深绿卷柏中木脂素成分及10个木脂素结构的修订
汤卓雅,潘月华,邹明锋,胡荣,唐贵华
中山大学药学院,广东广州510006
Selaginella doederleinii Hieron. (Selaginellaceae)is a small evergreen pteridophyte growing throughout south and southwestern China at an altitude of 200-1 000 (-1 400) m[1]. Its whole herbs are used as a traditional Chinese medicine (TCM) for the treatment of sore throat, jaundice, rheumatism, bleeding, and some kinds of cancer[2]. Biflavones are the characteristic chemical constituents of S. doederleinii[3]. In addition,previous phytochemical investigation of this plant has led to the isolation of several alkaloids and lignans[4-5].Most of chemical components from S. doederleinii possess various bioactivities such as anticancer, anti-Alzheimer′s disease,and cytotoxic properties[6-8].
Recently, the collective evidences suggested that thioredoxin reductase (TrxR) was a potential target for cancer chemotherapy. In our continuing search for natural TrxR inhibitors from TCM[9-10], the EtOH extract of S. doederleinii was subjected to chromatographic procedures to afford five lignans(1-5)(Fig.1). The inhibitory activities of these lignans against TrxR were evaluated, and compounds showed moderate activities with IC50values ranging from 10.1 to 39.0 μmol/L. However, during the structural elucidation of compounds 1-5 by analyses of their spectroscopic data and comparing with some key synthetic compounds, we found that the structures of ten previously reported compounds (Ⅰ-Ⅹ) (Fig. 2) were incorrect. Herein, this report describes details of the isolation, structural elucidation,and inhibitory activities of compounds 1-5 together with the structure revision of Ⅰ-Ⅹ.
1 Materials and methods
1.1 Instruments and reagents
Optical rotations were measured on a PerkinElmer 341 polarimeter, and UV spectra were recorded on a Shimadzu UV-2450 spectrophotometer. IR spectra were determined on a Bruker Tensor 37 infrared spectrophotometer with KBr disks. NMR spectra were measured on a Bruker AM-400 spectrometer at 25°C. ESIMS and HRESIMS were recorded on a Finnigan LC QDECAinstrument. A Shimadzu LC-20AT equipped with a SPD-M20A PDA detector was used for HPLC,and a YMC-pack ODS-A column (250 mm × 10 mm,S-5 μm, 12 nm) was used for semipreparative HPLC separation. Silica gel (300-400 mesh, Qingdao Haiyang Chemical Co. Ltd.), reversed-phase C18(Rp-C18)silica gel(12 nm,S-50 μm,YMC Co. Ltd),and Sephadex LH-20 gel (Amersham Biosciences) were used for column chromatography (CC). MeOH for HPLC was obtained from BCR International Trading Co., Ltd.,and other analytical grade solvents from Shanghai Titan Scientific Co., Ltd. TrxR was purchased from Sigma-Aldrich(St. Louis,USA).
1.2 Plant material
Plants of S. doederleinii were collected in March 2013 from Guangdong Province, P. R. China, and were identified by one of the authors (Gui-Hua Tang).A voucher specimen (accession number: 20130303)has been deposited at the School of Pharmaceutical Sciences,Sun Yat-sen University.
1.3 Extraction and isolation
The air-dried and powdered plants of S. doederleinii (1.5 kg) were extracted with 95% EtOH (3 L × 3 L) at room temperature to give 150.7 g of crude extract. The extract was suspended in H2O and successively partitioned with petroleum ether (PE)and EtOAc to yield two corresponding portions. The EtOAc fraction (17.0 g) was subjected to silica gel CC using PE/acetone (2∶1→1∶1→0∶1) to afford three fractions(Ⅰ-Ⅲ). Fr. II was chromatographed over Rp-C18silica gel column with MeOH/H2O (5∶5→10∶0) to give two fractions(IIa and IIb). Fr. IIa was separated by silica gel CC (CH2Cl2/MeOH, 200∶1→100∶1) to give compound 5 (12.0 mg) and four subfractions (IIa1-IIa4). Compound 3 (3.0 mg) was obtained from Fr.IIa2 by semi-preparative HPLC (MeOH/H2O, 4∶6, 3 mL/min). Fr. IIa3 was purified by a Sephadex LH-20 column(CHCl3/MeOH,1∶1)to give 4(5.0 mg). Compounds 1 (2.5 mg) and 2 (7.0 mg) were obtained from Fr. IIa4 by Rp-C18silica gel CC (MeOH/H2O, 4∶6)and silica gel CC(CHCl3/MeOH,100∶1).
