Study on differential gene expression profile of serum exosomes in patients with acute cerebral infarction
TANG Rongmei, CHEN Bowei, YI Jin, LIU Biyn,b*, LIN Hushn
a.Department of Neurology, The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan 410007, China
b.Department of Basic Medical Sciences, Yiyang Medical College, Yiyang, Hunan 413002, China
c.Institute of Precision Medicine, GE Healthcare, Changsha, Hunan 410005, China
Keywords Acute cerebral infarction (ACI)Exosomes mRNA Expression profile Gene chip H3F3B
ABSTRACT Objective To analyze the differential gene expression profile of serum exosomes in patients with acute cerebral infarction (ACI)and clarify the changes in gene expression related to cerebral infarction injury and the potential serum markers.Methods Four patients with ACI and five healthy people were enrolled in the Phase Ⅰ study.After serum isolation from peripheral blood, exosomes were extracted with exosomes kits, highthroughput detection of mRNA was performed with gene chips,and differentially expressed mRNAs were screened.Gene Ontology (GO) functional analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed simultaneously.Furthermore,real-time polymerase chain reaction (qRT-PCR) was used to verify the expression levels of the screened differential mRNAs in the serum exosomes collected in Phase Ⅱ from 32 patients each in the ACI case and normal control groups.Results In the Phase Ⅰ study, there were 248 differentially expressed mRNAs (fold change ≥ 2.0, P < 0.05) among five patients in the normal control group and four patients in the case group, of which the expression of 242 was upregulated and that of six was downregulated.The results of GO functional enrichment analysis mainly included behavior regulation, cell connection,and antioxidant activity.The results of KEGG pathway enrichment analysis mainly included ribosomes, proteasomes, oxytocin signaling pathways, and oxidative phosphorylation.After researching and screening based on relevant literature, it was found that among the genes with significant differential expression,H3F3B mRNA may be associated with and might play an important role in ACI.The qRT-PCR method was used to detect the H3F3B mRNA expression in serum exosomes of 32 patients each in the normal control and case groups in Phase Ⅱ; the expression was significantly higher in serum exosomes of the case group than in those of the normal control group (P < 0.001).H3F3B mRNA expression in serum exosomes of the case group positively correlated with age, the National Institutes of Health Stroke Scale(NIHSS) score, and the maximum infarct size (P < 0.05).Conclusion ACI can lead to changes in the serum exosomes mRNA expression profile, which may be closely related to the occurrence, development, and prognosis of this condition.These findings will provide direction for research on the molecular mechanism, diagnostic markers, and therapeutic targets of ACI.
1 Introduction
Cerebral stroke is a common cerebrovascular disease, and the incidence of ischemic stroke accounts for 87% of all cerebral strokes.At present, the main treatment methods for acute cerebral infarction(ACI) include thrombolytic drugs and interventional therapy.However, due to the urgency of the onset of cerebral infarction and the particularity of neuron function, it often leads to high disability and fatality rates, resulting in huge psychological and economic burdens to the patients, families, and society[1,2].Therefore, the search for more effective treatments for cerebral infarction has become a clinical problem that needs to be solved urgently.At present, mesenchymal stem cell therapy is considered a novel and effective treatment method[3,4].Mesenchymal stem cells play an important role in brain neuron generation, synapse formation, and axon connection[5-8].However, these cells cannot directly pass through the blood-brain barrier.Studies have shown that mesenchymal stem cells may indirectly participate in the regulation of brain nerve function in a paracrine manner, that is, they load active and signaling substances into a type of carrier-exosomes,and then the exosomes are excreted into the blood.After being ingested by brain nerves and cells, they help with signal transmission and component exchange between cells[7-9].However, the specific mechanism by which exosomes participate in the regulation of neurological function is still unclear.
Exosomes are small vesicles with a diameter of 30 to 100 nm that are rich in DNA, mRNA, miRNA, protein, and other biologically active substances.Exosomes are widely found in body fluids such as blood,saliva, and urine, and are important mediators of cell-to-cell communication that can protect internal signal molecules from degradation[10].Studies have been conducted on exosomal miRNA and lncRNA,which are considered to be involved in many physiological and pathological processes of the human body[11,12].A study has shown that the levels of exosomal miR-223 and miR-9 are significantly higher in patients with ACI than in healthy people, and that their expression positively correlates with the National Institutes of Health Stroke Scale (NIHSS) score[13],suggesting that exosomal miR-223 and miR-9 are potential biomarkers for diagnosing ACI and assessing the degree of ischemic injury.In addition, there is evidence that, by incubating the exosomes in the cerebrospinal fluid and neural stem cells together,the insulin-like growth factor/mammalian target of rapamycin (mTOR) signaling pathway of neural stem cells can be activated; this promotes the proliferation of neural stem cells and maintains the normal function of the immune system[14,15].However, the role of exosomal mRNA was once controversial; there is reliable evidence that mRNA can be loaded intact into exosomes and transported to target cells to play a role[16].TERT mRNA can be detected in the serum exosomes of a variety of different tumors but not in the serum exosomes of normal people[17].In vitroandin vivoexperiments confirmed that serum exosomal ECRG4 mRNA can inhibit cell proliferation and tumor angiogenesis, thereby serving the same role as tumor suppressor genes[18].In summary, various substances carried by serum exosomes in patients with ACI may be important factors in the onset,development,and outcome of this condition.However, as the core substance in the process of transcription, the specific mechanism of exosomal mRNA involved in the occurrence, development and prognosis of cerebral infarction remains to be further explored.The technique of gene chip-based highthroughput detection has been widely used in gene expression profile analysis, gene cloning, and the search for specific biomarkers of many diseases[19].In this study, high-throughput gene chips were used to detect the differential expression profiles of exosomal mRNA in peripheral blood of patients with ACI and normal healthy individuals, and elucidate the specific molecular mechanisms of such infarction.
