Surface molecular imprinting on g-C3N4 photooxidative nanozyme for improved colorimetric biosensing
Yunheng Wu,Qing Chen,Shung Liu,Hu Xio,Menglin Zhng,Xinfeng Zhng,,*
a College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China
b College of Environment and Ecology, Chengdu University of Technology, Chengdu 610059, China
Keywords:
Molecularly imprinted polymer
Graphitic carbon nitride
Nanoenzyme
Colorimetry
Biosensing
ABSTRACT
Graphitic carbon nitride(g-C3N4),as a visible-light-active organic semiconductor,has attracted growing attentions in photocatalysis and photoluminescence-based biosensing.Here, we demonstrated the intrinsic photooxidase activity of g-C3N4 and then surface molecular imprinting on g-C3N4 nanozymes was achieved for improved biosensing.Upon blue LED irradiation, the g-C3N4 exhibited superior enzymatic activity for oxidation of chromogenic substrate like 3,3′,5,5′-tetramethylbenzidine (TMB)without destructive H2O2.The oxidation was mainly ascribed to· O2- that was generated during light irradiation.The surface molecular imprinting on g-C3N4 can lead to an over 1000-fold alleviation in matrix-interference from serum samples, 4-fold improved enzymatic activity as well as enhanced substrate specificity comparing with bare g-C3N4 during colorimetric sensing.Also, the MIP-g-C3N4 possesses a high affinity to TMB with a Km value of only 22 μmol/L, much lower than other comment nanozymes like AuNPs, Fe3O4 NPs, etc.It was successfully applied for detection of cysteine in serum sample with satisfactory recoveries.
Nanomaterial-based artificial enzymes, termed nanozymes,have attracted growing interest due to their high stability,low cost of synthesis,and ease of recycling and reuse[1-3].The Nanozymes are attractive for various applications, ranging from biosensing[4,5], disease therapy [6], anti-bacteria [7]to pollutants degradation [8].Especially, the peroxidase-like nanozymes can catalytically oxidize chromogenic substrates such as 3,3′,5,5′-tetramethylbenzidine(TMB)and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)(ABTS)to produce a visual signal[9,10].The interest of developing such nanozymes rests on their application for amplified biosensing.
Different to the most frequently used peroxidases, photooxidase mimics that can be activated by light for the oxidation of substrate in the presence of dissolve oxygen,have showed to be a fascinating alternative, since it not only avoids the destructive H2O2but also makes the oxidation reaction easily be modulated by light.A variety of inorganic nanomaterials, such as TiO2[11], GO[12], AgX [13]and gold nanocluster [14], have severed as the photooxidase mimics.Nevertheless, weak absorption of visible light by these semiconductor nanocrystals limits their applications.Correspondingly, organic photocatalysts like fluorescein[15], phloxine B [16], dsDNA-SYBR Green [17], 9-mesityl-10-methylacridinium ion [18]with strong visible absorption, was exploited as photooxidase mimics.
The graphite carbon nitride (g-C3N4), as an emerging visiblelight-active organic semiconductor with a band gap of~2.7 eV(~460 nm in optical wavelength), has received worldwide attention due to its fascinating merits, such as proper electronic band structure, nontoxicity, low cost, good stability, and easy preparation [19].In recent years, g-C3N4has been extensively used for photocatalytic hydrogen generation [20], photocatalytic degradation[21,22],etc.The intrinsic fluorescence property of g-C3N4also lends itself to biosensing or bioimaging applications [23-26].Hence,g-C3N4is desired to be an ideal visible-light-active material as photooxidase nanozyme.
