Short- and long-term outcomes associated with enhanced recovery after surgery protocol vs conventional management in patients undergoing laparoscopic gastrectomy
Yu-Long Tian, Shou-Gen Cao, Xiao-Dong Liu, Ze-Qun Li, Gan Liu, Xing-Qi Zhang, Yu-Qi Sun, Xin Zhou, Dao-Sheng Wang, Yan-Bing Zhou
Abstract
Key Words: Enhanced recovery after surgery; Conventional management; Laparoscopic gastrectomy; Short-term outcomes; Survival
INTRODUCTION
Globally, gastric cancer is a common malignant tumour and has the third highest mortality rate. In 2018, there were more than 1.3 million new cases of gastric cancer and more than 780000 gastric cancer related deaths[1]. The treatments and outcomes of gastric cancer have improved substantially over recent decades because of the introduction of new surgical techniques and chemotherapeutic drugs[2-5]. Enhanced recovery after surgery (ERAS) pathways have been proven to improve postoperative recovery and reduce postoperative complications, the length of hospital stay, and medical costs after gastric cancer surgery[6-8]. In a previously published randomized controlled trial, we investigated the relationship between postoperative compliance to ERAS protocols and short-term postoperative outcomes, and similar results were obtained[9]. Despite the short-term outcomes achieved with the perioperative ERAS protocol, the postoperative complication rate remains at approximately 10% to 30%.
Emerging evidence suggests that the ERAS protocol can influence long-term oncological outcomes after colorectal cancer surgery and elective orthopaedic surgery[10-12]. The mechanism behind this effect may not only be related to the reduction of complications and immune suppression but also to changes in immune response leading to a higher recurrence rate and distant metastasis rate[13-16]. However, it is not clear whether the ERAS regimen can improve the long-term prognosis of gastric cancer. Therefore, the aim of this retrospective study was to determine the effect of the ERAS protocol after laparoscopic gastrectomy on long-term survival. Moreover, shortterm clinical outcomes and inflammatory parameters were compared between the ERAS and conventional protocols.
MATERIALS AND METHODS
Study design and participants
From January 2012 to December 2015, 1026 consecutive laparoscopic gastrectomies were performed at the Department of General Surgery of the Affiliated Hospital of Qingdao University, China. Data from these procedures were prospectively collected in a database and then retrospectively reviewed. The ERAS protocol was introduced in 2010 as a standard protocol for perioperative care in our department. Because the ERAS protocol was not be accepted by some patients at that time, based on the willingness of the patients, the department was divided into an ERAS ward (ERAS protocol) and a non-ERAS ward (conventional pathway). In the same period, we extensively performed laparoscopic surgery for gastric cancer. According to the postoperative pathological stage, patients with advanced gastric cancer were treated with S-1 combined with oxaliplatin for 6-8 cycles. All patients were followed by outpatient clinic visits and telephone interviews for up to 5 years after the primary operation.
After excluding some patients who did not meet the criteria, the ERAS group was matched in a ratio of 1:1 with the conventional group. Matching was achieved based on propensity scores including the following seven covariates: Age, American Society of Anesthesiology score, primary tumour location, histologic type, pathological stage, operation date, and adjuvant chemotherapy. The study design is shown in Figure 1.
Perioperative management and operation
Patients in the ERAS ward were managed according to the ERAS pathway during the perioperative period, the specific protocol includes 17 care elements (Table 1), and their compliance with all care elements was more than 80%, while the non-ERAS ward was managed according to the conventional pathway (Supplementary Table 1). To ensure recovery and reduce hospitalizations after gastric surgery, we paid particular attention to the “key components” of the ERAS programme, namely, the following six basic elements: Preoperative patient information and education, thoracic epidural anaesthesia combined with multimodal analgesia, target-oriented liquid management, no nasogastric tube, early oral feeding, and early mobilization.
Before surgery, chest computed tomography (CT), abdominal enhanced CT, and pelvic CT were performed to confirm the size and location of the tumour, and distant organ metastases were excluded according to the evaluation by two experienced radiologists. Echocardiography and pulmonary function tests were used to evaluate the tolerance of cardiopulmonary function to laparoscopic surgery. Nutrition risk screening 2002 was used to evaluate the nutritional status of patients; if the score was ≥ 3, the patients were given nutritional support.
