Central nervous system relapse in a pediatric anaplastic large cell lymphoma patient with CLTC/ALK translocation treated with alectinib:A case report
Jing Yang, Jun Li, Wei-Yue Gu, Ling Jin, Yan-Long Duan, Shuang Huang, Meng Zhang, Xi-Si Wang, Yi Liu,Chun-Ju Zhou, Chao Gao, Hu-Yong Zheng, Yong-Hong Zhang
Jing Yang, Jun Li, Ling Jin, Yan-Long Duan, Shuang Huang, Meng Zhang, Xi-Si Wang, Yi Liu,Chun-Ju Zhou, Chao Gao, Hu-Yong Zheng, Yong-Hong Zhang, Beijing Key Laboratory of Pediatric Hematology Oncology, National Discipline of Pediatrics, Ministry of Education,MOE Key Laboratory of Major Diseases in Children;Hematology Oncology Center, Beijing Children's Hospital, Capital Medical University, National Center for Children’s Health, Beijing 100045, China
Wei-Yue Gu, Chigene (Beijing) Translational Medical Research Center Co., Ltd., Beijing 101111, China
Abstract
Key words: Anaplastic;Lymphoma;Pediatric;CLTC/ALK;Central nervous system;Alectinib;Case report
INTRODUCTION
Anaplastic lymphoma kinase (ALK) expression is absent from all normal human postnatal tissues except rare cells in the brain.Expression of theALKgene in ALKpositive (ALK+) anaplastic large cell lymphoma (ALCL) is usually due to the(2;5)(p23;q35) chromosome translocation, which causes the fusion of theALKandNPM1genes, providing a promoter involved in the activation of ALK kinase, which plays a direct causative role in ALCL[1,2].
The aberrant expression of ALK can also result from a rearrangement of theALKgene with various partner genes[2-4].The ALK fusion partner determines the intracellular localization of the fusion protein.Immunohistochemistry with specific anti-ALK monoclonal antibodies indicates that NPM1-ALK fusion proteins are characterized by nuclear and cytoplasmic ALK staining, whereas the variant ALK fusion proteins are usually cytoplasmic and rarely show membranous ALK staining.Thus, immunohistochemistry can be used for screening the classical (2;5) translocation instead of molecular tests[1].
CLTC-ALKis a known recurrent fusion gene in ALK-positive diffuse large B-cell lymphoma but very rare in ALCL[2,5-7].Here, we report a case of ALCL withCLTC-ALKgene fusion arising from t(2;17)(p23;q23).The patient developed central nervous system (CNS) metastasis and achieved complete remission (CR) through chemotherapy and treatment with alectinib, which is a second-generation ALK tyrosine kinase inhibitor (TKI).This study was approved by the Beijing Children’s Hospital Institutional Ethics Committee.
CASE PRESENTATION
Chief complaints
An 8-year-old girl presented with fever, skin nodules with necrosis and ulceration, leg swelling, and abdominal pain over the preceding 6 mo (Figure 1).
Imaging examinations
She was admitted to our institution, and magnetic resonance imaging (MRI),computed tomography (CT), and positron emission tomography (PET) scans revealed that she had extensive involvement, including the skin, soft tissues, lungs, bones(skull, vertebrae, ribs, ilium, limb bones, and jawbone), bone marrow, lymph nodes(cervical, mediastinal, axillary, pelvic, and inguinal lymph nodes), ovaries, and pancreas, as well as a massive mesenteric tumor (Figure 2).