1.4 Bioactivity assay
The TrxR inhibitory activities of these five isolated lignans were evaluated by the 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) reduction assay. The procedure for inhibitory assays of TrxR were referred to those we described previously[10]. Briefly, all assays were preformed on 96-well plates with a final volume of 40 μL per well at 25 ℃. 0.3 μL of TrxR (0.04 mmol/L) was mixed with 35.5 μL of reaction buffer (1 mol/L potassium phosphate, pH 7.0; 500 mmol/L EDTA, pH 7.4; 0.48 mmol/L NADPH) and 1 μL of compound at a indicated concentration (3.125, 6.25,12.5,25,and 50 μmol/L) in each well. DMSO (2.5%,V/V) and curcumin were used as vehicle and positive controls,respectively. After mixing for 5 min,the reaction was started by the addition of 3.2 μL of DTNB (final concentration of 5.0 mmol/L). The increase in absorbance at 412 nm (εTNB412nm= 13.6 mmol·L-1·cm-1)was monitored in the initial 2 min.
2 Results and discussion
2.1 Structural identification
The whole plants of S. doederleinii were extracted with 95% EtOH. After removal of the EtOH by evaporation,the residue was suspended in H2O and then partitioned successively with petroleum ether and EtOAc.The EtOAc fraction was subjected to column chromatography over silica gel,Rp-C18,Sephadex LH-20,and semipreparative HPLC to obtain compounds 1-5.
The structures 1-5 (Fig. 1) were determined by analysis of their spectroscopic data (1D and 2D NMR data, HRMS, UV, IR, and specific rotation data) and comparison with literature data. All compounds (1-5)were known lignans,and 1-4 were isolated from S. doederleinii for the first time. (-)-erythro-(7′E)-4,9-Dihydroxy-3,3′,5′-trimethoxy-8,4′-oxyneolign-7′-en-9′-al (1), (-)-erythro-guaiacylglycerol-β-O-4′-sinapyl ether (2), and (7′E)-3,5,3′,5′-tetramethoxy-8,4′-oxyneolign-7′-ene-4,9,9′-triol (3) are represented as the type of 8,4′-oxyneolignan, (+)-(7R,8S)-5-methoxydihydrodehydrodiconiferyl alcohol (4) is the type of benzofuran, and syringaresinol (5) belongs to furofuranic lignan.
2.1.1 (-)-erythro-(7΄E)-4,9-Dihydroxy-3,3΄,5΄-trimethoxy-8, 4΄-oxyneolign-7΄-en-9΄-al (1) White amorphous power; [α] -6.7 (c 0.1, CH2Cl2); UV(MeOH)λmax(log ε)207(4.33),233(4.22),323(4.15)nm; IR (KBr) νmax3 450, 1 668, 1 621, 1 581,1 517,1 502, 1 460, 1 425, 1 335, 1 220, 1 125, 1 025 cm-1;1H NMR (400 MHz,CDCl3) δ 6.97 (1H,d,J = 1.8 Hz,H-2), 6.87 (1H, d, J = 8.2 Hz, H-5), 6.75 (1H, dd,J = 8.2, 1.8 Hz, H-6), 5.02 (1H, d, J = 3.7 Hz, H-7), 4.22 (1H, m, H-8), 3.94 (1H, dd, J = 12.0, 7.0 Hz, H-9a), 3.54 (1H, dd, J = 12.0, 2.1 Hz, H-9b),6.85 (2H, s, H-2΄and H-6΄), 7.42 (1H, d, J = 15.9 Hz, H-7΄), 6.67 (1H, dd, J = 15.9, 7.6 Hz, H-8΄),9.71(1H,d,J=7.6 Hz,H-9΄),3.90(3H,s,3-OMe),3.93 (6H, s, 3΄-OMe and 5΄-OMe);13C NMR data, see Table 1; ESIMS m/z 403 [M - H]-. These data were in consistent with those reported in the literature[11].Thus, compound 1 was determined as (-)-erythro-(7΄E)-4,9-dihydroxy-3,3΄,5΄-trimethoxy-8,4΄-oxyneolign-7΄-en-9΄-al.