Laparoscopic-assisted radical resection of distal gastric cancer (D2 Billroth-I/Billroth-II/Roux-en-Y) was performed under general anaesthesia. During the operation, we followed the basic principles of tumour treatment, mastered the appropriate scope of gastrectomy, performed fine lymph node dissection and gastrointestinal reconstruction, and recorded the volume of intraoperative infusion, volume of blood loss, operation time, and use of opioids and muscle relaxants.
Definition of surgical complications and mortality
Complications and mortality were defined as those occurring within 30 d after gastrectomy. Intraoperative complications were defined as bleeding due to vessel injury, injury to visceral organs, mechanical factor-related problems, cardiopulmonary dysfunction due to hypercapnia, and other complications. Wound infection was defined as a skin and subcutaneous tissue infection. A specific complication was diagnosed on the basis of either a medical imaging examination or obvious clinical evidence. Gastroparesis referred to the occurrence of emptying difficulties after surgery, combined with clinical features and imaging examinations for a final diagnosis. Anastomotic complications such as leakage, stenosis, or intracavitary haemorrhage were confirmed by gastrointestinal X-ray imaging, endoscopy, or angiography. Intra-abdominal collections and abscesses were proven by ultrasonography or CT scans and had concomitant systemic inflammatory responses that lasted for at least 24 h. Both intraoperative major bleeding and postoperative haemorrhage were defined as an amount of haemorrhage exceeding 300 mL. Traumatic pancreatitis was defined as increased serum amylase levels exceeding three times the upper limit of normal accompanied by obvious clinical symptoms and signs. Lymphatic leakage was confirmed by a chyle test when the abdominal drainage fluid volume exceeded 300 mL per day for 5 continuous days after postoperative day (POD)3. The severity of postoperative complications was assessed according to the Clavien-Dindo classification[17].

Table 1 Compliance with the enhanced recovery after surgery protocol in enhanced recovery after surgery group
Statistical analysis
Categorical variables are described as numbers and percentages and were compared between groups using Pearson’s chi-square test or Fisher’s exact test. Continuous variables are described as the mean ± standard deviation. Non-normally distributed continuous data are presented as the median and interquartile range, and Student’sttest was used for normally distributed continuous variables. Survival curves were plotted using the Kaplan-Meier method. The log-rank test was used to compare survival curves. Survival time was defined as the interval between the primary operation date and a new event or the last follow-up. Patients who did not experience any event and were still alive at 60 mo were censored at this time. Significance was defined asP< 0.05. All statistical tests were two-sided and performed using SPSS software version 24.0 (SPSS, Chicago, IL, United States).
Ethical statement
The study was approved by the Affiliated Hospital of Qingdao University Ethics Review Committee (No. QYFYKYLL-2018-34). All procedures were performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
RESULTS
Compliance to the ERAS protocol
Table 1 lists the specific ERAS programmes and compliance to its 17 care elements. The following three elements achieved 100% compliance: Health education, intraoperative safety check, and precision surgery scheme. As compliance to the ERAS programme is affected by multidisciplinary cooperation among surgeons, anaesthesiologists, and nurses as well as the physical status and willingness of patients, the compliance rate to partial elements is typically low. Fortunately, our ERAS team is united and cooperative, and the implementation rate of all elements was greater than 80%. According to the database, more than 90% of patients implemented four and more ‘key components’ of the ERAS protocol, which is essential for our research.

Figure 1 Flow diagram of patient selection process. GC: Gastric cancer; ASA: American Society of Anesthesiologists; ERAS: Enhanced recovery after surgery.
Demographics and baseline characteristics
After the ERAS group was matched in a 1:1 ratio to the conventional group, there were 365 patients in each group. The patient demographics and baseline characteristics are detailed in Table 2 (Pathologic stage according to the American Joint Committee on Cancer)[18]. The baseline characteristics were well balanced after matching. We can observe that with the advancement of surgical technology, the number of laparoscopic surgeries increased year by year. There were 245 and 233 patients in the ERAS group and the conventional group who received adjuvant chemotherapy, respectively.
