植物NAC转录因子的结构及功能研究进展
荣欢 任师杰 汪梓坪 王飞 周勇

摘要:NAC(NAM、ATAF1/2、CUC1/2)转录因子是植物特有的一类转录因子家族,在植物生长发育、生物及非生物胁迫反应中具有重要的调控作用。NAC蛋白的N端均存在1个高度保守的NAC结构域,而C端是变化的转录调控区。通过总结前人的研究进展,综述NAC转录因子在植物分生组织和器官边界的形成、根的发育、植物细胞次生壁的生长、植物衰老、激素调控和胁迫反应等过程中的重要调控作用,指出今后NAC转录因子的研究方向。
关键词:植物;NAC转录因子;生长发育;胁迫;NAC生理功能
中图分类号:S184 文献标志码: A
文章编号:1002-1302(2020)18-0044-10
收稿日期:2019-10-31
基金项目:江西省教育厅科技计划(编号:GJJ180172、GJJ160387)。
作者简介:荣 欢(1998—),男,江西萍乡人,主要从事生物科学与生物技术研究。E-mail:962610432@qq.com。
通信作者:周 勇,博士,讲师,主要从事植物功能基因组学研究,E-mail:yzhoujxan@163.com;王 飞,博士,副教授,主要从事微生物资源与蛋白质工程研究,E-mail:wangfei179@163.com。
植物在生长发育过程中极易受到逆境胁迫的影响。胁迫主要包括干旱、高盐、低温、高温等非生物胁迫和虫害、病原菌侵入等生物胁迫,这些胁迫通常会影响植物的正常生长发育。在长期的进化过程中,植物产生了一系列生理生化机制来适应、抵御或消除胁迫的影响。其中,基因表达调控是调节植物逆境胁迫最常见的一种方式。植物细胞感知逆境胁迫信号后,会通过某些信号途径将信号传递给胁迫应答的转录因子(transcription factor,简称TF),转录因子可以通过其DNA结合结构域(DNA binding domain,简称DBD)和靶基因上游启动子区域的特异DNA序列模体(顺式作用元件)结合,从而调控靶基因在植物的不同组织、不同细胞或不同环境条件下的特异表达,从而激活植物抗逆反应,降低胁迫对植物造成的伤害[1-3]。由于转录因子在植物生长发育和应对胁迫等过程中具有重要的调控作用,因此对转录因子的研究一直是功能基因组研究的重要内容。近几十年来,世界各国科研人员通过基因组测序和功能分析,相继从不同植物中克隆到了大量的转录因子[4],希望通过研究它们的功能,来揭示植物的抗逆机制。
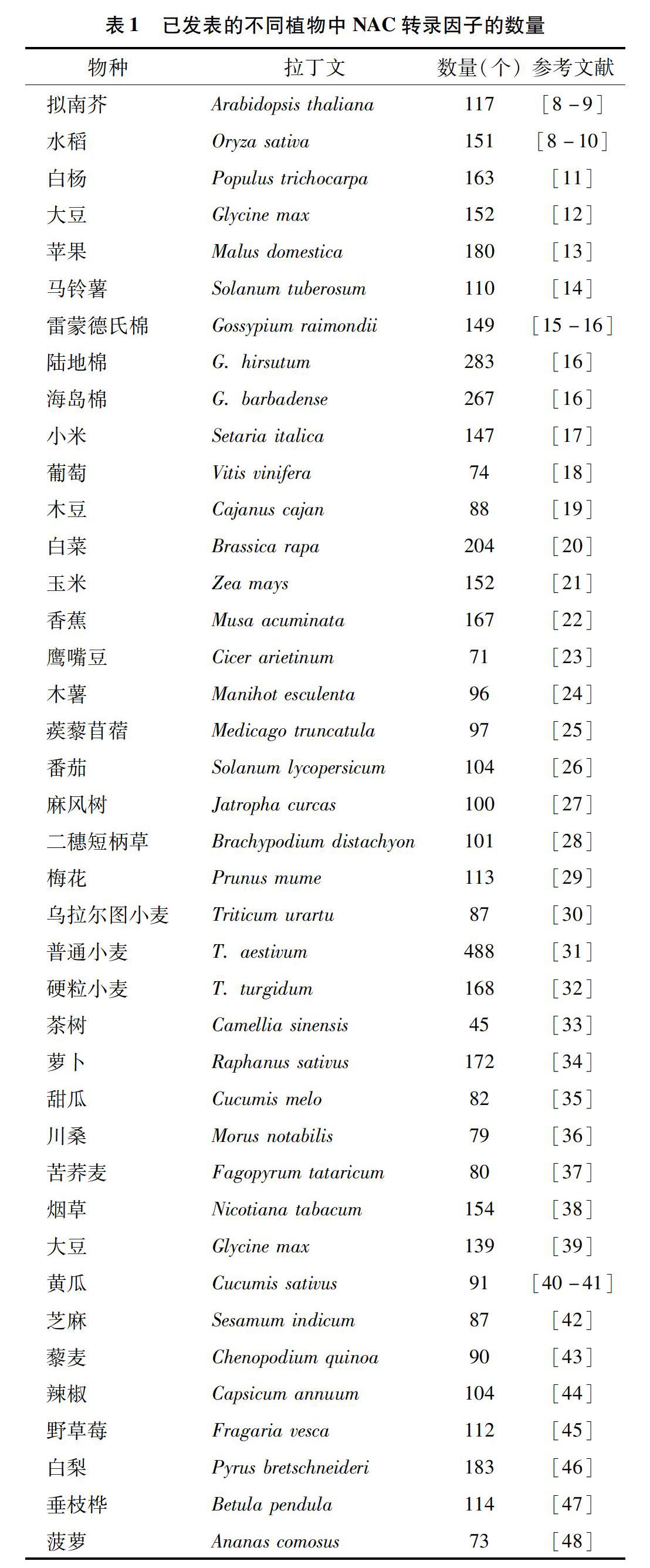
NAC转录因子是植物特有的一类转录因子家族,命名取自于矮牵牛(Petunia hybrida)的NAM(no apical meristem)基因、拟南芥(Arabidopsis thaliana)的ATAF1/2基因,以及CUC2(cup-shaped cotyledon)基因的首字母。1996年,Souer等研究人员从矮牵牛中克隆出第1个NAC转录因子家族成员NAM,它影响矮牵牛顶端分生组织的形成与分化[5]。随后,NAC转录因子相继在拟南芥、水稻、葡萄、小麦、大豆、木薯、番茄、黄瓜等物种中被发现(表1),是植物中最大的转录因子家族之一。很多研究表明,NAC转录因子不仅参与了植物根、茎、叶、花的生长发育、果实成熟、激素调控,还参与了生物及非生物胁迫等生理生化反应过程的调控[6-7]。
1 NAC转录因子的结构
NAC转录因子最显著的结构特点是在蛋白质的N端存在1个高度保守的NAC结构域(约150~160个氨基酸),而C端是变化的转录调控区(transcriptional activation region,简称TAR)(图1)。NAC结构域是NAC转录因子的结合域,可以分为5个亚结构域(A~E),其中亚结构域C和D高度保守且含有核定位信号,可能与DNA的结合有关,而亚结构域B和E则变化多样,可能会赋予NAC不同的功能[9]。有研究表明,亚结构域E能参与调控植物发育时期或组织特异性,并能够协同亚结构域D与DNA结合[49]。亚结构域A在不同的物种中也高度保守,可能与NAC蛋白形成二聚体有关[50]。NAC蛋白的C端具有高度的多样性,但会频繁出现一些简单氨基酸的重复排列,例如Thr(苏氨酸)、Ser(丝氨酸)、Pro(脯氨酸)、Glu(谷氨酸)或者酸性氨基酸残基等,这是植物转录激活结构域的典型特征。这些简单氨基酸的重复排列在NAC同一亚家族是保守的,在不同的亚家族之间却有明显的差异。一些特殊的NAC蛋白在C端会有一段跨膜区(transmembrane motifs,简称TMs),这种C端具有跨膜特性的NAC转录因子(NAC with transmembrane motif 1,简称NTM1)一般被称为NTL(NTM1-like)蛋白,必须从膜上被释放并转运到核中才能行使调控功能[51-52]。有些NAC转录因子只有NAC结构域,缺少转录调控区;更有的NAC结构域在C端,转录调控区在N端,中间含有一个保守的锌指结构。
通过X射线观察拟南芥ANAC019的NAC结构域的晶体结构,发现它是以数个螺旋元件包围一个螺旋状的结构,并和β-折叠组成一种未知的结构[50],而且NAC结构域可通过盐桥等作用形成一侧带正电荷的蛋白二聚体[50,53],这可能是它们结合DNA的基本形式。
2 NAC转录因子的生理功能
NAC转录因子因其在结构上有一定的共性和特性,其家族成员在功能上也有一定的共同点和多样性。但在植物不同部位、生长的不同时期,特定的NAC转录因子发挥的作用也不尽相同。总体来说,NAC转录因子对植物生长调控主要表现在如下几个方面。
2.1 参与植物分生组织和器官边界的形成
矮牵牛NAM基因主要在分生组织和器官原基边界的细胞内表达,nam突变体缺少茎顶端分生组织(shoot apical meristem,简称SAM),器官发育异常,导致幼苗大部分死亡,少部分存活下来的植株在成苗期花器官也会出现发育异常,说明NAM基因可能在分生组织器官原基的形成中起着一定的作用[5]。拟南芥AtNAM在胚胎SAM的整个区域均大量表达,暗示着参与AtNAM也参与SAM的形成[54]。CUC蛋白与矮牵牛NAM蛋白属于同一亚家族,拟南芥cuc1cuc2双基因突变体中子叶、萼片和雄蕊融合,难以形成SAM,而单基因的突变体却没有明显的表型,说明它们参与植物顶端分生组织的形成,且存在功能的冗余[55]。进一步研究发现,CUC1在拟南芥胚的顶端分生组织和花器官原基的边界处表达,处于STM(SHOOT MERISTEMLESS)基因的上游,可以激活很多SAM相关基因的表达,超量表达CUC1可以激活芽尖组织周缘细胞,诱导子叶不定芽的形成[56-57]。有趣的是,CUC1也可以通过一种不依赖STM的途径促进SAM的形成,该途径受到AS1(ASYMMETRIC1)和AS2基因的负调控[58]。此外,CUC1可以正调控LIGHT-DEPENDENT SHORT HYPOCOTYLS 4(LSH4)及其同源基因LSH3的表达,而在茎尖超量表达LSH4会抑制植物营养生长阶段叶片的生长,以及生殖生长阶段花中额外的芽或芽器官的形成[59]。CUC3基因主要在花器官原基边界表达,其表达量会被CUC1和CUC2所促进,超表达CUC3能促进胚后期的茎分生组织和器官边界的形成[60-61]。玉米ZmNAM1/2和ZmCUC3在胚芽鞘与叶原基的边界处大量表達,参与茎尖分生组织的形成[62]。由此可见,植物NAM亚族基因在分生组织和器官边界的形成中起着关键的作用。
