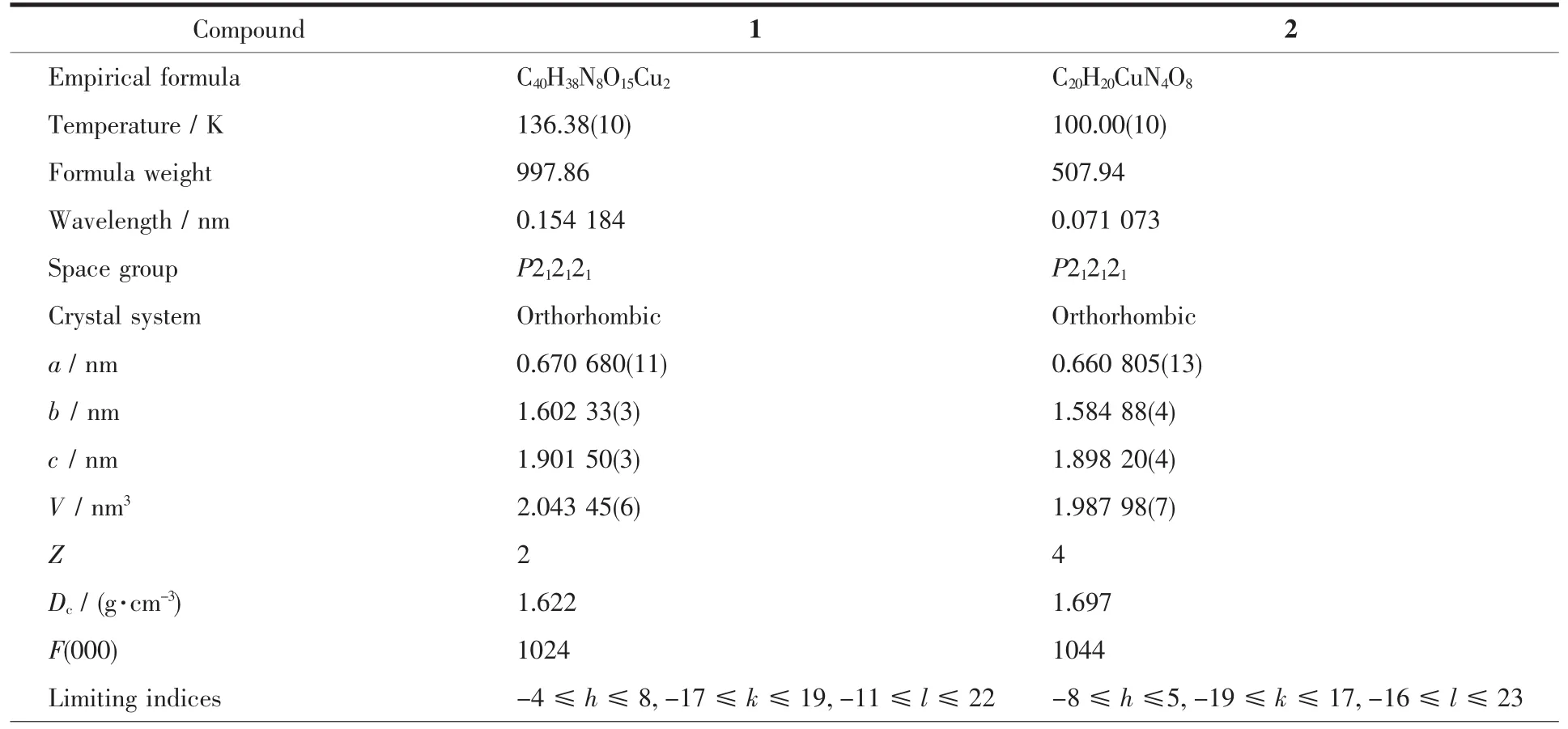
Table 1 Crystal data and structure refinements for 1 and 2