Surgical results
In the matched population, the surgical results of the ERAS and conventional groups are presented in Table 3. The mean time to first flatus in the conventional group was 0.9 days longer than that in the ERAS group (3.40 dvs2.50 d;P< 0.001). Both the time to first liquid intake (1.02 dvs3.64 d;P< 0.001) and time to ambulation (1.47 dvs2.99 d;P< 0.001) were earlier in the ERAS group than in the conventional group. Compared to the conventional group, the ERAS group had a significantly shorter postoperative hospital stay (7.09 dvs8.67 d;P< 0.001) and lower medical costs ($7621.75vs$7814.76;P= 0.009). There were no statistically significant differences between the two groups in the other surgical results, especially in 30-d reoperation and 30-d readmission.

Table 2 Patient demographics and baseline characteristics
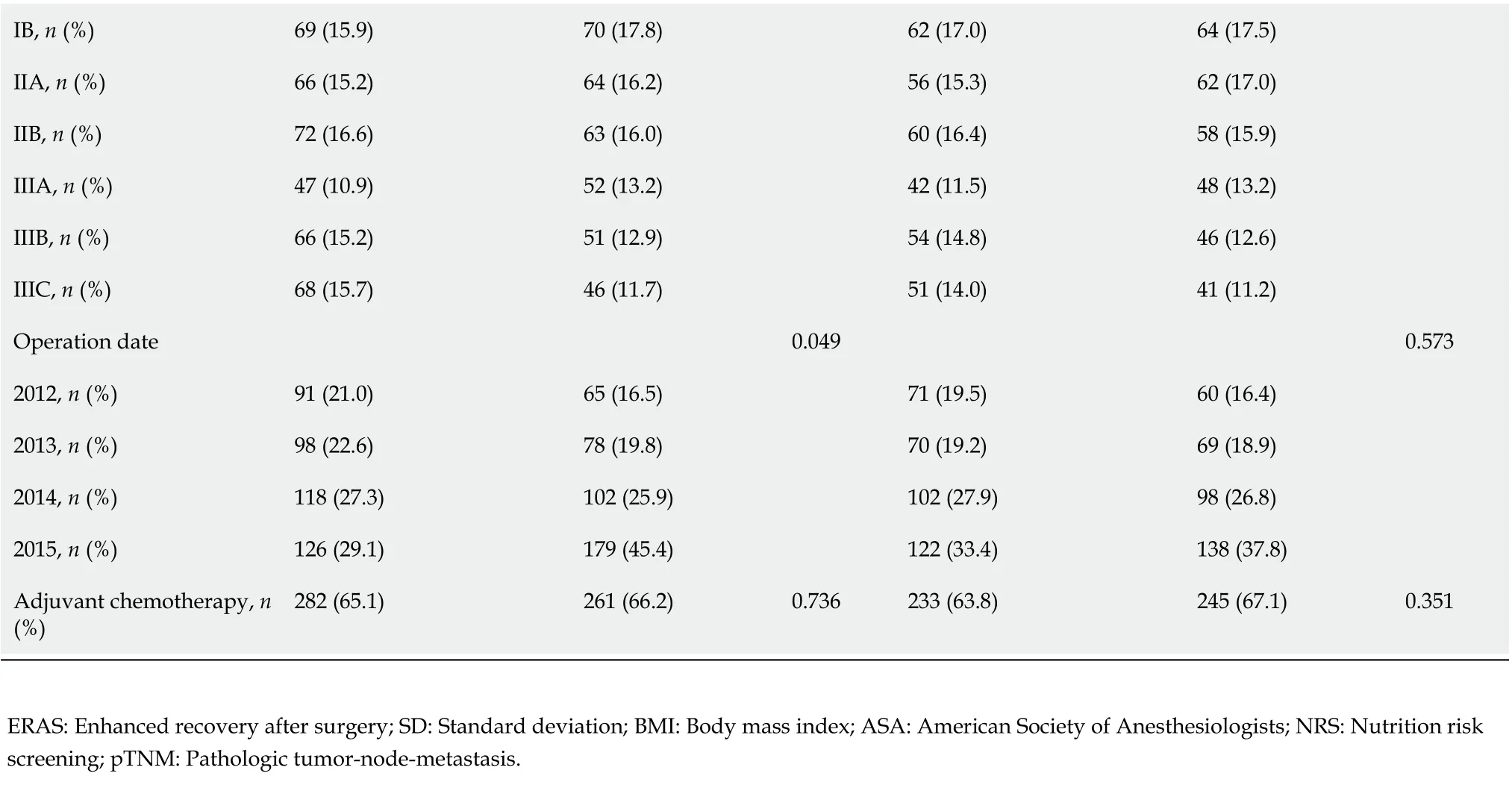
IB, n (%)69 (15.9)70 (17.8)62 (17.0)64 (17.5)IIA, n (%)66 (15.2)64 (16.2)56 (15.3)62 (17.0)IIB, n (%)72 (16.6)63 (16.0)60 (16.4)58 (15.9)IIIA, n (%)47 (10.9)52 (13.2)42 (11.5)48 (13.2)IIIB, n (%)66 (15.2)51 (12.9)54 (14.8)46 (12.6)IIIC, n (%)68 (15.7)46 (11.7)51 (14.0)41 (11.2)Operation date 0.049 0.573 2012, n (%)91 (21.0)65 (16.5)71 (19.5)60 (16.4)2013, n (%)98 (22.6)78 (19.8)70 (19.2)69 (18.9)2014, n (%)118 (27.3)102 (25.9)102 (27.9)98 (26.8)2015, n (%)126 (29.1)179 (45.4)122 (33.4)138 (37.8)Adjuvant chemotherapy, n (%)282 (65.1)261 (66.2)0.736 233 (63.8)245 (67.1)0.351 ERAS: Enhanced recovery after surgery; SD: Standard deviation; BMI: Body mass index; ASA: American Society of Anesthesiologists; NRS: Nutrition risk screening; pTNM: Pathologic tumor-node-metastasis.

Table 3 Surgical results for enhanced recovery after surgery and conventional groups
Postoperative complications
Postoperative morbidity and mortality are reported in Table 4. Apart from a significantly higher rate of postoperative complications among patients in the conventional group than among those in the ERAS group (18.1vs12.3;P= 0.030), no significant differences in intraoperative complications, mortality, or Clavien-Dindo classification could be detected between the two groups. Moreover, according to the Clavien-Dindo classification of surgical complications, the distribution of severity (grade 3 or more) was similar between the two groups (P=0.192). Remarkably, 20 (5.5%) patients in the ERAS group and 13 (3.6%) in the conventional group had pulmonary complications, with no statistically significant difference between the two groups (P= 0.212).