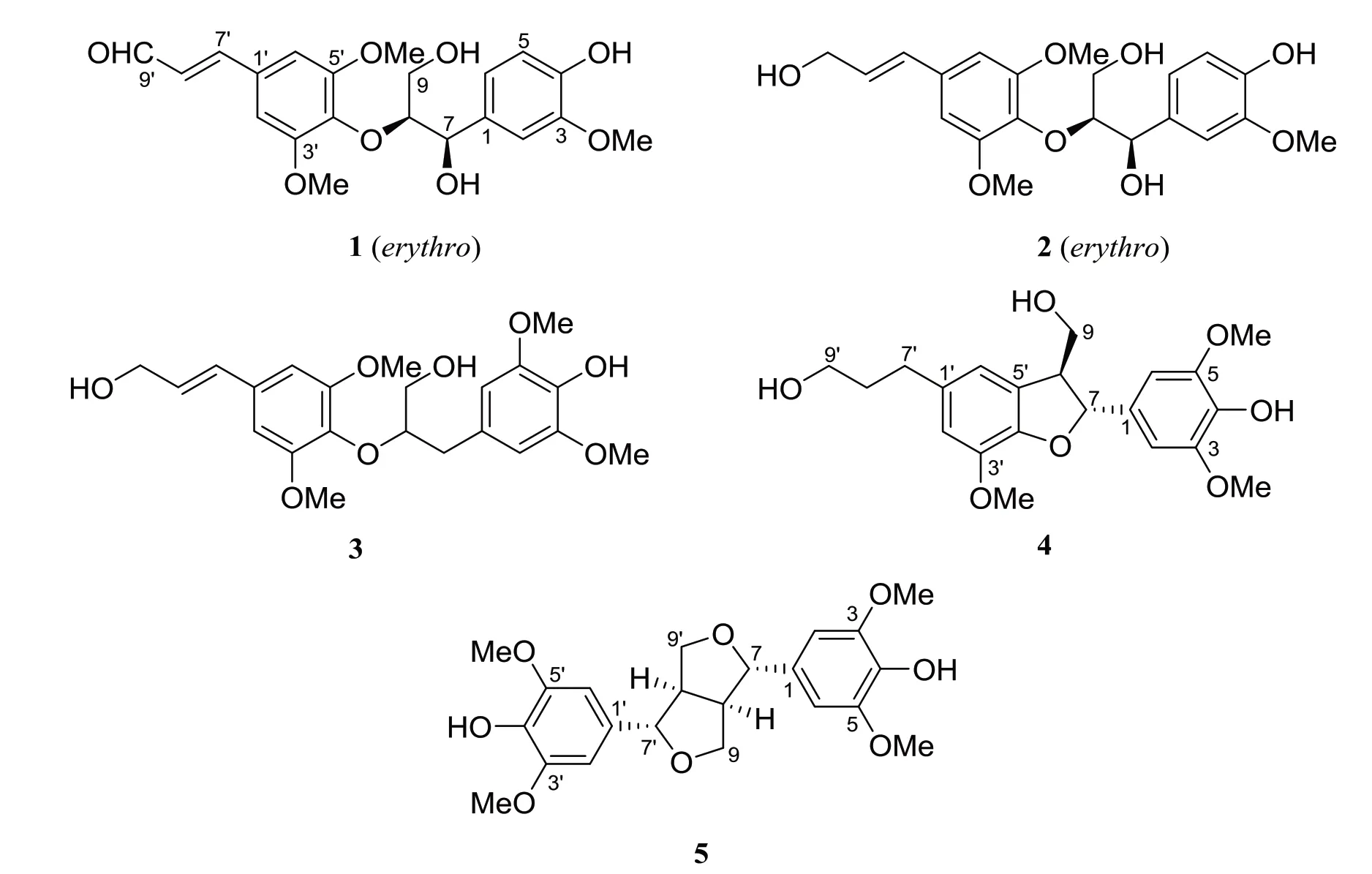
Fig.1 Structures of compounds 1-5 isolated from S. doederleinii
2.1.2 (-)-erythro-Guaiacylglycerol-β-O-4΄-sinapyl ether (2) White amorphous power; [α] -2.8(c 0.6, CH2Cl2); UV (MeOH) λmax(log ε) 222 (4.55),272 (4.27) nm; IR (KBr) νmax3 327, 1 668, 1 588,1 507, 1 453, 1 233, 1 124, 1 028 cm-1;1H NMR (400 MHz, CDCl3) δ 6.95 (1H, d, J = 1.5 Hz, H-2), 6.85(1H, d, J = 8.2 Hz, H-5), 6.75 (1H, dd, J = 8.2, 1.5 Hz, H-6), 5.00 (1H, d, J = 3.5 Hz, H-7), 4.13 (1H,m, H-8), 3.90 (1H, dd, J = 12.0, 7.0 Hz, H-9a),3.50 (1H, dd, J = 12.0, 2.1 Hz, H-9b), 6.66 (2H, s,H-2΄and H-6΄), 6.56 (1H, d, J = 15.9 Hz, H-7΄),6.31 (1H, dt, J = 15.9, 5.6 Hz, H-8΄), 4.34 (1H, d,J = 5.6 Hz, H-9΄), 3.88 (9H, s, 3-OMe, 3΄-OMe, and 5΄-OMe);13C NMR data, see Table 1; HRESIMS m/z 429.151 7 [M + Na]+(calcd for C21H26O8Na,429.152 5). Its NMR and MS data were identical with those reported in the literature[12-13]. Thus, this compound was assigned as (-)-erythro-guaiacylglycerolβ-O-4΄-sinapyl ether.