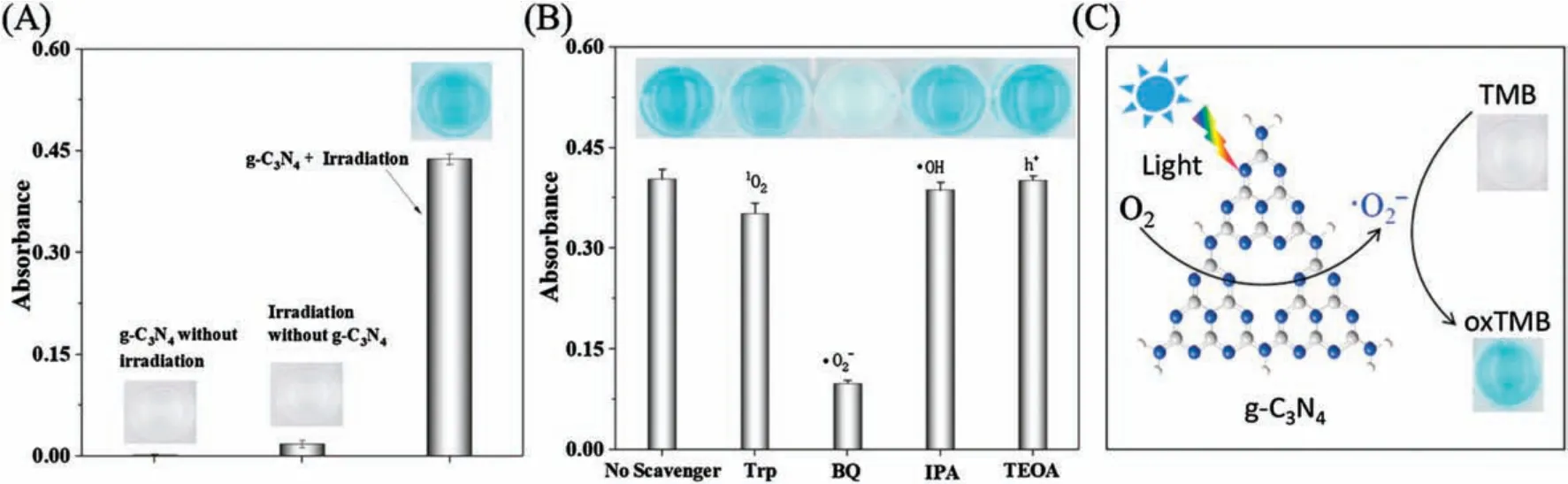
Fig.1.(A) The photooxidase activity of g-C3N4; (B) The effect of scavenger of oxidation on TMB; (C) The principle of g-C3N4-catalyzed oxidation of TMB.Experimental conditions: g-C3N4 concentration: 20 μg/mL; solution pH 4.0; TMB concentration: 0.5 mmol/L; LED color: blue.
During the biosensing applications of nanozymes,interference from biological sample matrix can always be suffered since the surface of nanozyme is active and can be readily interacted with biomolecules from small molecules to proteins[27].For examples,the 10-fold diluted serum sample can cause >600% positive interference during Fe3O4and AuNPs nanozyme-based biosensing,and >40%for even 1000-fold diluted serum sample(Figs.S1A and B in Supporting information).Such serious interferences make accurate analysis of biological samples difficult.Molecularly imprinted polymers (MIP), also known as plastic antibodies, can create target molecule-binding sites on large scales,and have been extensively used for solving matrix-interference[28,29].However,the MIP modification of oxidase mimics now is mainly utilized for enhancing the enzymatic activity or substrate selectivity [30-32].It is also expected to be a good approach for alleviating the matrixinterference during nanozyme-based biosensing.
In this work,we firstly demonstrated the intrinsic photooxidase activity of g-C3N4, and then molecular imprinting on g-C3N4surface was achieved.We found the MIP-g-C3N4nanozyme showed a top superiority in suppressing the matrix-interference,also being accompanied by enhancing the enzymatic activity and substrate selectivity.The new nanozyme was applied for detection of L-cysteine,a biomarker related to several important diseases like cancer and Alzheimer's disease.This MIP-g-C3N4is believed to be a promising photooxidase nanozyme for biosensing.
For probing the intrinsic photooxidase activity of g-C3N4,a wellknown chlorogenic substrate, TMB, was used for visual signaling.As shown in Fig.1A, no blue color can be observed for g-C3N4without light-irradiation, illustrating that g-C3N4did not possess oxidase activity; with blue LED irradiation, g-C3N4oxidized the TMB into a deep blue colored solution, demonstrating the activation of oxidase activity under light-irradiation.The Michaelis constant, Kmfor TMB, is calculated to be 152 μmol/L and the maximum reaction rate Vmaxcan achieve 0.74×10-8mol L-1s-1(Fig.S2 in Supporting information).