Inflammatory indexes
The serum white blood cell (WBC), C-reactive protein (CRP), and procalcitonin were used as indicators of the surgical stress response. On POD 1, the WBC, CRP, and procalcitonin levels increased significantly in both groups compared to the preoperative values (P< 0.05 for all). The WBC, CRP, and procalcitonin did not increase as much in the ERAS group as in the conventional group. In particular, the CRP (0.63 ± 0.33vs0.58 ± 0.30) and procalcitonin (90.61 ± 20.42vs78.35 ± 16.73) levels on POD 3/4 were significantly different between the two groups (P< 0.001, Figure 2A;P= 0.025, Figure 2B). There was no significant difference between the groups in terms of WBC (Figure 2C). Notably, from POD 4-6, all indicators of both groups gradually recovered.
Survival
The follow-up endpoint was December 31, 2019. The median follow-up duration was 67 (range, 3-92) mo. The deaths of 226 patients resulted in a 5-year overall survival (OS) rate of 72.9% (99 of 365) in the ERAS group and 65.2% (127 of 365) in the conventional group (log-rank test,P= 0.013, Figure 3A). ERAS interventions were associated with a significantly improved 5-year cancer-specific survival rate compared with that in the conventional group (P= 0.033, Figure 3B). When stratified by tumour stage, the 5-year OS rates were 91.8%, 83.3%, and 48.1% for stages I, II, and III disease in the ERAS group, respectively, but 90.2%, 76.7%, and 38.3% in the conventional group, respectively (Figure 3C). In particular, the 5-year OS rate of the ERAS group was better than that of the traditional group, but only the survival of patients with stage III disease was significantly different between the two groups (log-rank test,P= 0.044).
DISCUSSION
The results of this retrospective study suggest that ERAS might be a promising perioperative management protocol for gastric cancer in terms of short-term and longterm outcomes. To the best of our knowledge, this is the first propensity scorematched study to reveal that the ERAS protocol can improve the 5-year OS and cancerspecific survival rates of patients with gastric cancer.