2.1.3 (7΄E)-3,5,3΄,5΄-Tetramethoxy-8,4΄-oxyneolign-7΄-ene-4,9,9΄-triol (3) White amorphous power; [α] +17 (c 0.1, CH2Cl2); UV (MeOH) λmax(log ε)209 (4.38),271 (3.85) nm; IR (KBr) νmax3 500,1 666,1 587, 1 505, 1 450, 1 233, 1 122, 1 033 cm-1;1H NMR (400 MHz, CDCl3) δ 6.52 (2H, s, H-2 and H-6),3.19(1H,dd,J= 13.6,8.6 Hz,H-7a),2.98(1H,dd,J = 13.6, 5.4 Hz, H-7b), 4.21 (1H, m, H-8), 3.58(1H, m, H-9a), 3.45 (1H, m, H-9b), 6.63 (2H, s, H-2΄and H-6΄), 6.55 (1H, d, J = 15.8 Hz, H-7΄), 6.29(1H, dt, J = 15.8, 5.6 Hz, H-8΄), 4.32 (1H, d, J =5.6 Hz, H-9΄), 3.87 (6H, s, 3-OMe and 5-OMe),3.85 (6H, s, 3΄-OMe and 5΄-OMe);13C NMR data, see Table 1;HRESIMS m/z 443.167 5[M +Na]+(calcd for C22H28O8, 443.168 2). Its NMR and MS data were in accordance with literature data[14]. Therefore, compound 3 was determined to be (7΄E)-3,5,3΄,5΄-tetramethoxy-8,4΄-oxyneolign-7΄-ene-4,9,9΄-triol.

Table 1 13C NMR(100 MHz)data of 1-5 isolated from S. doederleinii(Recorded inCDCl3)
2.1.4 (+)-(7R,8S)-5-Methoxydihydrodehydrodiconiferyl alcohol (4) White amorphous power; [α] +2.7(c 0.6, CH2Cl2); UV (MeOH) λmax(log ε) 212 (4.54),281 (3.59) nm; IR (KBr) νmax3 367, 1 607, 1 456,1 327, 1 218, 1 115, 1 040 cm-1;1H NMR (400 MHz,CDCl3)δ 6.65(3H,s,H-2,H-6,and H-2΄),5.52(1H,d, J = 7.6 Hz, H-7), 3.60 (1H, m, H-8), 3.97 (1H,dd, J = 11.6, 6.0 Hz, H-9a), 3.89 (1H, dd, J = 11.0,4.9 Hz, H-9b), 6.67 (1H, s, H-6΄), 2.67 (2H, m,H-7΄), 1.87 (2H, m, H-8΄), 3.68 (2H, t, J = 6.3 Hz,H-9΄), 3.85 (6H, s, 3-OMe and 5-OMe), 3.88 (3H, s,3΄-OMe);13C NMR data, see Table 1; HRESIMS m/z 413.156 6 [M + Na]+(calcd for C21H26O7Na,413.157 6). Its NMR and MS data were identical with those reported in the literature[15]. Thus, the structure of 4 was defined as (+)-(7R,8S)-5-methoxydihydrodehydrodiconiferyl alcohol.
2.1.5 Syringaresinol (5) White amorphous power;1H NMR (400 MHz, CDCl3) δ 5.53 (2H, s, 4-OH and 4΄-OH), 6.58 (4H, s, H-2/6 and H-2΄/6΄), 4.73 (2H,d, J = 4.3 Hz, H-7 and H-7΄),3.09 (2H, m,H-8 and H-8΄), 4.28 (2H, m, H-9a and H-9΄a), 3.90 (2H, m,H-9b and H-9΄b), 3.89 (12H, s, 3/5-OMe and 3΄/5΄-OMe);13C NMR data,see Table 1;ESIMS m/z 417[MH]-. The NMR and MS data were in consistent with those reported in the literature[16]. In addition, the specific optical rotation of the compound was not tested,so its structure was determined to be syringaresinol.
2.2 The results of TrxR activity assay
The inhibitory activities of lignans 1-5 against TrxR were tested by the DTNB reduction assay. Compared with the positive control curcumin (IC50= 25.0 μmol/L), compounds 3, 2, and 5 represented the most active compounds with IC50values of 10.1, 13.4, and 20.2 μmol/L,respectively,while 4 showed weak activities with IC50values of 39.0 μmol/L and 1 was inactive.
