社会隔离对雌性小鼠动情周期及中枢ERα和TH免疫活性表达的影响
程广超 杨萍 赫晨
摘要:雌激素受体(ERα)和多巴胺(DA)可参与雌性动物动情周期调节,故本研究通过酪氨酸羟化酶(TH)标记DA神经元,探讨了社会隔离对雌性动情周期及ERα和DA的影响。成年雌性ICR小鼠社会隔离10 d后,检测其动情周期变化及动情期和动情间期中枢ERα-IR和TH-IR神经元表达。结果发现,社会隔离延长了雌鼠的动情前期和动情期(P<0.05),但对动情后期和动情间期没有影响;社会隔离和动情周期均会显著影响终纹床核(BNST)、内侧视前区(MPOA)和下丘脑弓状核(AR)的ERα-IR神经元表达(P<0.05),并对AR的ERα-IR表达和中脑腹侧被盖区(VTA)的TH-IR神经元表达有交互影响(P<0.05),表现为社会隔离减少动情期AR的ERα-IR表达(P<0.05),增加动情期和动情间期TH-IR表达(P<0.05),且对动情期的影响更明显。提示社会隔离会干扰雌鼠的动情周期,ERα和DA在特定脑区的变化对此具有重要调制作用。
关键词:隔离;雌性小鼠;动情周期;雌激素;多巴胺;免疫活性表达
中图分类号: Q492 文献标志码: A 文章编号:1002-1302(2019)14-0197-05
雌性哺乳动物的动情周期分为动情前期(proestrus)、动情期(estrus)、动情后期(metestrus) 和动情间期(diestrus)4 个阶段。在雌性的非妊娠生殖活动中,动情状态呈周期性变化,卵子的形成和性激素的分泌也呈周期性波动。研究发现,动情周期会影响雌性动物的行为和情绪,如配偶选择[1]、焦虑水平[2]、社会性学习[3]、空间记忆或目标识别[4-5]以及对成瘾药物的敏感性等[6-7]。内稳态紊乱如免疫或炎症应激会干扰大鼠、母羊和猕猴(rhesus macaques)的排卵周期[8-10]。此外,心理社会性应激也会抑制促性腺激素分泌。对猕猴研究发现,心理应激增加再结合其他应激源能干扰生殖激素分泌,破坏月经周期[11-12];母羊的动情周期对于急性及反复的心理社会应激具有低抗性[13]。动情周期的变化是下丘脑-垂 体- 性腺(hypothalamic-pituitary-gonadal,HPG)轴周期变化所致。雌激素α受体(estrogen receptor alpha,ERα)参与对HPG轴的调节,在调制生殖行为中具有关键作用[14-15]。多巴胺(dopamine,DA)也会影响动情周期和排卵[16]。作为一种心理社会性应激,长期社会隔离会影响啮齿动物的行为、情绪和神经内分泌反应[17-18]。酪氨酸羟化酶(tyrosine hydroxylase,TH)是DA合成的限速酶,本研究探讨了长期社会隔离对雌性小鼠动情周期的影响及ERα和TH在动情期和动情间期的变化,以期探讨隔离应激对动情周期影响的可能机制。
1 材料与方法
1.1 试验动物
SPF级ICR雌性小鼠由宁夏医科大学实验动物中心提供,饲养于北方民族大学生物科学与工程学院实验动物饲养房,塑料饲养笼(32 cm×21.5 cm×17 cm)饲养,饲料和饮水充足。室温25 ℃,光—暗周期12 h—12 h,食物、饮水充足。2016年5月开始试验,小鼠10周龄,共44只,平均体质量约32 g,其中22只进行单独饲养(社会隔离),另外22只进行群居饲养(每笼4~6只)。隔离10 d后,随机取隔离和群居鼠各10只检测动情周期,剩下24只检测动情期和动情间期的ERα和TH表达,共分为4组:隔离动情期组(IE,n=6)、隔离动情间期组(ID,n=6)、群居动情期组(CE,n=6)和群居动情间期组(CD,n=6)。
1.2 动情周期的检测