In this study, the perioperative ERAS protocol for gastric cancer surgery included at least 17 independent components. The formulation of consensus guidelines for enhanced recovery after gastrectomy, which consisted of 25 items in 2014, filled in the gap of an ERAS protocol in the perioperative period of gastric cancer surgery[19]. After more than 10 years of development, the ERAS protocol has been further improved and developed; we also summarized the protocol. Many studies have shown that compliance to the ERAS protocol is closely related to the surgical outcome[20,21]. In fact, fully implementing the ERAS protocol in clinical practice is indeed a challenge, and a nationwide survey in Korea also found similar problems[22]. There are many reasons behind these challenges, and they include a lack of knowledge, lack of acceptance, lack of ability, or lack of wish to change, or lack of clinical leadership; thus, many elements have to be implemented in a busy clinical department[23]. Fortunately, with total multidisciplinary collaboration among anaesthesiologists, surgeons, nurses, and physiotherapists, the patients' adherence to all components was greater than 80%. Research from the ERAS Compliance Group showed that increasing the compliance to an ERAS programme independently improved outcomes[24]. Next, we will pay more attention to focusing on how to improve patient compliance, including strengthening ERAS care training, strengthening education, improving clinical leadership, and simplifying the ERAS elements.

Table 4 Postoperative morbidity and mortality
ERAS protocols in elective radical gastrectomy for gastric cancer have been widely accepted, and the evidence behind the feasibility and safety of ERAS has repeatedly been shown[20,21]. After the ERAS group was matched with the conventional group, there were no differences in demographics or baseline characteristics between the two groups in this study. The present study showed that ERAS is a safe and feasible protocol for gastric cancer because this approach led to a faster recovery, shorter hospital stay, and lower medical costs. Moreover, the total incidence of postoperative complications in the ERAS group was significantly lower than that in the conventional group. Although there was no significant difference, the incidence of pulmonary complications and wound infections in the ERAS group was lower than that in the conventional group, which may be related to the use of pre-rehabilitation (health education, exercise advice, psychological guidance, organ function evaluation and intervention, and nutritional assessment and intervention), multimodal analgesia, and early mobilization and intake. In addition, it is worth mentioning that the 30-d reoperation and readmission and perioperative mortality rates were not different between the two groups.

Figure 2 Comparison of levels of inflammatory indexes in the two groups. A: C-reactive protein; B: Procalcitonin; C: White blood cell count. POD: Postoperative day; ERAS: Enhanced recovery after surgery.
Surgical trauma will stimulate the release of a large number of inflammatory molecules, such as CRP, procalcitonin, interleukin-6 (IL-6), and necrosis factor-alpha (TNF-α), which will lead to a systemic inflammatory response, affect immune function, and increase the incidence of postoperative symptoms. CRP is one of the most commonly used inflammatory proteins to study acute response, and its expression level is related to the stress response to surgery. In healthy people, procalcitonin levels are very low, but lipopolysaccharides, microbial toxins, and inflammatory mediators (IL-6 or TNF-α) can directly or indirectly cause its increase during inflammation. Therefore, procalcitonin also reflects systemic inflammatory response and stress activity. Although the CRP and procalcitonin levels on POD 3/4 were significantly different between the two groups, the WBC, CRP, and procalcitonin did not increase as much in the ERAS group as in the conventional group. The results of this study are similar to those of previous studies: The ERAS pathway can reduce the postoperative stress response[20].