2 Materials and methods
2.1 Clinical data
For Phase Ⅰof the study, four patients who were hospitalized in the Department of Neurology of the Affiliated Hospital of Yiyang Medical College and Taojiang County Hospital of Traditional Chinese Medicine from August to October 2019, and diagnosed with ACI by CT or MRI with an NIHSS score of 7 − 10 points, were enrolled as the case group, and five healthy subjects in this hospital during the same period were enrolled as the normal control group.Among the four patients in the case group, there were two males and two females; their age ranged from 45 to 70 years, with an average of (58.6 ± 9.1) years.The course period of the disease was 1 to 7 days, with an average of (3.6 ± 1.8) days.Among the five patients in the control group, there were three males and two females; their ages ranged from 45 to 72 years, with an average of (56.7 ± 8.5) years.The two groups of study subjects had no statistically significant differences in age, gender, and presence of concomitant hypertension, hyperlipidemia, and coronary heart disease (P>0.05), and they were comparable.
For Phase Ⅱ of the study, 32 patients who were hospitalized in the Department of Neurology of the Affiliated Hospital of Yiyang Medical College and Taojiang County Hospital of Traditional Chinese Medicine from January to May 2021, and diagnosed with ACI by CT or MRI with an NIHSS score of 1 − 42 points, were enrolled as the case group, and 32 healthy subjects in this hospital during the same period were enrolled as the normal control group.Among the 32 patients in the case group, there were 18 males and 14 females; their age ranged from 42 to 80 years, with an average of (65.2 ± 10.6) years.The course period of the disease was 1 to 14 days, with an average of (6.4 ± 3.7) days.Among the 32 patients in the control group, there were 17 males and 15 females; their ages ranged from 43 to 77 years, with an average of (63.8 ± 8.2) years.The two groups of study subjects had no statistically significant differences in age, gender, and presence of concomitant hypertension, hyperlipidemia, and coronary heart disease (P>0.05), and they were comparable.All the trial protocols were approved by the Ethics Committee of Yiyang Medical College and registered at www.chictr.org.cn(Registration No.ChiCTR2100052445).
2.2 Diagnostic criteria
ACI was diagnosed according to the “Guidelines for Diagnosis and Treatment of Acute Ischemic Stroke in China 2018”[20]: (1) acute onset; (2) presence of focal neurological deficits; (3) brain MRI/CT excluded cerebral hemorrhage and other non-hemorrhagic etiologies; (4) imaging showed responsible lesions or symptoms/signs lasting more than 24 h; (5) a few cases had comprehensive neurological deficits.
2.3 Inclusion criteria
(1) The patient was 35 to 80 years old; (2) this was the first onset of the disease, which was in line with the diagnostic criteria for ACI; (3) no thrombolysis or interventional treatment was performed after admission; (4) the patient or his/her family members had signed the informed consent form.
2.4 Exclusion criteria
(1) Those with infectious diseases; (2) cardiogenic cerebral embolism caused by atrial fibrillation; (3) those with a history of stroke, brain tumor, brain trauma,cerebral hemorrhage, etc.; (4) those with hematological diseases, malignant tumors, autoimmune diseases, and severe depression of function of heart,kidney, lung, liver, and other important organs;(5) breastfeeding or pregnant women; (6) those with allergies.
2.5 Specimen collection
10 mL of peripheral venous blood was drawn from all the subjects enrolled in the two phases (nine cases in Phase Ⅰ and 64 cases in Phase Ⅱ) on an empty stomach in the morning after admission, and centrifuged at 4 °C and 1 600 rpm for 10 min; the serum was collected, and stored at – 80 °C.