取小鼠阴道上皮组织涂片进行亚甲基蓝染色,根据上皮细胞形态特点确定动情周期各阶段[19],并记录各阶段的持续时间。
1.3 免疫组织化学试验
动物经腹腔注射戊巴比妥钠麻醉。先用4%多聚甲醛进行灌注固定;取出脑组织放入4%多聚甲醛后固定过夜(4 ℃),后4 ℃置于30%蔗糖溶液直至组织沉底。用冰冻切片机将脑作冠状切,片厚40 μm。用山羊血清封闭液37 ℃湿盒内封闭1 h。滴加由抗体稀释液稀释的一抗:ERα(1 ∶ 100;sc-542,Santa Cruz,中杉金桥生物技术有限公司分装)和TH(1 ∶ 2 000;ab112,Abcam,Hong Kong),4 ℃孵育72 h。0.01 mol/L PBS漂洗5 min/次,共3次。滴加生物素化羊抗兔IgG(博士德生物工程有限公司,武汉),37 ℃湿盒内孵育 1.5 h。0.01 mol/L PBS漂洗5 min/次,共3次。滴加SABC试剂(博士德生物工程有限公司,武汉),37 ℃湿盒内孵育2.5 h。采用0.01 mol/L的PBS漂洗10 min/次,共4次。DAB显色剂显色。常规乙醇脱水,二甲苯透明,中性树胶封片。
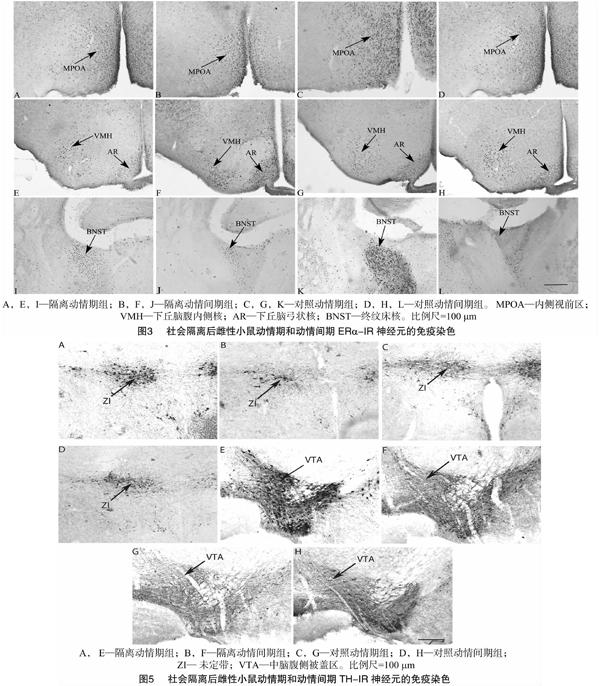
终纹床核(bed nucleus of the stria terminalis,BNST)、内侧视前区(medial preoptic area,MPOA)、下丘脑腹内侧核(ventromedial hypothalamic nucleus,VMH)和下丘脑弓状核(arcuate hypothalamic nucleus,AR)是ERα的重要分布区域。中脑腹侧被盖区(ventral tegmental area,VTA)、未定带(zona incerta,ZI)和下丘脑室旁核(paraventricular nucleus,PVN)是TH的重要分布區域,因此本研究检测了这些脑区的ERα和TH神经元表达。各脑区参照Paxinos & Franklin的著书以定位[20]。每只鼠选择3张连续的切片量化,利用显微测微尺,计算1 mm2内单侧脑区核团的阳性神经元数目。
1.4 统计方法
数据用SPSS 19.0软件进行统计分析。采用独立样本t检验比较隔离组与对照组(群居组)的动情周期。以隔离和动情周期作为2个固定因素,采用two-way ANOVA分析及post hoc检验ERα-IR和TH-IR神经元数量的组间差异。
2 结果与分析
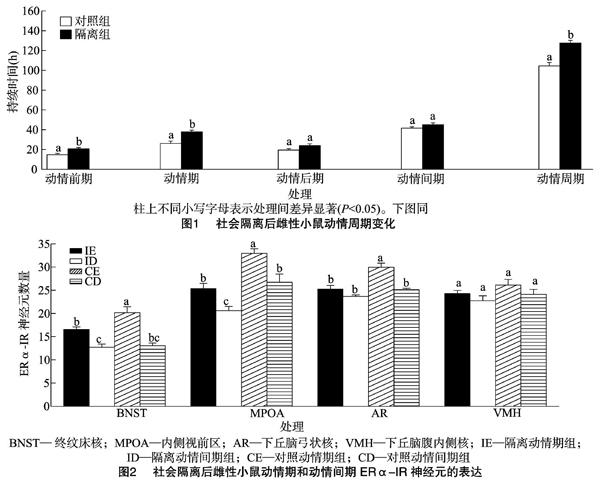
2.1 动情周期
3 讨论
动情周期的规律性是卵巢生殖功能正常的直接标志。本研究发现,社会隔离后雌性ICR小鼠的动情前期和动情期延长,动情后期和动情间期没有变化,总的动情周期变长。动情前期和动情期是排卵期,动情后期是分泌孕酮及黄体形成的时期,该结果暗示社会隔离延长了排卵时间。与本试验结果不同的是, 有研究发现隔离14 d的成年雌性C57BL/6J小鼠动情周期缩短并且肾上腺质量增加[21]。有种观点认为,在低群体密度下,延长动情期能增加受孕的可能性,这可能是对隔离应激的一种适应性反应[22]。生殖周期的形成是性类固醇激素通过作用于脑和垂体的正反馈和负反馈机制调制了黄体生成素(luteinizing hormone,LH)和促卵泡激素( follicle stimulating hormone,FSH)分泌产生的,其中雌二醇扮演了关键作用。对于成年雌性,促性腺激素释放激素(gonadotropin-releasing hormone,GnRH)的分泌受到性激素正反馈和负反馈调节,排卵前高水平的雌二醇通过正反馈使GnRH形成高峰,诱导LH释放并促进排卵[23]。应激对促性腺激素的分泌有损害,皮质酮和促肾上腺皮质激素释放因子(corticotropinreleasing factor,CRF)可以干扰促性腺激素对性激素生成细胞的刺激作用[24],并且心理社会应激可通过减少GnRH抑制LH的分泌[25]。因此,本試验中小鼠动情前期和动情期的延长,可能也是一种对隔离应激的适应,通过延长动情期和动情前期补偿排卵的下降。