2.2 调控根的发育
拟南芥NAC1基因受生长素(auxin)的诱导,主要在根尖和侧根生长原基表达,超量表达NAC1能促进侧根发育,而反义表达NAC1能抑制TIR1(transport inhibitor responsive protein 1)诱导的侧根发育,而生长素应答因子AIR3(auxin-induced in rootcultures 3)和DBP(DNA-binding protein)基因表达也受到NAC1的诱导,说明NAC1可以介导生长素信号以促进侧根的形成[63]。进一步研究表明,拟南芥SINAT5蛋白能促进E3泛素复合体与NAC1的连接,进而降低NAC1蛋白水平,减弱生长素信号,从而限制侧根的发育和伸长[64]。OsNAC2也可以通过整合生长素和细胞分裂素(cytokinin)信号途径来调控根的发育[65]。ANAC092/AtNAC2/ORE1基因也在根中特异表达,参与侧根的形成与发育[66]。进一步研究表明,ANAC092可以结合ARF8(AUXIN RESPONSE FACTOR 8)和PIN4(PIN-FORMED 4)的启动子,通过控制生长素信号途径来负调控根的发育[67]。TaRNAC1是小麦根中特异表达的NAC转录因子,在根中超量表达TaRNAC1的转基因小麦根长、生物量和干旱抗性明显增加[68]。在拟南芥中超量表达一些来自于其他物种的NAC基因,也能促进侧根的形成,如BnNAC14[69]、GmANC20[70]、GmNAC109[71]、CiNAC3和CiNAC4[72]基因等。
2.3 调节植物细胞次生壁的生长
一些NAC转录因子会调节细胞次生壁的生长。在拟南芥中,nst1nst2双突变体均表现出花药内皮层缺乏次生壁,花药异常开裂,表明NST1(NAC SECONDARY WALL THICKENING PROMOTING FACTOR1)和NST2参与花粉花药次生壁的形成,而且存在功能的冗余[73]。在拟南芥nst-1nst-3双敲除转基因植株中,除维管导管以外,维管束间纤维与木质部次生壁的加厚被完全抑制,表明NST1和NST3也参与调控木质组织中次生壁的正常形成[74],它们之间也存在部分功能的冗余[75]。苜蓿MtNST1是拟南芥NST1/2/3的同源基因,MtNST1的Tnt1逆转座子插入突变体出现花粉囊无法裂开,维管素纤维不再木质化[76]。拟南芥SND1(SECONDARY WALL-ASSOCIATED NAC DOMAIN PROTEIN1)在茎秆维管束间纤维和木质纤维中特异表达,异位超量表达SND1基因,会使非厚壁的正常细胞大量沉积次级细胞壁而成为厚壁细胞,表明SND1与纤维次级壁的厚度有关[77]。敲除SND1基因不能明显抑制次级纤维壁的加厚,而snd1nst1双突变体抑制的表型非常明显,细胞中纤维素、木聚糖、木质素等成分含量明显降低,说明SND1和NST1共同参与调控纤维素次生壁的生长[78]。拟南芥VND6(vascular-related NAC Domain 6)和VND7分别在主根的后生木质部和原生木质部中表达,超量表达VND6、VND7均能导致根的后生木质部细胞或原生木质部细胞发育异常,而抑制VND6、VND7的表达则会抑制后生木质部或原生木质部的发育,同时VND7能恢复snd1nst1双突变体抑制次级纤维壁加厚的表型,说明它们在调控拟南芥根原生木质部导管的分化中起着关键作用[79]。进一步研究表明,SND1及其同源蛋白NST1、NST2、VND6和VND7通过调控下游基因MYB类蛋白因子(如MYB46、MYB58、MYB63等)的表达,最终激活次生壁的纤维素、木聚糖和木质素合成的相关基因(如LAC4等),促进不同类型细胞次生壁的生物合成[80-83]。此外,一些SND1的同源基因(如PtVNSs/PtrWNDs等)能够恢复NST1和NST3双突变引起的维管束间纤维细胞次生壁的缺陷,它们的超量表达会引起杨树叶片和拟南芥幼苗的次生壁增厚[84-85]。水稻OsSWNs和玉米ZmSWNs也能互补拟南芥snd1nst1双突变体在次生细胞壁加厚方面缺陷的表型[86]。这些结果表明,在植物界中与SND1同源的NAC转录因子调控次生壁的生物合成机制可能是普遍存在的。
NAC转录因子对植物次生壁生长有着双向作用,既可能促进其生长,又可能抑制其生长。拟南芥ANAC012在开花茎和根的形成层区特异表达,超量表达ANAC012会显著抑制木纤维中次生壁的形成,但轻微地增加了木质部导管的细胞壁厚度[87]。拟南芥XND1(xylem NAC domain 1)在木质部中高度表达,超量表达XND1的转基因植株下胚轴原生木质部区域薄壁细胞的次生壁生长会受到明显的抑制,显示出极端矮化的表型[88]。
2.4 调控植物衰老
有研究表明,一些NAC转录因子能够间接或直接地加速或延缓植物衰老过程。NAM-B1是野生二粒小麦的一个NAC转录因子,能正调控衰老,促进营养成分从营养器官向籽粒转移[89]。AtNAP(NAC-like,activated by APETALA 3/PISTILLATA)是一个典型的叶片衰老相关基因,超量表达AtNAP的转基因植株明显早衰,atnap突变体则表现出延缓叶片衰老的表型[90]。进一步研究发现,AtNAP可以被脱落酸(abscisic acid,简称ABA)所诱导,可以和SAG113(SENESCENCE-ASSOCIATED GENE113)的启动子结合,形成一个ABA-AtNAP-SAG113蛋白调控链来控制叶片衰老时的气孔运动和失水速率,进而调控叶片衰老进程[91]。水稻中AtNAP的同源基因OsNAP可以互补atnap的表型,在调控水稻衰老发育过程中也发挥着重要作用[92-93]。此外,在金丝慈竹(Bambusa emeiensis ‘Viridiflavus)中的同源基因BeNAC1也能互补atnap的表型,在拟南芥中超量表达BeNAC1也会产生不同的早衰表型[94]。超量表达甜瓜CmNAC60基因的拟南芥转基因植株叶片衰老也明显加速[95]。另一个同源基因GhNAP也能通过调节ABA介导的叶片衰老途径来调控棉花的产量和纤维质量[96]。拟南芥ANAC092/AtNAC2/ORE1[97-98]、ANAC032[99]等既能正调控依赖年龄的叶片衰老,也在盐胁迫诱导的叶片衰老过程中起着重要的作用。一些NAC转录因子可以直接结合在叶绿素降解途径相关基因的启动子上,通过调节叶绿素的代谢来调控叶片衰老进程,如OsNAP[92]、ANAC016[100]、BrNAC055[101]、SlNAP2[102]等。大多数调控叶片衰老的NAC转录因子都是以正调控的方式来调控叶片衰老,但也有少量的NAC轉录因子是以负调控的方式进行调控的,如ONAC106[103]、DRL1[104]等。
2.5 参与激素调控