The adoption of ERAS principles is increasing due to the significantly better shortterm outcomes, but little is known about how ERAS can improve long-term prognosis. All things considered, the results of this study seem to have established that ERAS is superior to the conventional protocol in terms of oncological outcomes, particularly for stage III gastric cancer. Although there was no significant difference in survival of patients with stage II disease, the 5-year OS rate in the ERAS group was better than that in the conventional group. We conducted a retrospective study of gastric cancer patients who underwent standard radical gastrectomy from 2007 to 2012 and reached similar conclusions[25]. However, there are several potential explanations for why compliance to ERAS protocols better contributed to improved survival. First, perioperative complications have been shown to be strongly associated with impaired long-term outcomes[26,27]. This could be the result of either delayed adjuvant chemoradiation or no adjuvant chemoradiation in complicated cases[28]. Moreover, ERAS protocols can bring potential benefits to advanced gastric cancer that may be related to a lower stress response and reduction in insulin resistance, which are considered to be factors associated with a good cancer long-term prognosis[29-31]. Studies show that a relative perioperative cortisol deficiency appears to be an underrecognised contributor to perioperative organ injury[32]. Recent data from high-dose preoperative steroid administrations (i.e., greater than 8 mg dexamethasone) are promising and showed that this approach improves pain relief, reduces the inflammatory response and early fatigue, and enhances patient recovery[33]. Furthermore, environmental challenges that affect the patient perioperatively, such as psychological distress, intraoperative hypothermia, and use of anaesthetic drugs or blood transfusions, also trigger a variety of stress responses that can substantially affect the metastatic process through effects on distal malignant cells, their microenvironment, and interacting immunocytes[34-36]. In contrast, ERAS protocols advocate for preoperative education, pre-rehabilitation, an avoidance of intraoperative hypothermia, multimodal analgesia, and an avoidance of unnecessary blood transfusions to reduce the stress response of surgical patients[37]. The unavoidable damage to the patients’ tissues and the removal and manipulations of the primary tumour during surgery have been shown to increase tumour cell shedding into the blood and lymphatic systems and to decrease systemic levels of primary tumourassociated antiangiogenic factors (such as endostatin)[38,39]. In addition, the patients’ paracrine and neuroendocrine responses to surgery, including the release of prostaglandins and catecholamines, facilitate malignant cell survival, motility, invasion, and proliferation and the release of proangiogenic factors, and suppress antimetastatic immunity[40,41]. In summary, every element plays an important role in the ERAS protocol, and even a combination of small changes can have a large impact on the outcome.

Figure 3 Kaplan-Meier curves. A: 5-yr overall survival; B: 5-yr cancer-specific survival; C: 5-yr overall survival for stages I, II, and III disease between the two groups. ERAS: Enhanced recovery after surgery.
Our study has several limitations. First, we attempted to analyse the changes in stress response, but due to the limitations of retrospective data, there was no analysis of additional indicators (TNF-α and IL-6). Second, many components related to the time-varying ERAS protocol may have affected the results regarding both short-term and long-term outcomes; however, the weight of these components in the ERAS protocol requires further evaluation to determine the rational essential elements. Third, it is impossible to clarify the mechanism behind the influence of the ERAS protocol on short-term and long-term outcomes; detailed mechanistic studies are needed in the future. Finally, this was a single-centre retrospective study; therefore, multicentre randomised controlled trial studies should be performed to verify the reliability of the results. Fortunately, we have registered (Chinese Clinical Trial Registry, CHiCTR1900022438) and started such a project, and patients are currently being recruited[42]. We hope that our data will provide trustworthy evidence that the ERAS pathway improves survival in patients with gastric cancer.
CONCLUSION
In conclusion, the ERAS protocol has been proven to be a safe and effective perioperative management pathway in the current literature. In particular, the ERAS protocol has shown promising results in improving the survival of patients with gastric cancer after surgery.
ARTICLE HIGHLIGHTS


Research conclusions
The ERAS protocol has been proven to be a safe and effective perioperative management pathway in the current literature. In particular, the ERAS protocol has shown promising results in improving the survival of patients with gastric cancer after surgery.
Research perspectives
This was a single-centre retrospective study; therefore, multicentre randomised controlled trial studies should be performed to verify the reliability of the results. Fortunately, we have registered (Chinese Clinical Trial Registry, CHiCTR1900022438) and started such a project, and patients are currently being recruited. We hope that our data will provide trustworthy evidence that the ERAS pathway improves survival in patients with gastric cancer.
ACKNOWLEDGEMENTS
We thank the patients participating in the study as well as the nurses and the management staff at the hospital. We also thank Wang H and Wang LK for contribution to the data collection.
 World Journal of Gastroenterology2020年37期
World Journal of Gastroenterology2020年37期
- World Journal of Gastroenterology的其它文章
- Review of inflammatory bowel disease and COVID-19
- Hepatitis E virus: Epidemiology, diagnosis, clinical manifestations, and treatment
- Calcifying fibrous tumor of the gastrointestinal tract: A clinicopathologic review and update
- Application of artificial intelligence in the diagnosis and treatment of hepatocellular carcinoma: A review
- Antioxidant activity and hepatoprotective effect of 10 medicinal herbs on CCl45629 -induced liver injury in mice
- Periodontitis combined with smoking increases risk of the ulcerative colitis: A national cohort study