动情期隔离组与动情期群居组相比,在BNST、MPOA和AR的ERα-IR表达减少;动情间期隔离组与动情间期群居组相比ERα-IR表达仅在MPOA减少,说明社会隔离在动情期和动情间期均会在一定脑区减少ERα的表达,但动情期的影响似乎更广泛。BNST的活动与焦虑和应激有关,BNST的差异表明社会隔离更易引起动情期应激和情绪的变化。ERα 表达受到外周雌激素的影响,MPOA区的雌激素受体mRNA水平在动情期比动情间期高[26];对雌性布氏田鼠(Lasiopodomys brandtii)研究表明,动情期较动情间期和动情前期在MPOA有更高的ERα-IR细胞[27]。动情期和动情间期的雌二醇的基础水平不同,这可能是社会隔离引起ERα 在2个阶段表达水平不同的一个重要原因。MPOA主要调节性行为,并影响对异性气味的偏好[28]。AR可调节不同的神经内分泌功能,其功能依赖于内在的不同神经内分泌细胞,其中GnRH 神经元分泌GnRH[29],AR的ERα神经元对雌性小鼠动情周期和雌激素负反馈机制具有关键作用。雌二醇通过
AR细胞间接、跨突触地抑制GnRH神经元的活动,从而抑制GnRH分泌进入正中隆起(median eminence) [30]。因此,社会隔离可通过调节ERα继而影响动情周期。至于VMH,虽然参与雌性性行为[31],但没有证据表明VMH神经元参与GnRH神经元网络[30]。
隔离组的动情期和动情间期分别与群居组的相比,VTA的TH表达增加,隔离组的动情期与群居组的相比,ZI的TH表达也有增加。此外,社会隔离和动情周期对VTA的TH表达有交互作用,隔离动情期组和群居动情期组分别与隔离动情间期组及群居动情间期组相比TH表达显著增加。DA能神经元的胞体主要位于VTA,TH是DA合成的限速酶,因此,这些结果说明社会隔离增加了动情期和动情间期的VTA的DA合成,且在动情期这种影响更明显。对八齿鼠(Octodon degus)研究发现,早期亲本隔离及断乳隔离会增加伏核(NAc)核区和壳区的TH-IR神经纤维密度[32];大鼠长期隔离后额叶皮质中TH活性也会增加[33]。从断乳后至成年的社会隔离会引起大鼠在VTA和NAc的DA释放和摄取增强[34]。DA通过控制黄体生成激素释放因子(luteinizing hormone releasing factor,LRF)诱导LH释放[35],并可刺激促卵泡激素释放因子(follicle stimulating hormone releasing factor,FSF)分泌和LRF释放[36],LRF可促进排卵,而DA水平的改变可以影响动情周期的持续时间,例如,高水平DA会延长大鼠的动情期[16]。本研究中,隔离组在动情期高水平的TH表达,暗示了更多的DA释放,这可能是引起动情期延长的重要机制之一。此外,DA可以调节情绪和社会行为[37],边缘系统如BNST、MPOA、VMH的ERα也与调节社会行为有关[38]。因此,隔离应激引起的动情期和动情间期ERα和TH的不同表达,改变动情周期,并可能影响到雌性在不同动情阶段的情绪和行为。
参考文献:
[1]Zinck L,Lima S Q. Mate choice in mus musculus is relative and dependent on the estrous state[J]. PLoS One,2013,8(6):e66064.