In this part, photochemical investigation of the TCM S. doederleinii let the isolation of three 8,4′-oxyneolignans (1-3), a benzofuran (4), and one furofuran(5). Compounds 1-4 were isolated from S. doederleinii for the first time. The inhibitory activities of 1-5 against TrxR were evaluated, and compounds 4-5 showed moderate activities. These findings suggested that some lignans might be promising structural motif for the development of TrxR inhibitors.
2.3 Structure revision of ten lignans
We found that the structures of ten reported compounds Ⅰ-Ⅹ(Fig. 2) were incorrect and were therefore wrongly considered as the types of biphenylneolignans and 4,4΄-oxyneolignans. They were 2,6,2΄,6΄-tetramethoxy-4, 4΄-bis(2, 3-epoxy-1-hydroxypropyl)biphenyl (Ⅰ) from the roots of Cynanchum atratum[17]and the stems of Millettia griffithii[18], griffilignan A(Ⅱ) from the stems of Millettia griffithii[18], 2-hydroxy-3,2΄,6΄-trimethoxy-4΄-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-l-propenyl)biphenyl(Ⅲ),2-hydroxy-3,2΄-dimethoxy-4΄-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-1-propenyl)biphenyl ( Ⅳ), and 2, 2΄-dimethoxy-4- (3-hydroxy-l-propenyl) -4΄- (1, 2, 3-trihydroxypropyl)diphenyl ethers(Ⅴand Ⅵ)from the woods of Eurycoma longifolia[19], (7΄R,8΄R)-2,2΄-dimethoxy-4-(3-hydroxyl-propenyl)-4΄-(1,2,3-trihydroxypropyl)biphenyl ether(Ⅶ)from the aerial parts of Saussurea pulchella[16], utiline B (Ⅷ) from the stems of Zanthoxylum utile[20],and rhemaneolignans A and B (Ⅸand Ⅹ)from the roots of Rehmannia glutinosa[21],respectively.
By analysis of the13C NMR data of model compounds such as synthetic and natural products, it could be determined that some fragments of Ⅰ-Ⅹwere incorrect. The characteristic carbon signals for the moiety of hydroxy(oxiran-2-yl)methyl in the synthetic compound, (4-((R)-hydroxy((R)-oxiran-2-yl)methyl)-2-methoxyphenyl) carbonate (14) (Fig. 3), were at δC74.1(CH,C-1),55.8(CH,C-2),and 45.4(CH2,C-3)[22]. However, these characteristic carbon signals were not found in the13C NMR data of Ⅰ-Ⅳ,which indicated that they did not have the hydroxy(oxiran-2-yl)methyl unit. Then the comparison of their 1D NMR data with those of furofurans such as syringaresinol (5)and pinoresinol[23]and benzofurans such as (+)-(7R,8S) -5-methoxydihydrodehydroconiferyl alcohol (4)and simulanol[24]have resulted in their structure revision. Similarly, the characteristic carbon signals(Fig. 3) for the moiety of 1,2,3-trihydroxypropyl in the synthetic compounds, tert-butyl (2-methoxy-4-((1R,2R)-1,2,3-trihydroxypropyl)phenyl) carbonate (15)[22]and tert-butyl 1-phenylpropane-1, 2, 3-triol (16)[25](Fig. 3), were quite different from those for the same fragments in Ⅴ-Ⅹ. Especially, the present of the downfield-shifted13C NMR chemical shifts of an oxygenated methine [δCca. 86.5 (CH)] in Ⅴ-Ⅹsuggested that there must be a highly electrophilic O-substituent attached to this carbon instead of an OH. Therefore, the structure revision of Ⅴ-Ⅹwere achieved by comparative analysis of their NMR data with 1 and 2 as well as other 8,4′-oxyneolignans.
On the basis of spectroscopic data including NMR and specific rotation data comparison with the isolates(1-5)(Fig. 1) in this study together with some synthetic compounds and natural products, compounds Ⅰ-Ⅹwere revised to be(+)-syringaresinol(5)[16,26-27],(+)-pinoresinol (6)[23,26,28-29], (-)-simulanol (7)[24,30], (-)-dehydrodiconiferyl alcohol (8)[23],(-)-threo-guaiacylglycerolβ-O-4΄-coniferyl ether (9)[12,31-32],(+)-erythro-guaiacylglycerol-β-O-4΄-coniferyl ether(10a)[12,31,32],(-)-erythro-guaiacylglycerol-β-O-4΄-coniferyl ether (10b)[32],(+)-threo-guaiacylglycerol-β-O-4΄-sinapyl ether (11)[12], (-)-threo-methyl 4-O-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl) -1- (hydroxymethyl)ethyl]ferulate(12)[33], and (+)-erythro-methyl 4-O-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)-1-(hydroxymethyl)ethyl]ferulate (13)[33], respectively (Fig. 2). The revised structures are actually the types of furofurans (5 and 6),benzofurans (7 and 8), and 8,4΄-oxyneolignans (9-13),rather than biphenylneolignans (Ⅰand Ⅱ) and 4,4΄-oxyneolignans (Ⅲ-Ⅹ). Thus far, there is no evidence to support the existence of Ⅰ-Ⅹin nature.