2.6 Isolation of serum exosomes
Exosomes were firstly isolated from the sera collected from the subjects enrolled in the two phases(nine cases in Phase Ⅰ and 64 cases in Phase Ⅱ)using an exoRNeasy Serum-Plasma Midi Kit (Cat.No.7704, Qiagen, Germany) and ExoQuick exosome precipitation solution (Cat.No.EXOQ5A-1, Genetimes Excell, Shanghai) according to the operating manuals.Then, the TRIzol method was used to extract total RNA, including mRNA, miRNA, and other non-coding RNA, which were then stored for use after quality inspection by electrophoresis with the Agilent 2100 Bioanalyzer (Agilent, USA).
2.7 Exosomal mRNA high-throughput sequencing
The exosomes of nine subjects in Phase Ⅰ were subjected to mRNA high-throughput sequencing.An SBC Human 4 × 180K gene chip (Cat.No.074348, Agilent, USA) was operated according to the standard operating procedures stated in the manual, including links such as RNA fluorescent labeling, marker chip hybridization, and chip washing and scanning.
2.8 Identification of exosomal mRNA differential expression profile
The original data obtained by scanning the gene chips of nine study subjects in Phase Ⅰ were normalized using the R language “limma” software package, and the normalized data were screened for differentially expressed genes based on a fold change (FC) (absolute value of Log2FC) greater than 2.0 andP< 0.05.
2.9 Enrichment analysis of differentially expressed genes
Rpacages “Biomanager” and “ClusterProfiler”, and other software packages[21]were used to perform bioinformatics analysis on the target genes obtained from nine subjects in Phase Ⅰ, including Gene Ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis[22,23].
2.10 Real-time polymerase chain reaction (qRTPCR) method to detect exosomal H3F3B mRNA level
To evaluate the performance of the microarray data analysis, the serum exosomes of 32 patients each in the case and normal control groups were collected in Phase Ⅱ, and the differential expression of H3F3B mRNA was verified by qRT-PCR.Two sets of exosomes stored in a refrigerator at – 80 °C were taken out, the total RNA was extracted with TRIzol reagent(Thermo, USA), and cDNA was obtained by reverse transcription with the total mRNA as the template.Each sample was standardized using the U6 internal control gene, and H3F3B and U6 were amplified using a fluorescence quantitative RCP instrument(Thermo,USA).The primers were obtained as follows.The sequence of the target gene was searched on the NCBI database; Primer 5 software was used to design the primers, and they were synthesized by Tsingke.H3F3B-F: 5′-CAAGTGGTGGGGAGTCTTGT-3′; H3F3B-R: 5′-TCCCCCAGTTAGTGTTTGCATT-3′;product length: 175 bp.U6-F: 5′-CTCGCTTCGGCA GCACA-3′; U6-R: 5′-AACGCTTCACGAATTTGCGT-3′;product length: 94 bp.Sample loading system:template 2 μL,primers for amplification F/R(10 μmol/L) 1 μL for each, ddH2O 11 μL, 2 × SYBR Green PCR Master Mix 15 μL.Reaction program:95 °C, 10 min; 95 °C, 15 s; 60 °C, 30 s; 40 cycles; melting curve analysis: 60 − 95 °C.The result represented the average of three repetitions, and the expression level of exosomal H3F3B mRNA was quantitatively analyzed by the 2–ΔΔCtmethod.
2.11 Clinical characteristic indicators
In Phase Ⅱ of the study, patients in the case group(32 cases) were grouped based on their NIHSS score and the maximum infarct size after admission.Determination of neurological deficit score: the NIHSS score had a total of 42 points.The larger the score,the more severe the neurological deficit in patients with ACI.NIHSS score ≥ 15 was defined as severe(12 cases), 5 ≤ NIHSS score < 15 was defined as moderate (9 cases), and NIHSS score < 5 was defined as mild (11 cases).Determination of the maximum infarct size: all patients underwent head MRI + DWI within 24 h of admission, and the maximum infarct size was reflected by the maximum infarct diameter on the DWI sequence.The large-sized infarct group(10 cases) was defined as follows: more than one cerebral lobe, diameter >5 cm.The medium-sized infarct group (11 cases) was defined as follows: less than one cerebral lobe, 3 cm < diameter ≤ 5 cm.The small-sized infarct group (11 cases) was defined as follows: diameter ≤ 3 cm.
2.12 Statistical analysis
SPSS 23.0 statistical analysis software was used.The variables data were expressed as means ± standard deviations (mean ± SD), and the pairedttest was used.The Fisher test or Chi-squared test was used for statistical analysis of other attributes data.Pearson correlation was used for correlation analysis;P< 0.05 indicated that the difference was statistically significant.
3 Results
3.1 Principal component analysis (PCA) of nine samples from case and control groups in Phase Ⅰ
PCA was performed on the two sets of samples from the Phase Ⅰ study subjects.Figure 1 shows that the samples in the ACI group were significantly different from those in the normal control group.However, the intra-group differences between the samples of the two groups were small, indicating that the experimental design was reasonable, and that the uniformity of the biological replicate samples was good.