Reflection collected,unique 4 754,3 186(R int=0.013 3) 5 248,3 294(R int=0.022 4)Completeness toθ/% 99.7 99.7 Max.and min.transmission 1.000 00 and 0.824 28 1.000 00 and 0.995 98 Data,restraint,parameter 3 186,0,299 3 294,0,305 GOF 1.058 1.063 R1,wR2[I>2σ(I)] 0.029 7,0.099 2 0.033 2,0.083 5 R1,wR2(all data) 0.031 9,0.100 6 0.036 1,0.085 2 Absolute structure parameter -0.01(3) 0.000(14)Largest diff.peak and hole/(e·nm-3) -290 and-410 317 and-492 Flack parameter -0.01(3) 0.000(14)
2 Results and discussion
2.1 Crystal structure
Single crystal X-ray diffraction analysis revealed that compound 1 crystallizes in the Orthorhombic space group of P212121and possesses a 1D chain structure.As shown in Fig.1,the asymmetric unit is composed of one Cuギcation,one D-hpg-anion,one phen ligand,one and a half coordinated water molecule and one nitrate counter anion.Each Cuギexhibits a distorted octahedral geometry,which is six-coordinated by three oxygen atoms (O1,O6,O7)from one D-hpg-anion and two nitrate counter anions,and nitrogen atoms(N3,N1,N2)from one D-hpg-anion and one phen ligand.The O1,N1,N2 and N3 atoms form the equatorial plane,O6 and O7 atoms occupy the apical positions.The Cu-O bond lengths are 0.192 3(2)and 0.262 0(3)nm,and the Cu-N bond lengths range are between 0.199 9(2)and 0.201 3(3)nm,which are in accordance with the previously reported complexes[39].Selected bond lengths and angles are given in Table 2.

Fig.1 Coordination environment of Cuギfor 1(a)and 2(b)
For compound 1,nitrate counter anion bridges two Cuギions through two different oxygen atoms(O6,O7)to form a 1D infinite linear structure along the a-axis direction.As shown in Fig.2,the 1D chains are stacked in an ABAB fashion along the b-axis direction with the shortest Cu-Cu distance between adjacent chains being 0.867 54(7)nm.D-hpg-and phen coordinate with Cu2+in bidentate chelate coordination mode as modifying ligands,and arrange on both sides of the chain.It is worth noting that these chains are further extended through extensive hydrogen bonding interactions(Table 3):(1)hydrogen bonding between lattice water and lattice water(O(8)…O(7)#5 0.308 3(7)nm);(2) hydrogen bonding between lattice water and coordinated carboxyl oxygen atom (O(7)…O(1)#4 0.314 9(4)nm);(3)hydrogen bonding between lattice water and uncoordinated carboxyl oxygen atom(O(7)…O(2)#4 0.276 2(4)nm);(4)hydrogen bonding between lattice water and phenol hydroxyl oxygen atom(O(3)…O(7)#3 0.263 7(4)nm);(5)hydrogen bonding between the amino nitrogen atom of the coordination and the phenolic hydroxyl oxygen atom of another molecule(N(3)…O(3)#2 0.309 2(4)nm);(6)hydrogen bonding between the coordinated amino nitrogen atom and the coordinated nitrate anionoxygen atom(N(3)…O(5)#1 0.316 3(4)nm);(7)hydrogen bonding between lattice water and coordinated nitrate anion oxygen atom(O(7)…O(6)0.281 7(4)nm,O(8)…O(4)0.308 4(4)nm).Interestingly,between lattice water molecules and coordination nitrate anions there exist extensive hydrogen bonds(O(7)…O(6)0.281 7(4)nm,O(8)…O(4)0.308 4(4)nm,O(8)…O(7)#5 0.308 3(7)nm)resulting in a 1D left-handed helical chain along the b-axis direction(Fig.3).The adjacent chain form weak π-π stacking interaction through the phen ligand(Fig.4).The combination of hydrogen bonding and π-π stacking makes the 3D supramolecular structure of the compounds more stable.