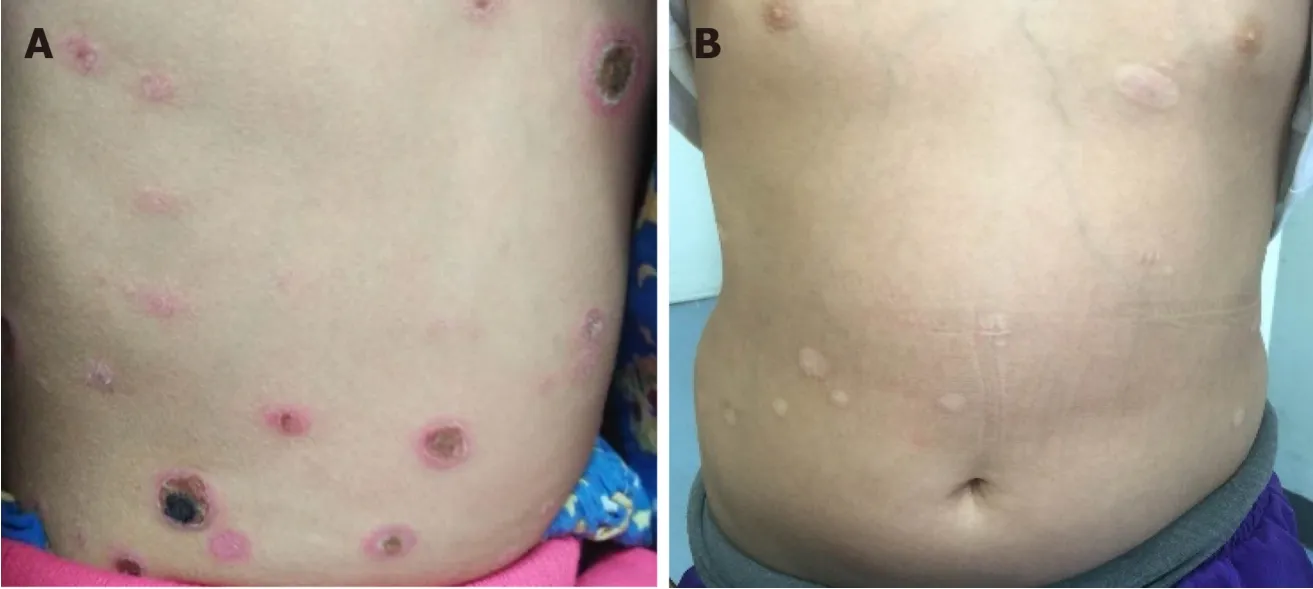
Figure 1 Skin rashes (A) before treatment and (B) after chemotherapy.
Laboratory examinations
Complete blood count showed mild anemia (hemoglobin 9.9 g/dL), while the white blood cell and platelet counts were normal.Lactose dehydrogenase was slightly elevated (720 IU/L).A bone marrow aspiration smear revealed 86% of atypical lymphocytes, which differed in size and shape.They had eccentric nuclei, prominent nucleoli, and abundant cytoplasm containing prominent vacuoles (Figure 3).
Pathological findings
Histopathological examination of the biopsied specimens from inguinal lymph nodes and bone marrow showed a diffuse infiltrate of irregularly shaped tumor cells.Immunophenotypically, the tumor cells were negative for CD3, CD5, CD8, and CD20,and positive for CD30, CD4, CD7, CD2, EMA, TIA-1, Gram-B, and Ki67 (80%+);ALK was strongly positive.Therefore, a diagnosis of ALK+ALCL was made.Rarely, the ALK protein was distributed in a restricted cytoplasmic staining pattern, indicating that the aberrant ALK expression was due to a partner gene other thanNPM1(Figure 4).
Further diagnostic work-up
Transcriptome sequencing of bone marrow mononuclear cells (BMMCs) by RNA-seq was performed to identify the fusion gene.Total RNA was extracted with TRIzol reagent.The transcriptome was constructed using the TruSeq Stranded mRNA library kit (Illumina, San Diego, CA, United States), and the library containing 200bp fragments was paired-end sequenced in duplicate with the Illumina HiSeq X Ten platform.Sequencing data were filtered and quality controlled to obtain clean data and aligned to the hg19 reference genome and its transcriptome.Differential gene expression (log2 fold change > 1 and FDR < 0.01) and its enrichment were obtained.SNP/InDel calling, annotation, and fusion gene analysis were subsequently performed using biostatistical software.A genetic variation of transcriptional fusion was identified with mutation point A in chr17:57768072 and mutation point B in chr2:29448328.We also confirmed theCLTC-ALKfusion by reverse transcriptionpolymerase chain reaction (RT-PCR).Total RNA was extracted from BMMCs and then reverse transcribed into cDNA.To amplifyCLTC-ALK, two rounds of PCR were performed;the amplification conditions and primer sequences were as previously reported[8](Figure 5).