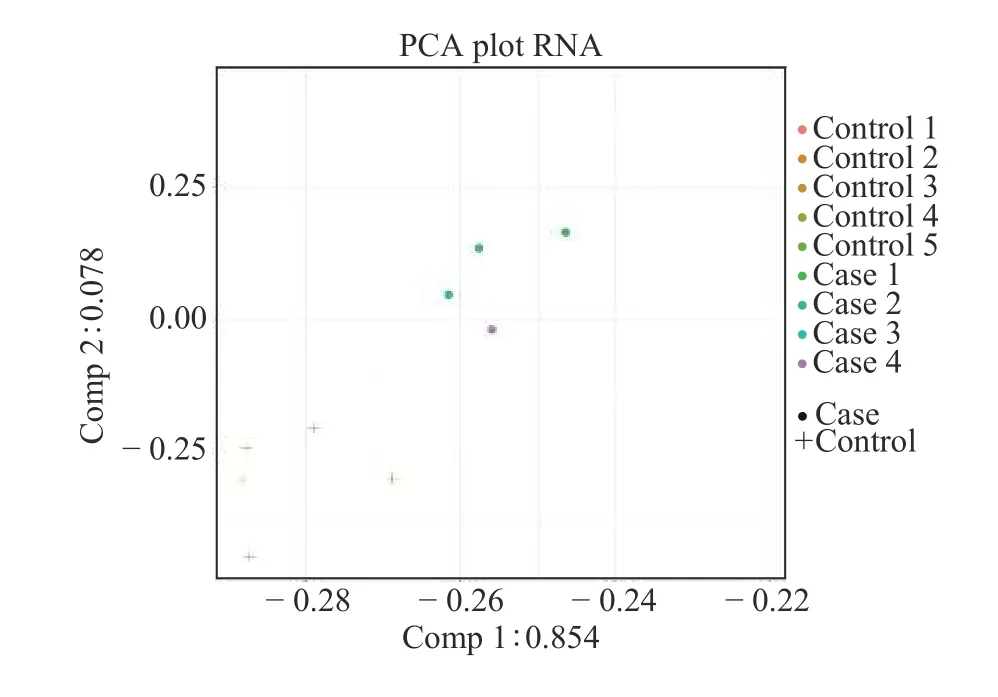
Figure 1 PCA of nine samples from case and control groups in Phase Ⅰ
3.2 Differential expression profile of serum exosomal mRNA in patients with ACI
After normalizing the original data of Phase Ⅰ study subjects and converting them into Log2values, an absolute value of Log2FC >2.0 andP< 0.05 were used to screen for differentially expressed genes.The results indicated that there were 248 differentially expressed mRNAs in the case and control groups, of which 242 were upregulated and six were downregulated.The volcano map of the two groups is shown in Figure 2,and the heat map of the two groups is shown in Figure 3.Among the differentially expressed mRNAs, 30 exhibited the most significant expression changes;these are shown in Table 1.
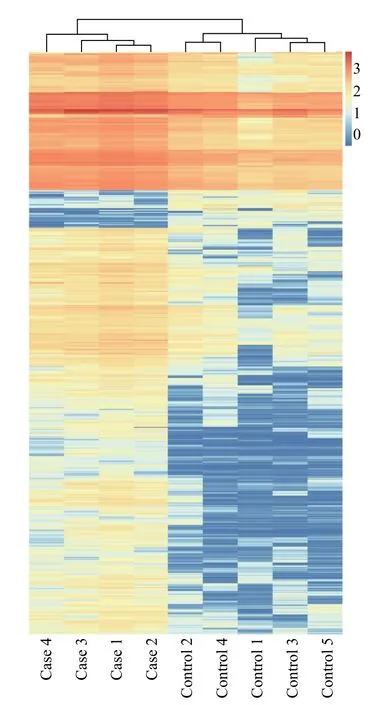
Figure 3 Heat map of differential expression of exosomal mRNA in nine samples from case and control groups in Phase Ⅰ
3.3 Functional characteristics of differentially expressed genes in exosomes
Enrichment analysis was carried out on the differentially expressed genes in the case and control groups in Phase Ⅰ.A total of 3 069 GO functional enrichment analysis results were obtained, including 2 270 biological processes, which were predominantly cotranslational protein targeting of the membrane and the nuclear-transcribed mRNA catabolic process; the functions involved were behavior regulation, adhesion, biological regulation, and cell damage.There were 387 cell components, which were predominantly cytoplasmic ribosomes and ribosomes; the functions involved were cell connection, cell membrane, cell synapse, etc.There were 412 molecular functions, which were predominantly the structural components and structural molecular activities of ribosomes; the functions involved were antioxidant activity, binding, and catalytic activity.Figure 4 contains the details.These results suggest that ACI is closely related to many biological processes and involves multiple factors and mechanisms.