很多NAC转录因子的表达量受到ABA的诱导,参与ABA的生物合成,或者介导ABA的信号转导途径。如拟南芥ATAF1可以直接调节ABA合成基因NCED3的表达,来调控ABA的生物合成[105]。拟南芥VNI2(VND-INTERACTING2)是一个NAC转录因子,其表达量受ABA诱导,可以结合RD(RESPONSIVE TO DEHYDRATION)和COR(COLD-REGULATED)基因的启动子,通过调控RD和COR基因的表达量来介导盐胁迫和叶片衰老途径[106]。在拟南芥中超量表达ANAC072/RD26能提高ABA诱导相关基因和胁迫诱导相关基因的表达量,对ABA的敏感性增强,且增强了采后果实的抗逆性,而在ANAC072/RD26受到抑制的植株中这些基因的表达量同样受到抑制,对ABA不敏感,表明ANAC072/RD26在胁迫应答和ABA信号转导途径中起着重要作用[107]。水稻SNAC2(stress-responsive NAC 2)基因也受到ABA的诱导表达,它的超量表达植株表现出耐冷和抗盐的表型,并对ABA敏感[108]。此外,OsNAP也可以通过介导ABA的信号转导途径来增强水稻的抗逆性,在OsNAP的超量表达转基因植株中,很多胁迫相关基因和胁迫相关转录因子的表达量明显上升[109]。由此可见,介导ABA的信号转导途径的NAC转录因子多数与逆境信号传导途径有关。
NAC转录因子是茉莉酸(jasmonic acid,简称JA)信号的调控因子。超量表达ANAC072/RD26的转基因植株也增强了对茉莉酸甲酯(methyl jasmonate,简称MeJA)的敏感性,因此ANAC072/RD26可能同时介导ABA和MeJA的信号转导途径[107]。拟南芥ATAF1是ABA信号通路的一个负调控因子,但也能诱导JA途径相关防御信号基因的表达[110]。OsNAP也可能通过MeJA信号传导途径正调控水稻叶片衰老途径[93]。NAC转录因子RIM1是水稻矮缩病毒繁殖的宿主因子,rim1突变体植株表现出根生长受抑制,编码JA生物合成相关基因的表达量明显上升,而且在JA处理下突变体植株和野生型植株一致,没有内源JA的积累,说明RIM1是JA信号的负调控因子[111]。
NAC也可以参与生长素、细胞分裂素、乙烯和赤霉素(gibberellins,简称GA)等的信号转导途径[65-66,112]。拟南芥NAC1基因受生长素诱导并且介导生长素信号以促进侧根生长发育[63]。拟南芥AtNAC2受高盐诱导,这种诱导在乙烯超量突变体eto1-1中被增强,在乙烯不敏感突变体etr1-1、ein2-1和生长素敏感突变體tir1-1中受到抑制,而在ABA敏感突变体abi2-1、abi3-1和abi4-1中没有显著变化,说明AtNAC2的盐胁迫响应参与了乙烯和生长素信号途径,与ABA信号途径无关[66]。在拟南芥中,NTL8(NTM 1-like 8)的表达受高盐诱导和GA的抑制,NTL8可以经过不依赖ABA的GA途径介导拟南芥种子萌发过程中盐的调节[113]。
2.6 参与胁迫反应
植物在生长发育过程中极易受干旱、低温、高温、高盐等非生物胁迫和虫害、病原菌等生物胁迫的影响,植物细胞会产生对这些外界胁迫的感知,并通过多种复杂的信号传导途径将其传递给控制胁迫应答的转录因子,从而激活植物抗逆反应,降低逆境对植物造成的损害。NAC转录因子在这些过程中扮演着重要的角色。
很多NAC基因的表达量直接受到非生物逆境的调控,如大豆中有超过1/3(58/152)的NAC基因是潜在的胁迫响应基因[12]。在非生物胁迫中,绝大多数的报道集中在耐冷、耐旱和抗盐等方面。在水稻中超量表达内源基因SNAC1[114]、OsNAC6 [115]、SNAC2 [108]、ONAC045 [116]、OsNAP [109]、ONAC106 [103]、ONAC022 [117]、OsNAC2 [118]等,或外源基因ATAF1 [119]、EcNAC67 [120]等,均能一定程度地表现出耐冷、耐旱和抗盐的单一表型或者综合表型。在拟南芥中异源超表达不同物种来源的NAC成员也有类似的结果[71,121-127]。绝大部分NAC是正调控胁迫反应,但也有少部分NAC能负调控胁迫反应。如OsNAC95在水稻抗旱和耐冷胁迫反应中表现出相反的角色,它可以负调控抗旱胁迫,正调控耐冷胁迫[128]。拟南芥ANAC069能通过降低活性氧(reactive oxygen species,简称ROS)的清除能力和脯氨酸含量,来负调控高盐和渗透胁迫[129]。苹果MdNAC029/MdNAP以C-repeat binding factor(CBF)依赖的方式负调控植物的抗冷能力[130]。玉米ZmNAC071也通过负调控ROS清除能力来负调控ABA反应和渗透胁迫[131]。NAC转录因子调控非生物胁迫反应绝大多数是通过ABA依赖的途径来进行的,也可以依赖其他激素的信号转导途径,如JA [93,132-133]、GA/油菜素内酯(brassinolide,简称BR)[134]等。
一些报道表明,NAC转录因子也参与生物胁迫。如水稻OsNAC6对抵抗稻瘟病有正调控作用[115]。OsNAC19可能在MeJA信号途径中参与水稻对稻瘟病菌的响应[135]。拟南芥中ATAF1 [136]和ATAF2 [137]分别对抗灰霉病和枯萎病有负调控作用。在大麦和拟南芥中超量表达ATAF1的同源基因HvNAC6可以增强耐渗透细胞对白粉病菌的抗性[138-139],而超量表达ATAF1在棉花中的同源基因GhATAF1却增强了对灰葡萄孢菌的敏感性[132]。
3 展望
NAC家族转录因子是植物特有的一类转录因子,广泛参与植物生长发育及胁迫反应。到目前为止,NAC转录因子已经在几十种植物中被发现,但不同物种来源的NAC成员可能具有不同的生物学功能,如调控淀粉合成[140-141]、种子活力[142]、果实发育[143-144]、大豆抗毒素合成[145]、开花[146-147]、锌的转运[148]等。因此,广泛研究NAC成员的功能不仅能揭示NAC蛋白的调控网络,而且通过控制NAC基因或NAC蛋白的表达,提高作物的抗逆性,进而提升产量。
參考文献:
[1]Singh K B,Foley R C,Onate-Sanchez L. Transcription factors in plant defense and stress responses[J]. Current Opinion in Plant Biology,2002,5(5):430-436.
[2]Chen W J,Tong Z. Networks of transcription factors with roles in environmental stress response[J]. Trends in Plant Science,2004,9(12):591-596.
[3]Huang G T,Ma S L,Bai L P,et al. Signal transduction during cold,salt,and drought stresses in plants[J]. Molecular Biology Reports,2012,39(2):969-987.