FINAL DIAGNOSIS
ALK-positive ALCL withCLTC-ALKfusion gene was found.According to the St Jude Children’s Research Hospital staging system, the disease was stage IV.
TREATMENT
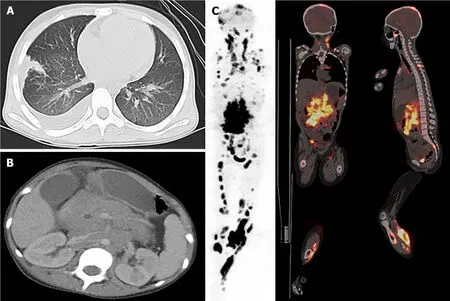
Figure 2 lmages at diagnosis.
The patient underwent chemotherapy as per the ALCL99 protocol[9]and showed a good response.PET-CT, performed after six courses of chemotherapy, indicated that there were only residual tumors in her left lower extremity.We also detectedCLTC/ALKexpression in her bone marrow (BM) and peripheral blood (PB) samples at follow-up by nested PCR.The outcome was still positive.Then, she received weekly vinblastine (VBL) injections as maintenance treatment, at an initial dose of 6 mg/m2(maximum, 10 mg) per injection, which was then reduced to 5 mg/m2for grade 4 neutropenia.Three months later, she achieved CR, and CLTC/ALK was undetectable in her BM and PB samples.
However, 1 mo later, she developed fever and headache and had frequent generalized seizures.The left pupil was dilated and did not react to light or accommodation.The cerebral spinal fluid (CSF) was slightly yellow (Figure 6Aa);CSF glucose level was 0.04 mmol/L, and CSF protein level was 4.5 g/L.A cytospin of CSF revealed approximately 72 tumor cells/high-power field (Figure 6Ab).MRI images of the brain revealed new lesions in the cerebral hemisphere, basal ganglia, thalamus,and brainstem.Intracranial segments of the bilateral optic nerves were enlarged(Figure 6Ba).Besides the CNS, there were no other signs of tumor relapse on CT and MRI scans.CLTC/ALK was positive again in one BM sample.
Two intrathecal triple therapies were administered, but the patient’s condition did not improve.More intensive chemotherapy and ALK-directed kinase inhibitor therapy were considered.She underwent treatment with both chemotherapy of COPADM according to LMB89 group C (including MTX 8 g/m2) and alectinib given orally[10].There is no information on alectinib use in pediatrics;the dose was extrapolated from the recommended adult dose (600 mg twice daily).Given the patient’s weight (34 kg) and body surface area (1.20 m2), alectinib was commenced at a total daily dose of 750 mg (divided into two doses).Written informed consent was obtained from her parents before commencing alectinib treatment.
Her fever resolved within one day;no seizures occurred thereafter.She received triple intrathecal therapy on the second day of chemotherapy.The CSF was much clearer than before, and no tumor cell was found on CSF cytospin.During the chemotherapy, complication by sepsis due toEscherichia coliand grade 4 neutropenia were observed.After the sepsis was cured, she received three more courses of chemotherapy of COPADM (MTX 8 g/m2), CYVE, and HD-MXT (5 g/m2)sequentially, with simultaneous alectinib.Thereafter, evaluation revealed that the involvement of the brain parenchyma and optic nerves was reduced (Figure 6Bb).She was discharged and started with maintenance treatment of VBL and alectinib at the clinic.The total daily dose of alectinib was reduced to 450-600 mg due to grade 4 neutropenia.The alectinib plasma trough level (Cmin) was monitored, and it ranged from 250 to 427 ng/mL.Two months later, she achieved CR, as shown by brain MRI(Figure 6Bc).CLTC/ALK was negative again in the BM and PB samples.Allogeneic hematopoietic stem cell transplantation was suggested, but it was declined by her parents.