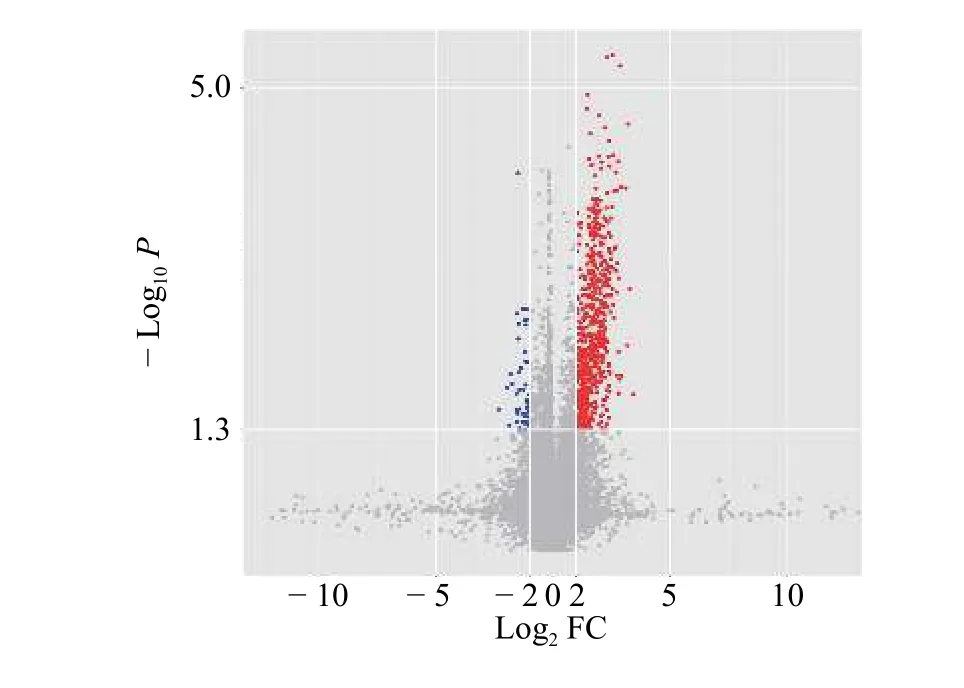
Figure 2 Volcano map of differential expression of exosomal mRNA in 9 samples from case and control groups in Phase Ⅰ
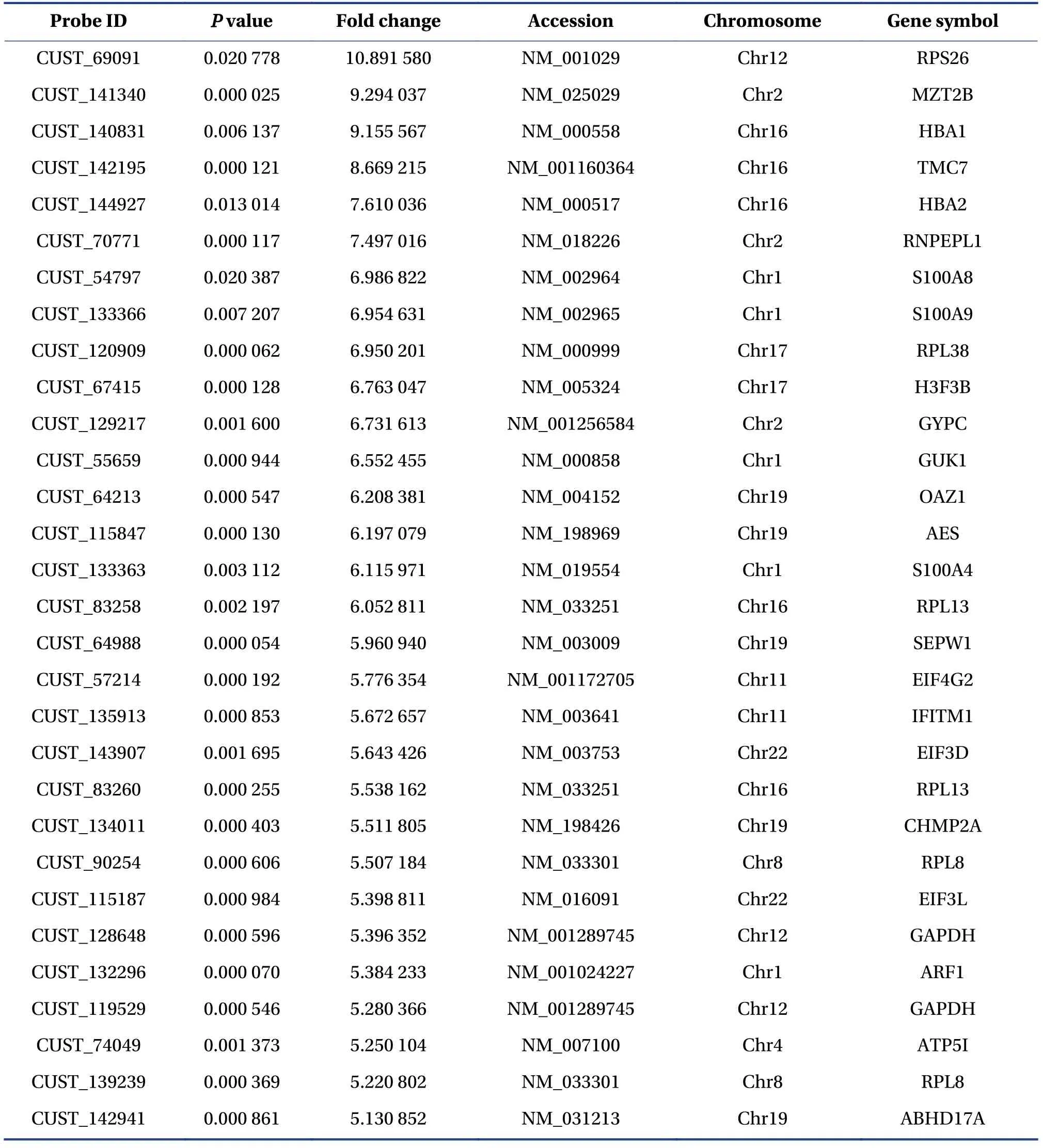
Table 1 Top 30 differently expressed exosomal mRNAs in case and control groups
3.4 Signaling pathways involving differentially expressed genes in exosomes
A total of 160 KEGG pathway enrichment analysis results were obtained for nine samples from the case and control groups in Phase Ⅰ; they were predominantly ribosomes, proteasomes, oxytocin signaling pathways, oxidative phosphorylation, Parkinson’s disease, and Alzheimer’s disease.Among them, 16 enrichment pathways had the most significant expression changes after screening according toP<0.05; they are shown in Figure 5.
3.5 Verification of H3F3B mRNA expression levels in exosomes of 64 samples from two groups in Phase Ⅱby qRT-PCR

Figure 4 Second-level GO functional enrichment analysis of differentially expressed mRNAs in exosomes of nine samples from case and control groups in Phase Ⅰ

Figure 5 KEGG enrichment analysis of differentially expressed mRNA in exosomes of nine samples from case and control groups in Phase Ⅰ
Among the genes with significant differential expression, it was found through research and screening based on relevant literature that the H3F3B gene may be the core gene of the lncRNA-miRNA-mRNA ternary transcription network of cerebral infarction[24];hence, subsequent analysis was focused on the H3F3B gene.The qRT-PCR method was used to detect the expression of H3F3B mRNA in serum exosomes collected in Phase Ⅱ from 32 patients each in the case and normal control groups.The results showed that the expression of H3F3B mRNA in serum exosomes (3.039 ± 1.965) in the case group was significantly higher than that in the normal control group(0.833 ± 0.700), and the differences in expression were statistically significant (P< 0.001).The expression patterns were consistent with the microarray data of the chip-based exosomal mRNA detection experiment in Phase Ⅰ.