[4]Jin P,Zhang H,Kong L,et al. PlantTFDB 3.0:a portal for the functional and evolutionary study of plant transcription factors[J]. Nucleic Acids Research,2014,42:1182-1187.
[5]Souer E,Vanhouwelingen A,Kloos D,et al. The no apical meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries[J]. Cell,1996,85(2):159-170.
[6]Nakashima K,Takasaki H,Mizoi J,et al. NAC transcription factors in plant abiotic stress responses[J]. Biochimica et Biophysica Acta-Gene Regulatory Mechanisms,2012,1819(2):97-103.
[7]Shao H B,Wang H Y,Tang X L. NAC transcription factors in plant multiple abiotic stress responses:progress and prospects[J]. Frontiers in Plant Science,2015,6:902.
[8]Nuruzzaman M,Manimekalai R,Sharoni A M,et al. Genome-wide analysis of NAC transcription factor family in rice[J]. Gene,2010,465(1/2):30-44.
[9]Ooka H,Satoh K,Doi K,et al. Comprehensive analysis of NAC family genes in Oryza sativa and Arabidopsis thaliana[J]. DNA Research:an International Journal for Rapid Publication of Reports on Genes and Genomes,2003,10(6):239-247.
[10]Fang Y J,Jun Y,Xie K B,et al. Systematic sequence analysis and identification of tissue-specific or stress-responsive genes of NAC transcription factor family in rice[J]. Molecular Genetics and Genomics,2008,280(6):547-563.
[11]Hu R B,Guang Q,Kong Y Z,et al. Comprehensive analysis of NAC domain transcription factor gene family in populus trichocarpa[J]. BMC Plant Biology,2010,10(1):145.
[12]Dung T L,Nishiyama R,Watanabe Y,et al. Genome-wide survey and expression analysis of the Plant-Specific NAC transcription factor family in soybean during development and dehydration stress[J]. DNA Research,2011,18(4):263-276.
[13]Su H Y,Zhang S Z,Yuan X W,et al. Genome-wide analysis and identification of stress-responsive genes of the NAM-ATAF1,2-CUC2 transcription factor family in apple[J]. Plant Physiology and Biochemistry,2013,71:11-21.
[14]Singh A K,Sharma V,Pal A K,et al. Genome-wide organization and expression profiling of the NAC transcription factor family in potato (Solanum tuberosum L.)[J]. DNA Research,2013,20(4):403-423.
[15]Shang H H,Li W,Zou C S,et al. Analyses of the NAC transcription factor gene family in Gossypium raimondii Ulbr: chromosomal location,structure,phylogeny,and expression patterns[J].J Integr Plant Biol,2013,55(7):663-676.
[16]Heng S,Hu M L,Li J Y,et al. Comprehensive analysis of NAC transcription factors uncovers their roles during fiber development and stress response in cotton[J]. BMC Plant Biology,2018,18(1):150.
[17]Puranik S,Sahu P P,Mandal S N,et al. Comprehensive genome-wide survey,genomic constitution and expression profiling of the NAC transcription factor family in foxtail millet (Setaria italica L.)[J]. PLoS One,2013,8(5):e64594.
[18]Nian W,Yu Z,Xin H P,et al. Comprehensive analysis of NAC domain transcription factor gene family in Vitis vinifera[J]. Plant Cell Reports,2013,32(1):61-75.
[19]Viswanathan S,Jagannadham P K,Parameswaran C,et al. NAC transcription factor genes:genome-wide identification,phylogenetic,motif and cis-regulatory element analysis in pigeonpea[Cajanus cajan (L.) Millsp.][J]. Molecular Biology Reports,2014,41(12):7763-7773.