Fig.2.(A)The schematic illustration of preparing MIP-g-C3N4;(B) FT-IR spectra of g-C3N4, MIP and MIP-g-C3N4; (C) UV-vis spectra of g-C3N4, MIP and MIP- g-C3N4;SEM images of g-C3N4 (D) and MIP-g-C3N4 (E).Scale bars in (D) and (E) are 500 nm.
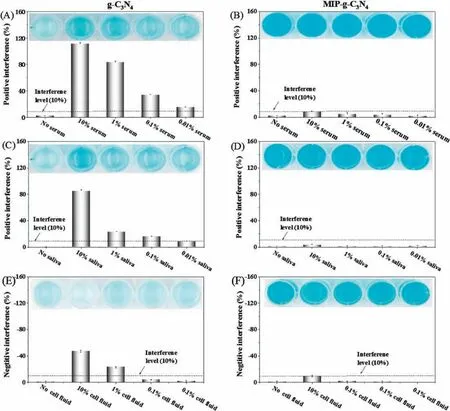
Fig.3.The matrix-interference from serum sample for g-C3N4(A)and MIP-g-C3N4(B),from saliva samples for g-C3N4(C)and MIP-g-C3N4(D),and from cell fluid for g-C3N4(E)and MIP-g-C3N4 (F).The experimental conditions were the same as those in Fig.1.
Subsequently, we further investigated the reactive oxygen species (ROS) produced during the g-C3N4photo-catalyzed oxidation of TMB by adding a series of free radica scavengers.Typically, tryptophan (Trp), isopropanol (IPA), p-benzoquinone(BQ)and triethanolamine(TEOA)were used as scavengers for1O2,·OH,·O2-and h+, respectively.As shown in Fig.1B, the·O2-scavenger BQ has an obvious inhibitory effect on the photooxidation of TMB.This result revealed that the TMB oxidation catalyzed by g-C3N4photooxidase nanozyme was mainly ascribed to·O2-that was generated during LED-irradiation of g-C3N4(Fig.1C).
Surface modification is essential for improving the performance of nanozyme [33].g-C3N4, similar to other nanozyme, is active to interact with biomolecules in sample matrix,leading to a positive or negative matrix interferences.We hope the MIP layer on g-C3N4only permit substrate molecule to binding sites and leave the biomolecules such as protein, amino acid in the sample matrix outside it.Such a selective binding to the MIP-g-C3N4can greatly suppress the matrix interference.To achieve this, the substrate TMB molecules were firstly adsorbed on the surface of g-C3N4.Then,NIPAAm as monomer and MBAAm as cross linker were added to the g-C3N4-TMB mixture.The system underwent thermalinduced polymerization at 80°C to yield MIP layer on g-C3N4.Finally, the TMB template was removed away to create binding sites (Fig.2A).
To confirm the successful formation of MIP material on g-C3N4surface, we firstly measured the FT-IR spectra of free g-C3N4, MIP and MIP-g-C3N4.As shown in Fig.2B,the g-C3N4materials have a series of peaks from 1640-1240 cm-1(1243,1325,1412,1575 and 1639 cm-1, respectively), corresponding to the typical stretching modes of CN heterocycles [34].And the peak at 810 cm-1is attributed to the ring-sextant out-of-plane bending vibration characteristic of both triazine or heptazine ring systems [35].Although the FT-IR spectra of MIP and MIP-g-C3N4are very similar,peaks at 1243, 1325 and 810 cm-1that belong to g-C3N4were clearly observed in MIP-g-C3N4, illustrating the formation of MIP on g-C3N4.The UV spectra of the three materials also demonstrated such result(Fig.2C).The SEM images of g-C3N4and MIP-g-C3N4are shown in Figs.2D and E.It can be seen that the g-C3N4nanosheetsare stacked together (Fig.2D).After imprinting, the sheet layer disappeared,since the g-C3N4materials were fully covered by tiny homogeneous polymer particles(Fig.2E).The compact and porous network of MIP provides numerous recognition sites for the substrate molecules.By measuring the fluorescence of g-C3N4,the imprinting efficiency for g-C3N4is estimated to be 86%.