2.3.1 (+ ) -Syringaresinol (5) Colorless needles;[α]D+75.0;1H NMR (400 MHz, CDCl3) δ 5.54 (2H, s,4-OH and 4΄-OH), 6.57 (4H, s, H-2/6 and H-2΄/6΄),4.78(2H,d,J=7.0 Hz(The reported data with differences: lit.[26]7.0 Hz, lit.[16,27]4.3 Hz), H-7 and H-7΄),3.09 (2H, m, H-8 and H-8΄), 4.26 (2H, m, H-9a and H-9΄a), 3.89 (2H, m, H-9b and 9΄b), 3.90 (12H, s,3/5-OMe and 3΄/5΄-OMe);13C NMR data, see Table 2.All data were from the reported for 2,6,2΄,6΄-tetramethoxy-4, 4΄-bis(2, 3-epoxy-1-hydroxypropyl)biphenyl(Ⅰ)[17], and the 1D NMR assignments of its revised structure were completed on the basis of the comparison of its NMR data with that of the correct compound[16,26-27].
2.3.2 (+)-Pinoresinol(6) Pale yellow oil;[α]D+63.2;1H NMR(400 MHz,CD3OD)δ 6.94(2H,d,J=1.6 Hz,H-2 and H-2΄),6.76(2H,d,J=8.0 Hz,H-5 and H-5΄),6.80 (2H, dd, J = 8.0, 1.6 Hz, H-6 and H-6΄), 4.70(2H,d,J=7.2 Hz(The reported data with differences:lit.[26,28]5.0 Hz,lit.[23,29]4.4 Hz),H-7 and H-7΄),3.12(2H,ddd,J=7.2,6.8,3.2 Hz,H-8 and H-8΄),4.22(2H,dd,J = 9.2, 6.8 Hz, H-9a and H-9΄a), 3.82 (2H, dd, J =9.2,3.2 Hz,H-9b and H-9΄b),3.84(6H,s,3-OMe and 3΄-OMe);13C NMR data, see Table 2. The above data were from the reported for griffilignan A (Ⅱ)[18],and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[23,26,28-29].

Fig.2 Structures of Ⅰ-Ⅹreported previously in literatures and their revised structures(5–13)
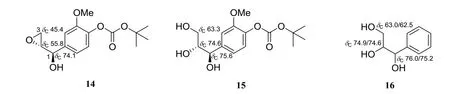
Fig.3 Structures of the reported synthetic compounds 14-16

Table 2 13C NMR data of compounds 5-13
2.3.3 (-)-Simulanol (7) Yellow needles;[α]D-2.6;1H NMR (400 MHz, pyridine-d5) δ 7.10 (2H, s, H-2 and H-6), 6.11 (1H, d, J = 7.0 Hz, H-7), 4.08 (1H,br. ddd, J = 6.2, 6.2, 6.2 Hz, H-8), 4.31 (1H, dd,J=10.8,5.3 Hz,H-9a),4.26(1H,dd,J=10.8,6.6 Hz, H-9b), 7.15 (1H, s, H-2΄), 7.33 (1H, s, H-6΄),6.91 (1H, d, J = 15.9 Hz, H-7΄), 6.57 (1H, dt, J =15.9,5.3 Hz,H-8΄),4.59(1H,d,J=5.3 Hz,H-9΄),3.73 (6H, s, 3-OMe and 5-OMe), 3.86 (3H, s, 3΄-OMe);13C NMR data, see Table 2. The above information were from the reported for 2-hydroxy-3,2΄,6΄-trimethoxy-4΄-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-l-propenyl)biphenyl(Ⅲ)[19],and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[24,30].