[20]Liu T K,Song X M,Duan W K,et al. Genome-wide analysis and expression patterns of NAC transcription factor family under different developmental stages and abiotic stresses in Chinese cabbage[J]. Plant Molecular Biology Reporter,2014,32(5):1041-1056.
[21]Shiriga K,Sharma R,Kumar K,et al. Genome-wide identification and expression pattern of drought-responsive members of the NAC family in maize[J]. Meta Gene,2014,2:407-417.
[22]Cenci A,Guignon V,Roux N,et al. Genomic analysis of NAC transcription factors in banana (Musa acuminata) and definition of NAC orthologous groups for monocots and dicots[J]. Plant Molecular Biology,2014,85(1/2):63-80.
[23]Ha C V,Esfahani M N,Watanabe Y,et al. Genome-wide identification and expression analysis of the CaNAC family members in chickpea during development,dehydration and ABA treatments[J]. PLoS One,2014,9(12):e114107.
[24]Wei H,Wei Y X,Xia Z Q,et al. Genome-wide identification and expression analysis of the NAC transcription factor family in cassava[J]. PLoS One,2015,10(8):e0136993.
[25]Lei L,Song L,Wang Y J,et al. Genome-wide analysis and expression patterns of the NAC transcription factor family in Medicago truncatula[J]. Physiology and Molecular Biology of Plants,2017,23(2):343-356.
[26]Su H Y,Zhang S Z,Yin Y L,et al. Genome-wide analysis of NAM-ATAF1,2-CUC2 transcription factor family in Solanum lycopersicum[J]. Journal of Plant Biochemistry and Biotechnology,2015,24(2):176-183.
[27]Wu Z Y,Xu X Q,Xiong W D,et al. Genome-wide analysis of the NAC gene family in physic nut (Jatropha curcas L.)[J]. PLoS One,2015,10(6):e0131890.
[28]Jun Y,Zhang L H,Bo S,et al. Systematic analysis and identification of stress-responsive genes of the NAC gene family in Brachypodium distachyon[J]. PLoS One,2015,10(3):e0122027.
[29]Zhuo X K,Zheng T C,Zhang Z Y,et al. Genome-wide analysis of the NAC transcription factor gene family reveals differential expression patterns and cold-stress responses in the woody plant Prunus mume[J]. Genes,2018,9(10):494.
[30]Ma J H,Tong D D,Zhang W L,et al. Identification and analysis of the NAC transcription factor family in Triticum urartu[J]. Yi Chuan,2016,38(3):243-253.
[31]Guérin C,Roche J,Allard V,et al. Genome-wide analysis,expansion and expression of the NAC family under drought and heat stresses in bread wheat (T. aestivum L.)[J]. PLoS One,2019,14(3):e0213390.
[32]Saidi M N,Mergby D,Brini F . Identification and expression analysis of the NAC transcription factor family in durum wheat (Triticum turgidum L. ssp. durum)[J]. Plant Physiology & Biochemistry,2017,112:117-128.
[33]Wang Y X,Liu Z W,Wu Z J,et al. Transcriptome-wide identification and expression analysis of the NAC gene family in tea plant[Camellia sinensis (L.) O. Kuntze][J]. PLoS One,2016,11(11):e0166727.
[34]Karanja B K,Xu L,Wang Y,et al. Genome-wide characterization and expression profiling of NAC transcription factor genes under abiotic stresses in radish(Raphanus sativus L.)[J].PeerJ,2017,5:e4172.
[35]Wei S W,Gao L W,Zhang Y D,et al. Genome-wide investigation of the NAC transcription factor family in melon (Cucumis melo L.) and their expression analysis under salt stress[J]. Plant Cell Reports,2016,35(9):1827-1839.
[36]Baranwal V K,Khurana P. Genome-wide analysis,expression dynamics and varietal comparison of NAC gene family at various developmental stages in Morus notabilis[J]. Molecular Genetics and Genomics,2016,291(3):1305-1317.
[37]Liu M Y,Ma Z T,Sun W J,et al. Genome-wide analysis of the NAC transcription factor family in Tartary buckwheat (Fagopyrum tataricum)[J]. BMC Genomics,2019,20(1):113.
[38]Wei L,Li X X,Chao J T,et al. NAC family transcription factors in tobacco and their potential role in regulating leaf senescence[J]. Frontiers in Plant Science,2018,9:1900.
[39]Hussain R M,Mohammed A,Xing F,et al. The essence of NAC gene family to the cultivation of drought-resistant soybean (Glycine max L. Merr.) cultivars[J]. BMC Plant Biology,2017,17(1):55.
[40]Zhang X M,Yu H J,Sun C,et al. Genome-wide characterization and expression profiling of the NAC genes under abiotic stresses in Cucumis sativus[J]. Plant Physiology and Biochemistry,2017,113:98-109.
[41]Liu X W,Wang T,Bartholomew E S,et al. Comprehensive analysis of NAC transcription factors and their expression during fruit spine development in cucumber(Cucumis sativus L.)[J].Hortic Res,2018,5(1):31.
[42]Zhang Y J,Li D H,Wang Y,et al. Genome-wide identification and comprehensive analysis of the NAC transcription factor family in Sesamum indicum[J]. PLoS One,2018,13(6):e0199262.
[43]Feng L,Guo X H,Liu J X,et al. Genome-wide identification,characterization,and expression analysis of the NAC transcription factor in Chenopodium quinoa[J]. Genes,2019,10(7):500.
[44]Diao W P,Snyder J,Wang S B,et al. Genome-wide analyses of the NAC transcription factor gene family in pepper (Capsicum annuum L.):chromosome location,phylogeny,structure,expression patterns,cis-elements in the promoter,and interaction network[J]. International Journal of Molecular Sciences,2018,19(4):1028.
[45]Moyano E,Martínez-Rivas F J,Blanco-Portales R,et al. Genome-wide analysis of the NAC transcription factor family and their expression during the development and ripening of the Fragaria × ananassa fruits[J]. PLoS One,2018,13(5):e0196953.
[46]Gong X,Zhao L Y,Song X F,et al. Genome-wide analyses and expression patterns under abiotic stress of NAC transcription factors in white pear (Pyrus bretschneideri)[J]. BMC Plant Biology,2019,19(1):161.
[47]Song C,Xin L,Zhang D W,et al. Genome-wide analysis of NAC gene family in Betula pendula[J]. Forests,2019,10(9):741.
[48]He Q,Liu Y H,Zhang M,et al. Genome-wide identification and expression analysis of the NAC transcription factor family in pineapple[J]. Tropical Plant Biology,2019,12(4):255-267.
[49]Olsen A N,Ernst H A,Leggio L L,et al. Preliminary crystallographic analysis of the NAC domain of ANAC,a member of the plant-specific NAC transcription factor family[J]. Acta Crystallographica Section D-Biological Crystallography,2004,60(1):112-115.
[50]Ernst H A,Olsen A N,Larsen S,et al. Structure of the conserved domain of ANAC,a member of the NAC family of transcription factors[J]. EMBO Reports,2004,5(3):297-303.