Table 1 Comparison of MIP-g-C3N4 with other nanozyme on anti-interference ability for serum sample.

Fig.4.(A) UV-vis spectra of oxidation of TMB catalyzed three different nonozymes and (B) their kinetics monitored at 652 nm; (C) The specificity of MIP-g-C3N4 photo oxidase and (D) MIPs with different template molecule of oxidation of TMB.
Human serum, a typical biological sample, was firstly used for the investigation of matrix-interference on bare g-C3N4and MIP-g-C3N4enzymatic activity.Fig.3A showed that serum matrix has a serious positive interference on the enzymatic activity of bare g-C3N4, and there is still 18% positive interference even for 0.01%serum samples.After imprinting, the MIP-g-C3N4nanozyme greatly enhanced the anti-interference ability, as shown in Fig.3B.Up to 10% of serum samples cause <10% interference in the coloration using MIP-g-C3N4nanozyme.Hence, the matrix interference from serum sample can be alleviated over 1000 fold after imprinting.The matrix interference from other biological samples like saliva and cell fluid can be also greatly reduced by imprinting ( Figs.3C-F).
The anti-interference ability of MIP-g-C3N4was compared with other oxidase/peroxidase-like nanozyme.Table 1 listed the tolerant serum percentage for various nanozymes (<10% in variation of absorbance value).It can be seen that the MIP-g-C3N4has a much better anti-interference ability than other nanozymes.Although the non-imprinted polymer modified g-C3N4(NIP-g-C3N4) was also covered with polymers, its tolerant serum percentage was only 0.1%, still 100-flod lower than that of MIP-g-C3N4.Such result demonstrated that the template molecule imprinting was quite essential for improving the anti-interference ability.For the common peroxidase-like Fe3O4NPs and AuNPs nanozyme, the tolerant percentage of serum is only 0.01% or less(Figs.S3 and S4 in Supporting information);the serum sample can cause a negative interference to C-dot phooxidase (Fig.S5 in Supporting information) and the tolerant percentage of serum is 1%; for PB, the serum matrix yield a quite serious positive interference, and the inference is >50% even for 0.01% serum(Fig.S6 in Supporting information).In all, the anti-interference ability of MIP-g-C3N4was also greatly improved comparing with the common peroxidase/oxidase-like nanozyme.
The created MIP binding sites on g-C3N4not only improved the enzyme affinity but also enhanced its enzymatic activity.To investigate the effect of imprinting enzymatic activity, all the oxidation experiments were performed at the same concentration of g-C3N4(10 μg/mL), regardless of g-C3N4, NIP-g-C3N4or MIP-g-C3N4.Fig.4A exhibited that g-C3N4and NIP-g-C3N4possessed similar enzymatic activities.The result indicates that the synthesized polymer does not lead to a positive effect on the photocatalytic ability of the g-C3N4.Comparing with g-C3N4and NIP-g-C3N4, the oxidation activity of MIP-g-C3N4increased over 4-fold,revealing that molecular imprinting can indeed improve the catalytic ability.The reaction kinetics in Fig.4B also demonstrated the much higher enzymatic activity for MIP-g-C3N4.The Vmaxfor MIP-g-C3N4-catalyzed oxidation of TMB can reach as high as 3.5×10-8mol L-1s-1(Fig.S7 in Supporting information).
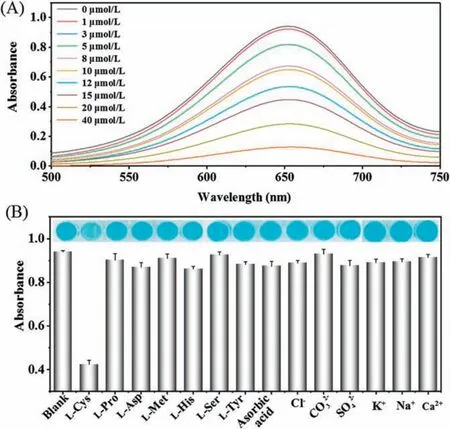
Fig.5.(A) The decreased oxidation of TMB with the increase of L-cysteine concentrations; (B) The selectivity of MIP-g-C3N4-based assay for detection of Lcysteine.