[2]Marcondes F K,Miguel K J,Melo L L,et al. Estrous cycle influences the response of female rats in the elevated plus-maze test[J]. Physiology & Behavior,2001,74(4/5):435-440.
[3]Choleris E,Clipperton-Allen A E,Gray D G,et al. Differential effects of dopamine receptor D1-Type and D2-Type antagonists and phase of the estrous cycle on social learning of food preferences,feeding,and social interactions in mice[J]. Neuropsy Chopharmacology,2011,36(8):1689-1702.
[4]Frick K M,Berger-Sweeney J. Spatial reference memory and neocortical neurochemistry vary with the estrous cycle in C57BL/6 mice[J]. Behavioral Neuroscience,2001,115(1):229-237.
[5]Paris J J,Frye C A. Estrous cycle,pregnancy,and parity enhance performance of rats in object recognition or object placement tasks[J]. Reproduction,2008,136(1):105-115.
[6]Sell S L,Dillon A M,Cunningham K A,et al. Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats[J]. Behavioural Brain Research,2005,161(1):69-74.
[7]Anker J J,Carroll M E. Females are more vulnerable to drug abuse than males:evidence from preclinical studies and the role of ovarian hormones[M]//Biological basis of sex differences in psychopharmacology. Berlin,Heidelberg:Springer:73-96.
[8]Nappi R E,Rivest S. Effect of immune and metabolic challenges on the luteinizing hormone-releasing hormone neural system in cycling female rats:an evaluation at the transcriptional level[J]. Endocrinology,1997,138(4):1374-1384.
[9]Battaglia D F,Krasa H B,Padmanabhan V,et al. Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes[J]. Biology of Reproduction,2000,62(1):45-53.
[10]Xiao E,Xia-Zhang L,Barth A,et al. Stress and the menstrual cycle:relevance of cycle quality in the short-and long-term response to a 5-day endotoxin challenge during the follicular phase in the rhesus monkey[J]. The Journal of Clinical Endocrinology and Metabolism,1998,83(7):2454-2460.
[11]Xiao E,Linna X Z,Ferin M. Inadequate luteal function is the initial clinical cyclic defect in a 12-day stress model that includes a psychogenic component in the rhesus monkey[J]. Journal of Clinical Endocrinology & Metabolism,2002,87(5):2232-2237.
[12]Williams N I,Berga S L,Cameron J L. Synergism between psychosocial and metabolic stressors:impact on reproductive function in cynomolgus monkeys[J]. American Journal of Physiology-Endocrinology and Metabolism,2007,293(1):270-276.
[13]Wagenmaker E R,Breen K M,Oakley A E,et al. The estrous cycle of the ewe is resistant to disruption by repeated,acute psychosocial stress[J]. Biology of Reproduction,2010,82(6):1206-1215.
[14]Couse J F,Yates M M,Walker V R,et al. Characterization of the hypothalamic-pituitary-gonadal (HPG) axis in female estrogen receptor knockout mice[J]. Biol Reprod,2002,66:98.
[15]Ogawa S,Eng V,Taylor J,et al. Roles of estrogen receptor alpha gene expression in reproduction-related behaviors in female mice[J]. Endocrinology,1998,139(12):5070-5081.
[16]Uemura H,Kobayashi H. Effects of dopamine implanted in the median eminence on the estrous cycle of the rat[J]. Endocrinologia Japonica,1971,17(6):91.
[17]Berry A,Bellisario V,Capoccia S,et al. Social deprivation stress is a triggering factor for the emergence of anxiety-and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice[J]. Psychoneuroendocrinology,2012,37(6):762-772.