Table 2 Selected bond distances(nm)and angles(°)for compound 1

Table 3 Hydrogen bond parameters for compound 1

Fig.2 ABAB stacking along b axis for 1(a)and 2(b)
By comparing the crystallography data of 1 and 2 showed in Table 1 as well as Fig.1a and Fig.1b,their different chirality is caused by the different chirality of main ligands,namely D-Hhpg and L-Hhpg.As shown in Fig.3b,the difference of their crystal structures is that L-hpg-ligands form right-handed supramolecular helices along the b-axis for 2 by the extensive hydrogen bond between lattice water molecules and coordination nitrate anion(O(7)…O(5)#2 0.278 1(4)nm,O(8)…O(7)#1 0.299 1(5)nm,O(8)…O(4)0.303 9(5)nm;Table 4).In addition,the Cu-O bond lengths are 0.192 5(2)and 0.261 3(2)nm,and the Cu-N bond lengths are 0.199 6(3)and 0.200 7(3)nm for 2.Selected bond lengths and angles are given in Table 5.

Fig.3 (a)One dimensional left-handed double stranded helices of compound 1;(b)One dimensional right-handed double stranded helices of compound 2

Fig.4 Three dimensional supramolecular layers formed by hydrogen bonding and π-π stacking for 1(a)and 2(b)

Table 4 Hydrogen bond parameters for compound 2

Table 5 Selected bond distances(nm)and angles(°)for compound 2
2.2 Powder X-ray diffraction analyses and IR spectra

Fig.5 PXRD patterns for 1(a)and 2(b)

Fig.6 IR spectra of 1(a)and 2(b)
The PXRD patterns for 1 and 2 are presented in Fig.5.The main peaks of simulated spectra of 1 and 2 matched well with their experimental spectra,demonstrating the crystallization degree and the purity of crystalline phase are both good.Theνas(COO-)and νs(COO-)vibrations of D-hpg-/L-hpg-can be observed at 1 635 and 1 427 cm-1.The peak at 1 249 cm-1is due toν(C-O)vibrations of phenolic hydroxyl group,and the peaks at 1 327 cm-1are due toνas(C-N)vibrations of amino-groups which connect to the carbon atoms in amino-acids.The(C-C)and(C=N)vibrations of phen can be observed at 1 519 and 1 388 cm-1,which had a certain degree of red-or blue-shift compared to the related absorption peak of phen at 1 504 and 1 419 cm-1,respectively.Meanwhile,the(C-H)bending vibration of phen at 840 and 732 cm-1have moved to 848 and 725 cm-1,respectively.This result indicates that the phen molecules have coordinated with metal.In addition,the strong peaks at 3 244~3 348 cm-1are due toν(N-H)vibrations of D-hpg-/L-hpg-ligands(Fig.6).
2.3 Circular dichroism spectra properties

Fig.7 Solid-state CD spectra of D-Hhpg/L-Hhpg and compounds 1 and 2
The solid-state circular dichroism(CD)spectra of compounds 1 and 2 were investigated at room temperature,as shown in Fig.7.Chiral ligand D-Hhpg displayed positive peak value at 245,261 and 283 nm,while L-Hhpg displayed negative peak value at 240,268 and 284 nm.Compound 1 displayed negative peak value at 237,275 and 305 nm,and compound 2 displays positive peak value at 254,282 and 306 nm.From the CD spectra,we can clearly see that 1 and 2 both show obvious Cotton effect,which confirms the two compounds are chiral compounds.Besides the opposite CD signal in the same peak position from compounds 1 and 2,we can come up with a conclusion is that their CD spectra are image symmetrical,while the CD signal direction of the compound is affected by chiral ligands(Fig.7).
2.4 Thermal analyses
The TG/DTG-DSC methods were used to describe thermal decomposition of synthesized compounds in air as shown in the Fig.8.The thermal decomposition results are presented in Table 6.Compound 1 is fairly stable before 121.37℃.The first weight loss in a temperature range of 121.37~181.37 ℃ is consistent with the removal of one and a half lattice water molecules(Obsd.4.55%,Calcd.5.41%).On the DSC curve,endothermic peak was observed at about 173.37℃,which can prove the loss of one and a half lattice water molecules.The second and third decomposition steps occurred in a temperature range of 181.37~251.37℃ and 251.37~481.15℃ with a weight loss of 21.49%and 24.51%,respectively,against calculated weight loss of 45.32%,corresponding to the loss of D-Hhpg and part of nitrate anion.Therewere a strong exothermic peak(195.87℃)and a weak exothermic peak (301.87℃)on the DSC curve,corresponding to the decomposition and degradation D-Hhpg and part of nitrate anion.The fourth decomposition steps took place at the temperature of 481.37℃,and until 850℃ no platform appeared,which indicates that the compound has not completely lost weight.