2.3.4 (-)-Dehydrodiconiferyl alcohol (8) Yellow needles; [α]D-9.7;1H NMR (400 MHz, pyridine-d5)δ 7.32 (1H, s, H-2), 7.22 (2H, overlapped, H-5 and H-6), 6.08 (1H, d, J = 6.7 Hz, H-7), 3.97 (1H, br.ddd, J = 6.2, 6.2, 6.2 Hz, H-8), 4.27 (1H, dd, J =10.7,5.5 Hz,H-9a),4.26(1H,dd,J=10.7,6.7 Hz,H-9b), 7.14 (1H, s, H-2΄), 7.32 (1H, s, H-6΄), 6.91(1H, d, J = 15.9 Hz, H-7΄), 6.57 (1H, dt, J = 15.9,5.3 Hz, H-8΄), 4.59 (1H, d, J = 5.3 Hz, H-9΄), 3.66(3H, s, 3-OMe), 3.28 (3H, s, 3΄-OMe);13C NMR data,see Table 2. All data were from the reported for 2-hydroxy-3,2΄-dimethoxy-4΄-(2,3-epoxy-1-hydroxypropyl)-5-(3-hydroxy-1-propenyl)biphenyl (Ⅳ)[19], and the 1D NMR assignments of its revised structure were completed on the basis of the comparison of its NMR data with that of the correct compound[23].
2.3.5 (-)-threo-Guaiacylglycerol-β-O-4΄-coniferyl ether (9) Yellow needles; [α]D-2.5;1H NMR (400 MHz, pyridine-d5) δ 7.19 (1H, d, J = 1.2 Hz, H-2),7.50(1H,d,J=8.3 Hz,H-5),7.07(1H,dd,J=8.3,1.2 Hz, H-6), 5.60 (1H, d, J = 5.7 Hz, H-7), 4.98(1H, br. ddd, J = 5.6, 5.6, 5.6 Hz, H-8), 4.40 (1H,dd, J = 11.8, 3.6 Hz, H-9a), 4.11 (1H, dd, J = 11.8,5.7 Hz, H-9b), 7.59 (1H, d, J = 1.7 Hz, H-2΄), 7.27(1H,d,J=7.8 Hz,H-5΄),7.42(1H,dd,J=7.8,1.7 Hz, H-6΄), 6.89 (1H, d, J = 15.9 Hz, H-7΄), 6.58(1H, dt, J = 15.9, 5.2 Hz, H-8΄), 4.58 (1H, d, J =5.2 Hz, H-9΄), 3.75 (3H, s, 3-OMe), 3.78 (3H, s, 3΄-OMe);13C NMR data, see Table 1. The above information were from the reported for 2,2΄-dimethoxy-4-(3-hydroxy-l-propenyl)-4΄-(1,2,3-trihydroxypropyl)diphenyl ether (Ⅴ)[19], and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[12,31,32].
2.3.6 (+ )-erythro-Guaiacylglycerol- β-O-4΄-coniferyl ether (10a) Yellow needles; [α]D+1.3;1H NMR (400 MHz, pyridine-d5) δ 7.14 (1H, d, J =1.9 Hz, H-2), 7.37 (1H, d, J = 8.3 Hz, H-5), 7.04(1H, dd, J = 8.3, 1.9 Hz, H-6), 5.60 (1H, d, J = 5.1 Hz, H-7), 5.03 (1H, br. ddd, J = 4.9, 4.9, 4.9 Hz,H-8), 4.55 (1H, dd, J = 11.8, 3.8 Hz, H-9a), 4.41(1H, dd, J = 11.8, 5.4 Hz, H-9b), 7.59 (1H, d, J =2.0 Hz, H-2΄), 7.24 (1H, d, J = 8.0 Hz, H-5΄), 7.38(1H, dd, J = 8.0, 2.0 Hz, H-6΄), 6.85 (1H, d, J =15.9 Hz, H-7΄), 6.55 (1H, dt, J = 15.9, 5.3 Hz, H-8΄), 4.56 (1H, d, J = 5.3 Hz, H-9΄), 3.72 (3H, s, 3-OMe), 3.74 (3H, s, 3΄-OMe);13C NMR data, see Table 2. The above data were from the reported for 2,2΄-dimethoxy-4-(3-hydroxy-l-propenyl)-4΄-(1,2,3-trihydroxypropyl)diphenyl ethers (Ⅵ)[19], and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[12,31,32].