[18]劉朝宝,王建礼,詹 泊. 断乳后隔离对BALB/c小鼠的焦虑水平、社会行为及血清应激激素影响的性别差异[J]. 生命科学研究,2016,20(4):325-332,357.
[19]Byers S L,Wiles M V,Dunn S L,et al. Mouse estrous cycle identification tool and images[J]. PLoS One,2012,7(4):e35538.
[20]Paxinos G,Franklin K. The mouse brain in stereotaxic coordinates[M]. 2nd ed. New York:Academic Press,2001:26-109.
[21]Bronson F H,Champman V M. Adrenal-estrus relationship in grouped or isolated mice[J]. Nature,1968,218(5140):483-484.
[22]Shamolina T S,Pivina S G,Ordian N E. Effects of social isolation during puberty on reproductive functions and behavior of prenatally stressed female rats[J]. Rossiiskii Fiziologicheskii Zhurnal Imeni I.M. Sechenova,2010,96(6):598-608.
[23]Moenter S M,Chu Z,Christian C A. Neurobiological mechanisms underlying oestradiol negative and positive feedback regulation of gonadotrophin-releasing hormone neurones[J]. Journal of Neuroendocrinology,2009,21(4):327-333.
[24]Rivier C,Rivest S. Effect of stress on the activity of the hypothalamicpituitary-gonadal axis:peripheral and central mechanisms[J]. Biology of Reproduction,1991,45(4):523-532.
[25]Wagenmaker E R,Breen K M,Oakley A E,et al. Psychosocial stress inhibits gonadotropin-releasing hormone pulses independent of cortisol action on the type Ⅱ glucocorticoid receptor[J]. Endocrinology,2009,150(2):762-769.
[26]Shughrue P J,Bushnell C D,Dorsa D M. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle:a comparison with ovariectomized females and intact males[J]. Endocrinology,1992,131(1):381-388.
[27]Pan Y,Xu L,Wang Z,et al. Expression of oestrogen receptor alpha in the brain of brandts voles (Lasiopodomys brandtii):sex differences and variations during ovarian cycles[J]. Journal of Neuroendocrinology,2011,23(10):926-932.
[28]Martinez L A,Petrulis,A. The medial preoptic area is necessary for sexual odor preference,but not sexual solicitation,in female Syrian hamsters[J]. Hormones and Behavior,2013,63(1):606-614.
[29]Bouret S G,Draper S J,Simerly R B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice[J]. Journal of Neuroscience,2004,24(11):2797-2805.
[30]Yeo S H,Herbison A E. Estrogen-negative feedback and estrous cyclicity are critically dependent upon estrogen receptor-α expression in the arcuate nucleus of adult female mice[J]. Endocrinology,2014,155(8):2986-2995.
[31]Holder M K,Hadjimarkou M M,Zup S L,et al. Methamphetamine facilitates female sexual behavior and enhances neuronal activation in the medial amygdala and ventromedial nucleus of the hypothalamus[J]. Psychoneuroendocrinology,2010,35(2):197-208.
[32]Gos T,Becker K,Bock J,et al. Early neonatal and postweaning social emotional deprivation interferes with the maturation of serotonergic and tyrosine hydroxylase-immunoreactive afferent fiber systems in the rodent nucleus accumbens,hippocampus and amygdala[J]. Neuroscience,2006,140(3):811-821.
[33]Toru M. Increased tyrosine hydroxylase activity in frontal cortex of rats after long-term isolation stress[J]. LEncéphale,1982,8(2):315-317.
[34]Yorgason J T,Calipari E S,Ferris M J,et al. Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum[J]. Neuropharmacology,2016,101:471-479.
[35]Kamberi I A,Mical R S,Porter J C. Luteinizing hormone-releasing activity in hypophysial stalk blood and elevation by dopamine[J]. Science,1969,166(3903):388-390.
[36]Kamberi I A,Schneider H P,Mccann S M. Action of dopamine to induce release of FSH-releasing factor(FRF)from hypothalamic tissue in vitro[J]. Endocrinology,1970,86(2):278-284.
[37]王建禮,邰发道,赵清梅. 社会互作奖赏效应的多巴胺依赖机制[J]. 生命科学,2011,23(5):423-428.
[38]Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network[J]. Ann N Y Acad Sci,1999,877:242-257.