3.6 Correlation analysis of H3F3B mRNA expression levels in exosomes
To further investigate the relationship between the expression of exosomal mRNA and the clinical characteristics of ACI, the patients in the case group in Phase Ⅱ were classified according to age, gender,NIHSS score, and the maximum infarct size.We found that the expression of H3F3B in serum exosomes of patients in the case group positively correlated with age (r=0.491,P=0.004), NIHSS score (Figure 6A,r=0.823,P< 0.001), and the maximum infarct size (Figure 6B,r=0.737,P< 0.001), but the correlation with gender was not significant (r=– 0.332,P=0.064).
4 Discussion
The injury and repair mechanisms of cerebral infarction are very complicated; it is a “waterfall-style”pathological change caused by the interaction of multiple factors and mechanisms, including nerve cell energy metabolism disorder and neuroinflammatory injury[4].Therefore, the effective transmission of neurotrophic factors quickly through the blood-brain barrier after cerebral infarction occurs is of great significance to restoring damaged brain tissue.mRNA is the key hub of protein translation;however, owing to its single-stranded structure, it is easily degraded during transportation and cannot stably exist in the blood[25].Studies have shown that the brain contains a large number of exosomes, the concentration of which increases significantly after the onset of cerebral ischemia[26,27].As a type of extracellular vesicle, exosomes can encapsulate mRNAs,protect them from degradation, and quickly pass through the blood-brain barrier to exert neuroprotective effects[28].