Substrate specificity is also important to nanozyme mimics.In order to prove the specificity, the nanozymes was used for catalyzed-oxidization of different substrate,i.e.,TMB,ABTS and DA.As shown in Fig.4C,the bare g-C3N4can oxidize all the substrate.After TMB-imprinting, the oxidation activity for TMB was improved about 4.7-fold, whereas only 1.1 and 1.3 for ABTS and DA.To test further whether the specificity of MIP is substratedependent, MIP with different templates TMB (MIPTMB), ABTS(MIPABTS) and DA (MIPDA) was also used for oxidation of TMB.Fig.4D showed that MIPABTSand MIPDApossess a similar enzymatic activity comparing with NIP, whereas MIPTMB-g-C3N4-catalyzed oxidation of TMB yielded the deepest blue color.
The MIP-g-C3N4nanozyme was applied for detection of Lcysteine,animportant marker of several diseases,such as cancer and Alzheimer's disease.Since the oxidation reaction of TMB can be inhibited by L-cysteine, a simple sensing assay for L-cysteine detection is developed based on the inhibition activity of TMB oxidation(Fig.S8 in Supporting information).As depicted in Fig.5A,the absorbance values of the oxTMB gradually decrease with the increase of L-cysteine concentrations.The linear response for Lcysteine detection ranged from 1 μmol/L to 20 μmol/L (Fig.S9 in Supporting information)with a limit of detection about 0.2 μmol/L.The MIP-g-C3N4-based sensing system were more sensitive than most of the reportednanozyme-basedassays for L-cysteinedetection(Table S1 in Supporting information).In addition, the selectivity experiment for L-cysteine detection was also evaluated by testing the response to as erieso fo the raminoacids and ions.Fig.5Bshowedthat only the target analyte L-cysteine can lead to inhibit the oxidation of TMB,demonstrating the good selectivity of the sensor.The MIP-g-C3N4-based assay was also applied for detection of L-cysteine in spiked serum samples with recoveries ranging from 91.6%to 94.1%.
In summary,we have demonstrated the intrinsic photooxidase activity of g-C3N4and then surface molecular imprinting can significantly enhance the performance of nanozyme.The most impressive feature of MIP-g-C3N4photooxidase nanozyme is the much improved (1000-fold) anti-interference ability for biosamples comparing with the present nanozymes.Also,the MIP-g-C3N4possesses a good affinity toTMB with a Kmvalue of only 22 μmol/L,much lower than comment nanozymes like AuNPs,Fe3O4NPs,etc.After imprinting, the enzymatic activity and substrate selectivity was also enhanced significantly.It was also applied for sensitive detection of L-cystine in serum sample successfully.Hence, the MIP-g-C3N4photooxidase nanozyme with the advantages of improved anti-interference ability, high substrate affinity and enzymatic activity, is an appealing new tool for biosensing.
Acknowledgments
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (No.21475013)and the Sichuan Science and Technology Project(No.2018JY0466).
Appendix A.Supplementary data
Supplementary material related to this article can be found,in the online version,at doi:https://doi.org/10.1016/j.cclet.2019.08.014.
 Chinese Chemical Letters2019年12期
Chinese Chemical Letters2019年12期
- Chinese Chemical Letters的其它文章
- A roadway of exploring polymer science, a lifetime of nurturing polymer scientists
- A personal journey on using polymerization in aqueous dispersed media to synthesize polymers with branched structures
- Amphiphilic block copolymers directed synthesis of mesoporous nickel-based oxides with bimodal mesopores and nanocrystal-assembled walls
- Synthesis of magnetic polyphosphazene-Ag composite particles as surface enhanced Raman spectroscopy substrates for the detection of melamine
- Photothermal performance of MFe2O4 nanoparticles
- Enhanced electrochemical performance and mechanism study of AgLi1/3Sn2/3O2 for lithium storage