Table 6 Thermal decomposition data for compounds 1 and 2

Fig.8 TG-DTG and DSC curves of compounds 1(a)and 2(b)
Compound 2 is relatively stable before 124.38℃.The first weight loss in the temperature range of 124.38~159.38 ℃ is consistent with the removal of two lattice water molecules (Obsd.7.39%,Calcd.7.09%).On the DSC curve,endothermic peak was observed at about 173.88℃,which can prove the decomposition of one and two lattice water molecules.The second and third decomposition steps occurred in a temperature range of 159.38~180.88 ℃ and 180.88~256.88 ℃ with a weight loss of 2.90%and 21.02%,respectively,against calculated weight loss of 24.42%,corresponding to the loss of two nitrate anion.There were a weak exothermic peak(167.38℃)and a strong exothermic peak (194.88℃)on the DSC curve,corresponding to the decomposition two nitrate anion.The fourth decomposition steps took place at the temperature of 256.88℃,and no platform appeared until 850℃,indicating that the compound has not completely lost weight.
2.5 Cyclic voltammetry
We investigated the electrochemical behavior of the compounds by cyclic voltammetry(CV).Typically,the carbon paste electrodes modified by 1 and 2 were constructed and then electrochemical properties were studied in 1 mol·L-1H2SO4solution at different scan rates in a voltage range of-0.75~1.00 V.It can be seen that the potential of oxidation peak increased and that the potential of reduction peak decreased with the scan rate increasing (Fig.9a,c).Analysis of cyclic voltammetry at varying scan rates (0.03~0.13 V·s-1)demonstrates a linear relationship between the anode peakⅠ (or anodic peakⅠ′)current and the scan rate (Fig.9b,d),indicating a surface-controlled electrochemical process.

Fig.9 Cyclic voltammograms of compounds 1(a)and 2(c);Change of anodic and anode current(Ia)vs scan rate for compounds 1(b)and 2(d)
3 Conclusions
In summary,one pair of chiral coordination compounds{[Cu(D-hpg)(phen)(NO3)]·1.5H2O}n(1)and{[Cu(L-hpg)(phen)(NO3)]·2H2O}n(2)have been described in detail.They are 1D chain structure,which is extended into a 3D supramolecular structure by the hydrogen bond.Interestingly,for compounds 1 and 2,there exist left-handed or right-handed supramolecular helix chains along thebaxis direction by the hydrogen bond,respectively,which is extended by the hydrogen bond between coordinated nitrate anion and lattice water.The TG-DTG and DSC curves reveal that the frameworks of compounds 1 and 2 are thermally stable before 120℃.These phenomenon may be attributed to the coexistence of strong hydrogen bonding interaction,π-π stacking interaction and coordination interaction.The cyclic voltammetry of the compounds at varying scan rates (0.03~0.13 V·s-1)demonstrates a linear relationship between the anode peakⅠ (or anodic peak Ⅰ′)current and the scan rate,indicating a surface-controlled electrochemical process.