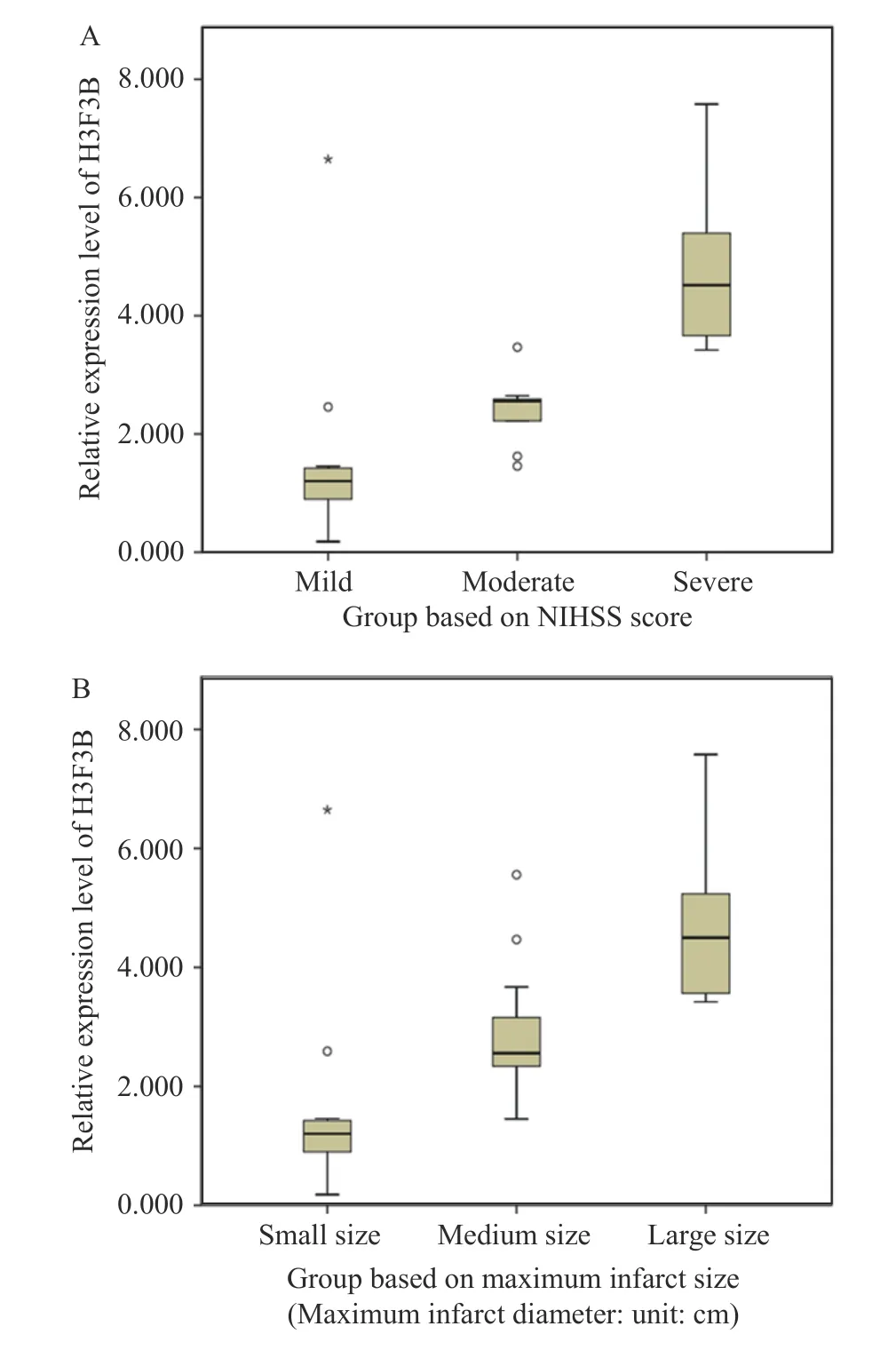
Figure 6 Comparison of NIHSS score subgroup
At present, research on revealing the biomarkers and therapeutic targets of cerebral infarction disease from the perspective of exosomes and transcriptomics is still in the preliminary exploratory stage.Traditional Chinese medicine(TCM) treatment of cerebral infarction has a long history, and its curative effect is remarkable.Previous studies have found that various active ingredients of Chinese medicine, such asnotoginsenosidesand Ciwujia (Acanthopanax senticosus), can promote the recovery of neurological function after cerebral ischemia by regulating various mRNAs[29,30].TIAN et al.[31]found that exosomes that were modified and then loaded with curcumin to target the ischemic brain injury area of a transient middle cerebral artery occlusion (MCAO) mouse model could reduce the inflammatory response.Buyang Huanwu Decoction (补阳还五汤) is a classic prescription for the treatment of cerebral infarction,and its efficacy has been confirmed using evidencebased medicine[32].Previous studies have shown that Buyang Huanwu Decoction can exert an anti-cerebral ischemia effect by promoting nerve regeneration and angiogenesis[33,34].After experimental spinal cord injury in rats, Buyang Huanwu Decoction promoted the recovery of nerve function by mediating the mTOR signaling pathway and autophagy.Buyang Huanwu Decoction combined with bone marrow mesenchymal stem cell transplantation can reduce ischemic injury by upregulating angiogenesis[35].In vivoandin vitroexperiments have confirmed that intervention with Buyang Huanwu Decoction can enhance the expression of VEGF and miRNA related to angiogenesis in exosomes secreted by mesenchymal stem cells and promote angiogenesis in the rat brain[36].However,TCM compounds such as Buyang Huanwu Decoction have complex ingredients.Conventional research methods in the past often only focused on a single pathway or target and could not reveal the characteristics of multi-target and multi-channel synergistic treatment of cerebral infarction at an overall level.Therefore, clarifying the transcription of genes carried by exosomes in the body after the occurrence of cerebral infarction can provide new ideas and a preliminary experimental basis for identifying the therapeutic targets and mechanism of action of Buyang Huanwu Decoction as well as other TCMs for the treatment of cerebral infarction.