[51]Kim Y S,Kim S Y,Park J E,et al. A Membrane-bound NAC transcription factor regulates cell division in Arabidopsis[J]. The Plant Cell,2006,18(11):3132-3144.
[52]Ya-Ni C,Slabaugh E,Brandizzi F. Membrane-tethered transcription factors in Arabidopsis thaliana:novel regulators in stress response and development[J]. Current Opinion in Plant Biology,2008,11(6):695-701.
[53]Chen Q F,Wang Q,Xiong L Z,et al. A structural view of the conserved domain of rice stress-responsive NAC1[J]. Protein & Cell,2011,2(1):55-63.
[54]Duval M,Hsieh T F,Kim S Y,et al. Molecular characterization of AtNAM:a member of the Arabidopsis NAC domain superfamily[J]. Plant Molecular Biology,2002,50(2):237-248.
[55]Aida M,Ishida T,Fukaki H,et al. Genes involved in organ separation in Arabidopsis:an analysis of the cup-shaped cotyledon mutant[J]. The Plant Cell,1997,9(6):841-857.
[56]Ken-Ichiro H,Takada S,Tasaka M. CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation[J]. The Plant Journal,2003,36(5):687-696.
[57]Takada S,Hibara K,Ishida T,et al. The CUP-SHAPED COTYLEDON1 gene of Arabidopsis regulates shoot apical meristem formation[J]. Development (Cambridge,England),2001,128(7):1127-1135.
[58]Ori N,Eshed Y,Chuck G,et al. Mechanisms that control knox gene expression in the Arabidopsis shoot[J]. Development (Cambridge,England),2000,127(24):5523-5532.
[59]Takeda S,Hanano K,Kariya A,et al. CUP-SHAPED COTYLEDON1 transcription factor activates the expression of LSH4 and LSH3,two members of the ALOG gene family,in shoot organ boundary cells[J]. Plant Journal,2011,66(6):1066-1077.
[60]Ken-Ichiro H,Karim M R,Takada S,et al. Arabidopsis CUP-SHAPED COTYLEDON3 regulates postembryonic shoot meristem and organ boundary formation[J]. The Plant Cell,2006,18(11):2946-2957.
[61]Vroemen C W,Mordhorst A P,Albrecht C,et al. The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis[J]. Plant Cell,2003,15(7):1563-1577.
[62]Zimmermann R,Werr W. Pattern formation in the monocot embryo as revealed by NAM and CUC3 orthologues from Zea mays L.[J]. Plant Molecular Biology,2005,58(5):669-685.
[63]Xie Q,Frugis G,Colgan D F,et al. Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development[J]. Genes & Development,2000,14(23):3024-3036.
[64]Qi X,Hui-Shan G,Dallman G,et al. SINAT5 promotes ubiquitin-related degradation of NAC1 to attenuate auxin signals[J]. Nature,2002,419(693):167-170.
[65]Mao C,He J,Liu L,et al. OsNAC2 integrates auxin and cytokinin pathways to modulate rice root development[J]. Plant Biotechnology Journal,2019,18(2):429-442.
[66]He X J,Mu R L,Cao W H,et al. AtNAC2,a transcription factor downstream of ethylene and auxin signaling pathways,is involved in salt stress response and lateral root development[J]. The Plant Journal,2005,44(6):903-916.
[67]Xi D,Xu C,Wang Y X,et al. Arabidopsis ANAC092 regulates auxin-mediated root development by binding to the ARF8 and PIN4 promoters[J]. Journal of Integrative Plant Biology,2019,61(9):1015-1031.
[68]Chen D,Chai S C,Mcintyre C L,et al. Overexpression of a predominantly root-expressed NAC transcription factor in wheat roots enhances root length,biomass and drought tolerance[J]. Plant Cell Reports,2018,37(2):225-237.
[69]Hegedus D,Yu M,Baldwin D,et al. Molecular characterization of Brassica napus NAC domain transcriptional activators induced in response to biotic and abiotic stress[J]. Plant Molecular Biology,2003,53(3):383-397.
[70]Hao Y J,Wei W,Song Q X,et al. Soybean NAC transcription factors promote abiotic stress tolerance and lateral root formation in transgenic plants[J]. The Plant Journal,2011,68(2):302-313.
[71]Yang X F,Kim M Y,Ha J M,et al. Overexpression of the soybean NAC gene GmNAC109 increases lateral root formation and abiotic stress tolerance in transgenic Arabidopsis plants[J]. Frontiers in Plant Science,2019,10:1036.
[72]Han X M,Feng Z Q,Xing D,et al. Two NAC transcription factors from Caragana intermedia altered salt tolerance of the transgenic Arabidopsis[J]. BMC Plant Biology,2015,15(1):208.
[73]Mitsuda N,Seki M,Shinozaki K,et al. The NAC transcription factors NST1 and NST2 of Arabidopsis regulate secondary wall thickenings and are required for anther dehiscence[J]. The Plant Cell,2005,17(11):2993-3006.
[74]Mitsuda N,Iwase A,Hiroyuki Y,et al. NAC transcription factors,NST1 and NST3,are key regulators of the formation of secondary walls in woody tissues of Arabidopsis[J]. The Plant Cell,2007,19(1):270-280.
[75]Mitsuda N,Ohme-Takagi M. NAC transcription factors NST1 and NST3 regulate pod shattering in a partially redundant manner by promoting secondary wall formation after the establishment of tissue identity[J]. The Plant Journal,2008,56(5):768-778.
[76]Zhao X,Gallego-Giraldo L,Wang H Z,et al. An NAC transcription factor orchestrates multiple features of cell wall development in Medicago truncatula[J]. The Plant Journal,2010,63(1):100-114.
[77]Zhong R Q,Demura T,Ye Z H. SND1,a NAC domain transcription factor,is a key regulator of secondary wall synthesis in fibers of Arabidopsis[J]. The Plant Cell,2006,18(11):3158-3170.
[78]Zhong R Q,Richardson E A,Ye Z H. Two NAC domain transcription factors,SND1 and NST1,function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis[J]. Planta,2007,225(6):1603-1611.
[79]Yamaguchi M,Mitsuda N,Ohtani M,et al. VASCULAR-RELATED NAC-DOMAIN7 directly regulates the expression of a broad range of genes for xylem vessel formation[J]. The Plant Journal,2011,66(4):579-590.
[80]Zhong R Q,Richardson E A,Ye Z H. The MYB46 transcription factor is a direct target of SND1 and regulates secondary wall biosynthesis in Arabidopsis[J]. The Plant Cell,2007,19(9):2776-2792.
[81]Mccarthy R L,Zhong R,Ye Z H. MYB83 is a direct target of SND1 and Acts redundantly with MYB46 in the regulation of secondary cell wall biosynthesis in Arabidopsis[J]. Plant and Cell Physiology,2009,50(11):1950-1964.
[82]Zhou J L,Lee C,Zhong R Q,et al. MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis[J]. The Plant Cell,2009,21(1):248-266.
[83]Zhong R Q,Lee C,Zhou J L,et al. A battery of transcription factors involved in the regulation of secondary cell wall biosynthesis in Arabidopsis[J]. The Plant Cell,2008,20(10):2763-2782.