2.3.7 (-)-erythro-Guaiacylglycerol- β-O-4΄-coniferyl ether (10b) Colorless oil; [α]D-8.0;1H NMR(500 MHz, CD3OD) δ 7.04 (1H, d, J = 1.8 Hz, H-2),6.77(1H,d,J=8.0 Hz,H-5),6.88(1H,dd,J=8.0,1.8 Hz, H-6), 4.90 (1H, d, J = 5.0 Hz, H-7), 4.32(1H,q,J=5.0 Hz,H-8),3.79(1H,dd,J=12.0,4.0 Hz, H-9a), 3.50 (1H, dd, J = 12.0, 5.0 Hz, H-9b),7.07 (1H, d, J = 2.0 Hz, H-2΄), 7.01 (1H, d, J = 8.0 Hz, H-5΄), 7.38 (1H, dd, J = 8.0, 2.0 Hz, H-6΄),6.56 (1H, d, J = 15.9 Hz, H-7΄), 6.30 (1H, dt, J =15.9,5.7 Hz,H-8΄),4.56(1H,d,J=5.7 Hz,H-9΄),3.83 (3H, s, 3-OMe), 3.89 (3H, s, 3΄-OMe);13C NMR data, see Table 2. The above information were from the reported for (7΄R,8΄R)-2,2΄dimethoxy-4-(3-hydroxyl-propenyl) -4΄- (1, 2, 3-trihydroxypropyl)biphenyl ether (Ⅶ)[16], and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[32].
2.3.8 (+)-threo-Guaiacylglycerol-β-O-4΄-sinapyl ether (11) Colorless oil; [α]D+6.6;1H NMR (400 MHz, CD3OD) δ 7.04 (1H, d, J = 1.2 Hz, H-2), 6.79(1H, d, J = 8.0 Hz, H-5), 6.89 (1H, dd, J = 8.0, 1.6 Hz, H-6), 5.01 (1H, d, J = 7.2 Hz, H-7), 4.00-4.06(1H,m,H-8),3.77(1H,dd,J=12.0,4.0 Hz,H-9a),3.30-3.34 (1H, m, H-9b), 6.77 (2H, d, J = 1.7 Hz,H-2΄and H-6΄), 6.57 (1H, d, J = 15.6 Hz, H-7΄),6.36 (1H, dt, J = 15.6, 5.6 Hz, H-8΄), 4.24 (1H, d,J= 5.6 Hz,H-9΄),3.84 (3H,s,3-OMe),3.90 (3H,s,3΄-OMe and 5΄-OMe);13C NMR data, see Table 2. All data were from the reported for utiline B (Ⅷ)[20],and the 1D NMR assignments of its revised structure were completed on the basis of the comparison of its NMR data with that of the correct compound[12].
2.3.9 (-)-threo-Methyl 4-O-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl) -1- (hydroxymethyl)ethyl]ferulate (12) Colorless amorphous power; [α]D-4.2;1H NMR(500 MHz,CD3OD)δ 7.02(1H,d,J=1.2 Hz,H-2),6.70(1H,d,J=8.0 Hz,H-5),6.83(1H,dd,J=8.0,2.0 Hz,H-6),4.81(1H,d,J=6.0 Hz,H-7),4.48(1H,m,H-8),3.83(1H,m,H-9a),3.76(1H,m,H-9b),7.15(1H,d,J=2.0 Hz,H-2΄),6.95(1H,d,J=8.5 Hz,H-5΄),7.06(1H,dd,J=8.5,2.0 Hz,H-6΄),7.59(1H,d,J=16.0 Hz,H-7΄),6.38(1H,d,J=16.0 Hz,H-8΄),3.77(3H,s,3-OMe),3.81(3H,s,3΄-OMe),3.76(3H,s,9΄-OMe);13C NMR data,see Table 2. The above data were from the reported for rhemaneolignan A (IX)[21],and the 1D NMR assignments of its revised structure were completed by comparing of its NMR data with that of the correct structure[33].
2.3.10 (+)-erythro-Methyl 4-O-[2-hydroxy-2-(4-hydroxy-3-methoxyphenyl) -1- (hydroxymethyl)ethyl]ferulate (13) Colorless amorphous power; [α]D+1.8;1H NMR (500 MHz, DMSO-d6) δ 6.95 (1H, d, J = 2.0 Hz, H-2), 6.66 (1H, d, J = 8.0 Hz, H-5), 6.74 (1H,dd, J = 8.0, 2.0 Hz, H-6), 4.68 (1H, d, J = 4.0 Hz,H-7), 4.37 (1H, m, H-8), 3.56 (1H, m, H-9a), 3.23(1H, m, H-9b), 7.32 (1H, d, J = 2.0 Hz, H-2΄), 7.04(1H,d,J=8.0 Hz,H-5΄),7.18(1H,dd,J=8.0,2.0 Hz, H-6΄), 7.51 (1H, d, J = 16.0 Hz, H-7΄), 6.52(1H, d, J = 16.0 Hz, H-8΄), 3.71 (3H, s, 3-OMe),3.80 (3H, s, 3΄-OMe), 3.69 (3H, s, 9΄-OMe);13C NMR data, see Table 2. All data were from the reported for rhemaneolignan B (Ⅹ)[21], and the 1D NMR assignments of its revised structure were completed on the basis of the comparison of its NMR data with that of the correct compound[33].