This study took a different approach, using the perspective of exosomes combined with transcriptomics to study the expression pattern of exosomal mRNA in ACI for the first time, and was aimed at providing new targets for the diagnosis and treatment of cerebral infarction in the future.A total of 248 mRNAs were differentially expressed in the case and control groups, of which 242 were upregulated and six were downregulated.Among them, typical genes included H3F3B, ATP5A1, EIF4G2, EIF3D, and EIF3L.Related studies have also confirmed the role of the above genes.For example, based on the endogenous competitive RNA hypothesis, ZOU et al.[24]constructed an lncRNA-miRNA-mRNA ternary transcription network for cerebral infarction and found that H3F3B may be the core gene of the network .In this study, we found that an increase in serum exosomal H3F3B mRNA levels was closely related to.In this study, we found that an increase in serum exosomal H3F3B mRNA levels was closely related to the occurrence of ACI, and that the increase was more obvious in patients with higher NIHSS scores and bigger largest infarct sizes, suggesting that the level of H3F3B mRNA in serum exosomes in patients with ACI can reflect the severity of the disease to a certain extent.ATP5A1 is widely distributed on the inner mitochondrial membrane.Research has shown that abnormal expression of ATP5A1 can cause central nervous system diseases such as Alzheimer’s disease and amyotrophic lateral sclerosis key pathway that regulates cell proliferation and protein synthesis[37].This pathway plays an important role in the process of angiogenesis, nerve regeneration, and maintenance of synaptic plasticity after the occurrence of cerebral ischemia[38,39].In addition, it has also been reported that OAZ1 can be used as a reference gene forin vitrocerebral ischemia models[40].These results suggest that the differential genes we detected in the serum exosomal mRNA expression profile may be involved in the injury mechanism of cerebral infarction.Using GO functional and KEGG pathway enrichment analyses, we found that these differentially expressed genes are closely related to many biological processes, suggesting that the disease mechanism of cerebral infarction involves multiple factors and mechanisms.These mRNAs may be potential targets of ACI; nevertheless, it is necessary to conduct further localizing and functional research throughin vivoandin vitrostudies.
In summary, this study preliminarily clarified the changes in the mRNA expression profile of serum exosomes in patients with ACI, and indicated that H3F3B, ATP5A1, EIF4G2, EIF3D, EIF3L, and other genes that are significantly differentially expressed in serum exosomes may be closely related to ACI.In addition, bioinformatics methods were used to analyze the molecular functions, cellular components, biological processes, and signal pathways of the differentially expressed mRNAs.The main results of GO functional enrichment analysis were behavior regulation,cell connection, and antioxidant activity.The main results of KEGG pathway enrichment analysis were ribosomes, proteasomes, oxytocin signaling pathways, and oxidative phosphorylation.Finally, by expanding the sample size, the differential expression of H3F3B mRNA was confirmed, and it positively correlated with age, NIHSS score, and the maximum infarct size.These results provide a potential direction for elucidating the disease development mechanism of cerebral infarction.
Acknowledgements
We thank for the funding support from the National Natural Science Foundation of China (No.82074251),the Hunan Natural Science Foundation of China (No.2018JJ2413), and the Hunan Provincial Health and Health Commission Project (No.c2018032).
Competing interests
The authors declare no conflict of interest.
 Digital Chinese Medicine2021年4期
Digital Chinese Medicine2021年4期
- Digital Chinese Medicine的其它文章
- P-glycoprotein mediated interactions between Chinese materia medica and pharmaceutical drugs
- Systematic review of robust experimental models of rheumatoid arthritis for basic research
- Traditional Chinese medicine compounds for the treatment of functional dyspepsia: an updated meta-analysis of randomized,double-blind, placebo-controlled trials
- Rational drug design, synthesis, and biological evaluation of novel N-(2-arylaminophenyl)-2,3-diphenylquinoxaline-6-sulfonamides as potential antimalarial, antifungal, and antibacterial agents
- Effects of Fuke Qianjin Formula on hormones and their receptors and metabonomics study in uterine fibroids model rats
- Protective effects of Fufang Ejiao Jiang against aplastic anemia assessed by network pharmacology and metabolomics strategy