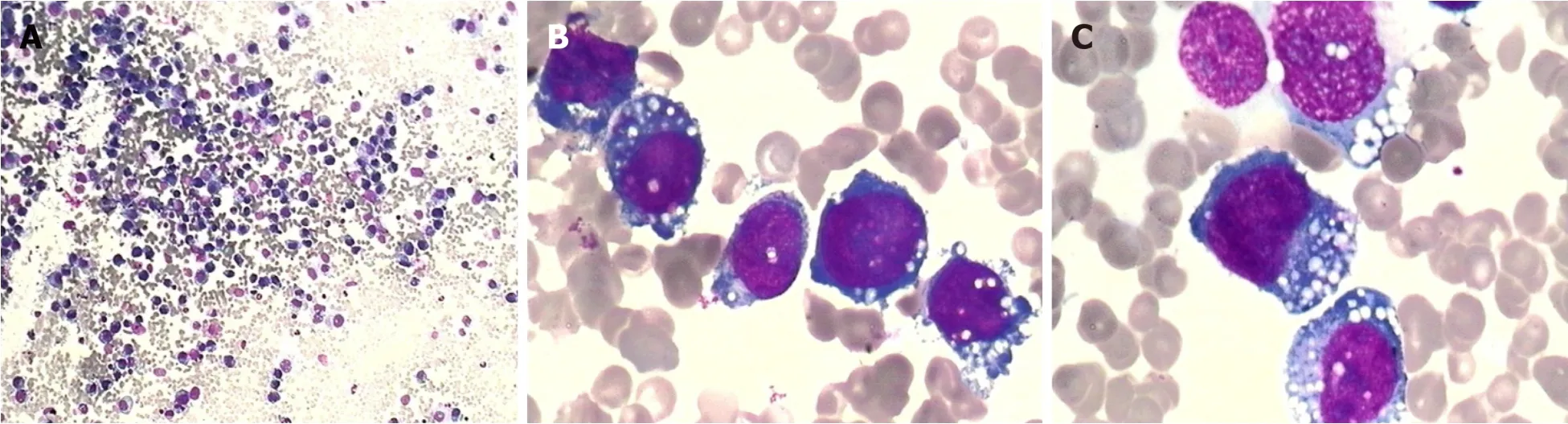
Figure 3 Wright-Giemsa-stained bone marrow smear.
OUTCOME AND FOLLOW-UP
Thus far, she has completed treatment with VBL and alectinib for 17 mo.The treatment was tolerated well, without significant adverse effects, except grades 1-4 neutropenia.She is still in CR, but her vision has not recovered.
DISCUSSION
Here, we present an extraordinary and rare case of ALCL with theCLTC-ALKfusion gene.The clinical relevance of various translocations has not been confirmed.Five XALK variants have been reported to display different proliferative, migratory, and invasive properties, which seem to be due to differential activation of various signaling pathways[11].This patient’s invasive and refractory clinical process suggests the possibility of a more aggressive property related to the CLTC/ALK variant in ALCL, although more cases are needed to confirm this.
VBL has shown remarkable activity as a single agent even in the treatment of relapsed ALCL[12].There are limited data on VBL passing the blood-brain barrier(BBB).Besides this patient, there are other reports on CNS progression during VBL therapy for high-risk ALK+ALCL[13].These cases indicate that the CNS/CSF penetration of VBL is insufficient for the control of high-risk ALCL relapse, and CNSdirected therapy ought to be considered during VBL monotherapy.
The reported incidence of CNS involvement in ALK+ALCL is about 2.6%.Patients with CNS involvement show an inferior prognosis[9].The first-generation ALKinhibitor crizotinib has poor CSF penetrance.Alectinib is not a P-glycoprotein substrate;hence, it could penetrate the BBB[14].The reported CSF penetration rates of crizotinib and alectinib are 0.26% and 86%, respectively[15].Alectinib has demonstrated potent CNS activity in ALK-positive non-small-cell lung cancer patients[16,17].It also showed good CNS response in two reported ALK+ALCL patients with CNS involvement[18,19].However, pediatric dosing data are lacking.A population pharmacokinetic analysis showed that a higher-than-median steady-state alectinib Cmin of ≥ 435 ng/mL is associated with a greater reduction in tumor size[20].The current patient did not reach this target concentration because of grade 3 or 4 neutropenia.We speculate that this might be related to the co-administration of VBL,which can also cause myelosuppression.Nonetheless, the disease was still well controlled, and no significant adverse events occurred.