[84]Zhong R,Lee C,Ye Z H. Functional characterization of poplar wood-associated NAC domain transcription factors[J]. Plant Physiology,2010,152(2):1044-1055.
[85]Ohtani M,Nishikubo N,Bo X,et al. A NAC domain protein family contributing to the regulation of wood formation in poplar[J]. The Plant Journal,2011,67(3):499-512.
[86]Zhong R,Lee C,Mccarthy R L,et al. Transcriptional activation of secondary wall biosynthesis by rice and maize NAC and MYB transcription factors[J]. Plant and Cell Physiology,2011,52(10):1856-1871.
[87]Jae-Heung K,Seung H Y,Andrew H P,et al. ANAC012,a member of the plant-specific NAC transcription factor family,negatively regulates xylary fiber development in Arabidopsis thaliana[J]. The Plant Journal,2007,50(6):1035-1048.
[88]Zhao C S,Avci U,Emily H G,et al. XND1,a member of the NAC domain family in Arabidopsis thaliana,negatively regulates lignocellulose synthesis and programmed cell death in xylem[J]. The Plant Journal,2007,53(3):425-436.
[89]Uauy C,Distelfeld A,Fahima T,et al. A NAC gene regulating senescence improves grain protein,Zinc,and Iron content in wheat[J]. Science,2006,314(583):1298-1301.
[90]Guo Y F,Gan S S. AtNAP,a NAC family transcription factor,has an important role in leaf senescence[J]. The Plant Journal:for Cell and Molecular Biology,2006,46(4):601-612.
[91]Zhang K W,Gan S S. An abscisic acid-AtNAP transcription factor-SAG113 protein phosphatase 2C regulatory chain for controlling dehydration in senescing Arabidopsis leaves[J]. Plant Physiology,2012,158(2):961-969.
[92]Liang C Z,Wang Y Q,Zhu Y N,et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice[J]. Proceedings of the National Academy of Sciences of the United States of America,2014,111(27):10013-10018.
[93]Yong Z,Huang W F,Li L,et al. Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence[J]. BMC Plant Biology,2013,13(1):132.
[94]Chen Y X,Kai Q,Kuai B K,et al. Identification of an NAP-like transcription factor BeNAC1 regulating leaf senescence in bamboo (Bambusa emeiensis‘Viridiflavus)[J]. Physiologia Plantarum,2011,142(4):361-371.
[95]Cao S X,Zhang Z B,Wang C H,et al. Identification of a novel melon transcription factor CmNAC60 as a potential regulator of leaf senescence[J]. Genes,2019,10(8):584.
[96]Kai F,Bibi N,Gan S S,et al. A novel NAP member GhNAP is involved in leaf senescence in Gossypium hirsutum[J]. Journal of Experimental Botany,2015,66(15):4669-4682.
[97]Kim J H,Woo H R,Kim J,et al. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis[J]. Science,2009,323(5917):1053-1057.
[98]Balazadeh S,Siddiqui H,Allu A D,et al. A gene regulatory network controlled by the NAC transcription factor ANAC092/AtNAC2/ORE1 during salt-promoted senescence[J]. The Plant Journal:for Cell and Molecular Biology,2010,62(2):250-264.
[99]Mahmood K,El-Kereamy A,Sung-Hyun K,et al. ANAC032 positively regulates age-dependent and stress-induced senescence in Arabidopsis thaliana[J]. Plant and Cell Physiology,2016,57(10):2029-2046.
[100]Sakuraba Y,Su-Hyun H,Sang-Hwa L,et al. Arabidopsis NAC016 promotes chlorophyll breakdown by directly upregulating STAYGREEN1 transcription[J]. Plant Cell Reports,2016,35(1):155-166.
[101]Fan Z Q,Tan X L,Chen J W,et al. BrNAC055,a novel transcriptional activator,regulates leaf senescence in Chinese flowering cabbage by modulating reactive oxygen species production and chlorophyll degradation[J]. Journal of Agricultural and Food Chemistry,2018,66(36):9399-9408.
[102]Ma X M,Zhang Y J,Veronika T,et al. The NAC transcription factor SlNAP2 regulates leaf senescence and fruit yield in tomato[J]. Plant Physiology,2018,177(3):1286-1302.
[103]Sakuraba Y,Piao W L,Lim J H,et al. Rice ONAC106 inhibits leaf senescence and increases salt tolerance and tiller angle[J]. Plant and Cell Physiology,2015,56(12):2325-2339.
[104]Zhu Z G,Li G R,Yan C H,et al. DRL1,encoding a NAC transcription factor,is involved in leaf senescence in grapevine[J]. International Journal of Molecular Sciences,2019,20(11):2678.
[105]Jensen M K,Lindemose S,Masi F D,et al. ATAF1 transcription factor directly regulates abscisic acid biosynthetic gene NCED3 in Arabidopsis thaliana[J]. FEBS Open Bio,2013,3(1):321-327.
[106]Yang S D,Seo P J,Yoon H K,et al. The Arabidopsis NAC transcription factor VNI2 integrates abscisic acid signals into leaf senescence via the COR/RD genes[J]. The Plant Cell,2011,23(6):2155-2168.
[107]Fujita M,Fujita Y,Maruyama K,et al. A dehydration-induced NAC protein,RD26,is involved in a novel ABA-dependent stress-signaling pathway[J]. Plant Journal,2004,39(6):863-876.
[108]Hu H,You J,Fang Y,et al. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice[J]. Plant Molecular Biology,2008,67(1/2):169-181.
[109]Chen X,Wang Y F,Lv B,et al. The NAC family transcription factor OsNAP confers abiotic stress response through the ABA pathway[J]. Plant and Cell Physiology,2014,55(3):604-619.
[110]Lu P L,Chen N Z,An R,et al. A novel drought-inducible gene,ATAF1,encodes a NAC family protein that negatively regulates the expression of stress-responsive genes in Arabidopsis[J]. Plant Molecular Biology,2007,63(2):289-305.
[111]Yoshii M,Yamazaki M,Rakwal R,et al. The NAC transcription factor RIM1 of rice is a new regulator of jasmonate signaling[J]. The Plant Journal,2010,61(5):804-815.
[112]Xu C,Lu S C,Wang Y F,et al. OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice[J]. The Plant Journal,2015,82(2):302-314.
[113]Kim S G,Lee A K,Yoon H K,et al. A membrane-bound NAC transcription factor NTL8 regulates gibberellic acid-mediated salt signaling in Arabidopsis seed germination[J]. The Plant Journal,2008,55(1):77-88.
[114]Hu H,Dai M,Yao J,et al. Overexpressing a NAM,ATAF,and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice[J]. Proceedings of the National Academy of Sciences of the United States of America,2006,103(35):12987-12992.
[115]Nakashima K,Tran L S,Van Nguyen D,et al. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice[J]. The Plant Journal,2007,51(4):617-630.
[116]Zheng X N,Chen B,Lu G J,et al. Overexpression of a NAC transcription factor enhances rice drought and salt tolerance[J]. Biochemical and Biophysical Research Communications,2009,379(4):985-989.
[117]Hong Y B,Zhang H J,Huang L,et al. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in rice[J]. Frontiers in Plant Science,2016,7:4.