CONCLUSION
In summary, this report describes a rare pediatric ALCL patient with theCLTC-ALKfusion gene.After CNS relapse, she achieved a durable second remission with highdose chemotherapy and continuous alectinib.Alectinib showed significant and durable CNS effects in this patient.To our knowledge, this is the first published pediatric case report describing the use of alectinib for the treatment of ALK+ALCL.However, our findings are based on a single patient, and more cases are needed to prove the efficacy and safety of alectinib for pediatric patients.

Figure 4 Histological and immunophenotypic results .
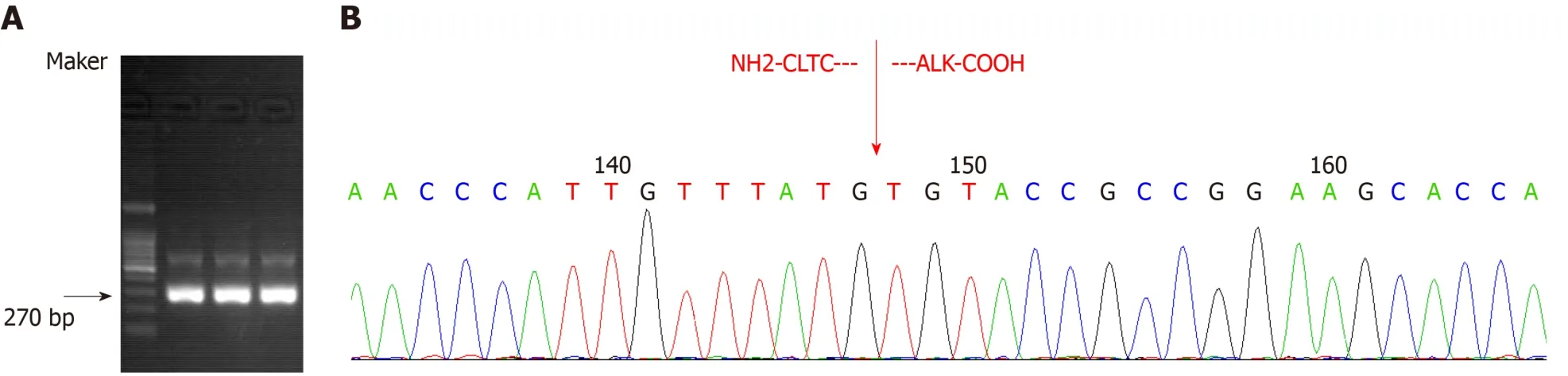
Figure 5 The sample at the diagnosis time-point showing the expected 270 bp CLTC-ALK reverse transcription-polymerase chain reaction product (three bands represent three repetitions) (A) and direct sequencing results of polymerase chain reaction products (B).
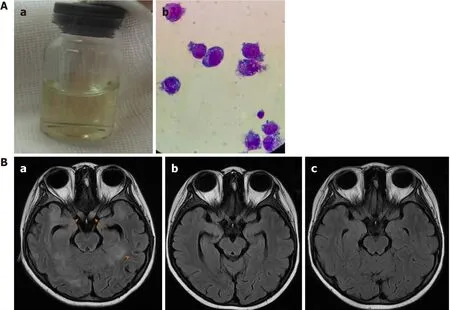
Figure 6 Central nervous system recurrence after 13 mo of treatment.
ACKNOWLEDGEMENTS
The authors would like to thank the patient and her family.
 World Journal of Clinical Cases2020年9期
World Journal of Clinical Cases2020年9期
- World Journal of Clinical Cases的其它文章
- Nutrition management in acute pancreatitis:Clinical practice consideration
- Bone disease in chronic pancreatitis
- Role of microRNAs in the predisposition to gastrointestinal malignancies
- Recurrent anal fistulas:When, why, and how to manage?
- Removal of biofilm is essential for long-term ventilation tube retention
- Neutrophil gelatinase-associated lipocalin does not predict acute kidney injury in heart failure