[118]Shen J B,Lv B,Luo L Q,et al. The NAC-type transcription factor OsNAC2 regulates ABA-dependent genes and abiotic stress tolerance in rice[J]. Scientific Reports,2017,7(1):40641.
[119]Liu Y C,Jie S,Wu Y R. Arabidopsis ATAF1 enhances the tolerance to salt stress and ABA in transgenic rice[J]. Journal of Plant Research,2016,129(5):955-962.
[120]Rahman H,Ramanathan V,Nallathambi J,et al. Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice[J]. BMC Biotechnology,2016,16(S1):35.
[121]Guan H R,Xin L,Fei N,et al. OoNAC72,a NAC-Type Oxytropis ochrocephala transcription factor,conferring enhanced drought and salt stress tolerance in Arabidopsis[J]. Frontiers in Plant Science,2019,10:890.
[122]Pang X Y,Xue M,Ren M Y,et al. Ammopiptanthus mongolicus stress-responsive NAC gene enhances the tolerance of transgenic Arabidopsis thaliana to drought and cold stresses[J]. Genetics and Molecular Biology,2019,42(3):624-634.
[123]Yong Y,Zhang Y,Lyu Y. A Stress-Responsive NAC transcription factor from tiger lily (LlNAC2) interacts with LlDREB1 and LlZHFD4 and enhances various abiotic stress tolerance in Arabidopsis[J]. International Journal of Molecular Sciences,2019,20(13):3225.
[124]Borgohain P,Saha B,Agrahari R,et al. SlNAC2 overexpression in Arabidopsis results in enhanced abiotic stress tolerance with alteration in glutathione metabolism[J]. Protoplasma,2019,256(4):1065-1077.
[125]He K,Zhao X,Chi X Y,et al. A novel Miscanthus NAC transcription factor MlNAC10 enhances drought and salinity tolerance in transgenic Arabidopsis[J]. Journal of Plant Physiology,2019,233:84-93.
[126]Yang X W,Kang H,Chi X Y,et al. Miscanthus NAC transcription factor MlNAC12 positively mediates abiotic stress tolerance in transgenic Arabidopsis[J]. Plant Science,2018,277:229-241.
[127]Cao H S,Li W,Muhammad A N,et al. Ectopic expression of pumpkin NAC transcription factor CmNAC1 improves multiple abiotic stress tolerance in Arabidopsis[J]. Frontiers in Plant Science,2017,8:2052.
[128]Lei H,Hong Y B,Zhang H J,et al. Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance[J]. BMC Plant Biology,2016,16(1):203.
[129]He L,Shi X X,Wang Y M,et al. Arabidopsis ANAC069 binds to C[A/G]CG[T/G]sequences to negatively regulate salt and osmotic stress tolerance[J].Plant Mol Biol, 2017,93(4/5):369-387.
[130]An J P,Rui L,Qu F J,et al. An apple NAC transcription factor negatively regulates cold tolerance via CBF-dependent pathway[J]. Journal of Plant Physiology,2018,221:74-80.
[131]Lin H,Jing B,Xu J Y,et al. Novel maize NAC transcriptional repressor ZmNAC071 confers enhanced sensitivity to ABA and osmotic stress by downregulating stress-responsive genes in transgenic Arabidopsis[J]. Journal of Agricultural and Food Chemistry,2019,67(32):8905-8918.
[132]Xin H,Zhu L F,Lian X,et al. GhATAF1,a NAC transcription factor,confers abiotic and biotic stress responses by regulating phytohormonal signaling networks[J]. Plant Cell Reports,2016,35(10):2167-2179.
[133]Fang L C,Su L Y,Sun X M,et al. Expression of Vitis amurensis NAC26 in Arabidopsis enhances drought tolerance by modulating jasmonic acid synthesis[J]. Journal of Experimental Botany,2016,67(9):2829-2845.
[134]Shahnejat-Bushehri S,Tarkowska D,Sakuraba Y,et al. Arabidopsis NAC transcription factor JUB1 regulates GA/BR metabolism and signalling[J]. Nature Plants,2016,2(3):16013.
[135]Lin R M,Zhao W S,Meng X B,et al. Rice gene OsNAC19 encodes a novel NAC-domain transcription factor and responds to infection by Magnaporthe grisea[J]. Plant Science,2007,172(1):120-130.
[136]Wang X E,Basnayake B S,Zhang H J,et al. The Arabidopsis ATAF1,a NAC transcription factor,is a negative regulator of defense responses against necrotrophic fungal and bacterial pathogens[J]. Molecular Plant-Microbe Interactions,2009,22(10):1227-1238.
[137]Delessert C,Kazan K,Wilson I W,et al. The transcription factor ATAF2 represses the expression of pathogenesis-related genes in Arabidopsis[J]. The Plant Journal,2005,43(5):745-757.
[138]Chen Y J,Perera V,Christiansen M W,et al. The barley HvNAC6 transcription factor affects ABA accumulation and promotes basal resistance against powdery mildew[J]. Plant Molecular Biology,2013,83(6):577-590.
[139]Jensen M K,Jesper H R,Gregersen P L,et al. The HvNAC6 transcription factor:a positive regulator of penetration resistance in barley and Arabidopsis[J]. Plant Molecular Biology,2007,65(1/2):137-150.
[140]Peng X J,Wang Q,Yu W,et al. A maize NAC transcription factor,ZmNAC34,negatively regulates starch synthesis in rice[J]. Plant Cell Reports,2019,38(12):1473-1484.
[141]Zhang Z Y,Dong J Q,Chen J,et al. NAC-type transcription factors regulate accumulation of starch and protein in maize seeds[J]. Proceedings of the National Academy of Sciences of the United States of America,2019,116(23):11223-11228.
[142]Li W J,Xue H,Yi C,et al. A wheat transcription factor positively sets seed vigour by regulating the grain nitrate signal[J]. New Phytologist,2020,225(4):1667-1680.
[143]Gao Y,Wei W,Zhao X D,et al. A NAC transcription factor,NOR-like1,is a new positive regulator of tomato fruit ripening[J]. Horticulture Research,2018,5(1):75.
[144]Carrasco-Orellana C,Stappung Y,Mendez-Yaez A,et al. Characterization of a ripening-related transcription factor FcNAC1 from Fragaria chiloensis fruit[J]. Scientific Reports,2018,8(1):10524.
[145]Jahan M A,Harris B,Lowery M,et al. The NAC family transcription factor GmNAC42-1 regulates biosynthesis of the anticancer and neuroprotective glyceollins in soybean[J]. BMC Genomics,2019,20(1):149.
[146]Guo S Y,Dai S J,Prashant K S,et al. A membrane-bound NAC-like transcription factor OsNTL5 represses the flowering in Oryza sativa[J]. Frontiers in Plant Science,2018,9:555.
[147]Zhang H H,Cui X Y,Guo Y X,et al. Picea wilsonii transcription factor NAC2 enhanced plant tolerance to abiotic stress and participated in RFCP1-regulated flowering time[J]. Plant Molecular Biology,2018,98(6):471-493.
[148]Bin Z,Huo D A,Hong X X,et al. The Salvia miltiorrhiza NAC transcription factor SmNAC1 enhances Zinc content in transgenic Arabidopsis[J]. Gene,2019,688:54-61.

