室内木柴燃烧排放水溶性离子粒径分布特征
樊泽薇,孔少飞*,严 沁,郑淑睿,郑 煌,姚立全,吴 剑,张 颖,牛真真,吴方琪,程 溢, 曾 昕 ,覃 思,刘 玺,燕莹莹,祁士华,2
室内木柴燃烧排放水溶性离子粒径分布特征
樊泽薇1,孔少飞1*,严 沁1,郑淑睿1,郑 煌1,姚立全1,吴 剑1,张 颖1,牛真真1,吴方琪1,程 溢1, 曾 昕1,覃 思1,刘 玺1,燕莹莹1,祁士华1,2
(1.中国地质大学(武汉)环境学院,湖北 武汉 430074;2.中国地质大学(武汉),生物地质与环境地质国家重点实验室,湖北 武汉 430074)
基于实验室模拟燃烧和稀释通道采样系统,采用荷电低压撞击采样器采集了6种典型木柴燃烧排放的14级粒径段颗粒物.采用离子色谱分析了8种水溶性离子,获得水溶性离子的分粒径排放因子和排放特征.结果表明,Ca2+的排放因子呈双峰分布,在0.25~0.38和2.5~3.6µm粒径段出现峰值,分别为0.14和0.16mg/kg.其余离子的排放因子为单峰分布.NH4+、NO3−和SO42−的排放因子在0.25~0.38µm粒径段出现峰值,分别为0.41、0.58和0.84mg/kg. K+和Cl−的排放因子在0.15~0.25µm内出现峰值,分别为0.89和0.99mg/kg.木柴燃烧排放总水溶性离子的质量中值粒径为(0.30±0.07) µm,各离子的质量中值粒径范围为0.24~0.44µm.PM0.094、PM0.94、PM2.5和PM10中水溶性离子的排放因子变化范围分别为1.04~9.33、5.00~48.87、5.46~52.00和6.14~53.68mg/kg.木柴燃烧排放颗粒物中K+/Cl−、K+/NO3−、K+/SO42−和SO42−/NO3−比值随粒径变化而变化,其排放初始值在应用于源解析和生物质燃烧排放气溶胶传输老化研究时需引起关注.木柴燃烧排放PM10中的阴阳离子当量比值为0.80±0.11,颗粒物的酸度随颗粒物粒径而改变,亚微米颗粒物和细颗粒物的酸度高于超细颗粒物和粗颗粒物的酸度.本研究对构建生物质燃烧排放分粒径水溶性离子清单,更新和改进相关气候和空气质量模型的参数设置,识别烟气传输过程中的老化具有重要意义.
室内木柴燃烧;稀释通道;水溶性离子;粒径分布;排放因子
生物质燃烧是大气污染物的重要来源之一,其排放的颗粒物对区域气候和大气环境有重要影响,如增强区域大气辐射强迫[1],导致区域霾事件频发[2-3]等.Cheng等[2]通过WRF-CMAQ模拟发现生物质燃烧贡献了长江三角洲地区大气污染期间37%的PM2.5,70%的有机碳和61%的元素碳.Zhang等[4]基于PMF模型解析发现美国东南部冬季27%的PM2.5源自生物质燃烧.在发展中国家,生物质作为家庭能源被广泛使用[5-6].中国47.6%的农村家庭将生物质作为日常做饭的主要燃料[6].生物质在低效且缺少排放控制措施的炉具中燃烧会导致严重的室内空气污染.2016年室内空气污染导致全球380万人死亡,占全球死亡率的7.7%[7].对生物质燃烧排放污染物开展研究是对其进行防控的基础.
建立准确的一次污染物排放清单是识别相应污染物对环境和健康影响的基础.排放因子的缺乏严重制约着排放清单的准确性.国内外学者开展了生物质燃烧水溶性离子排放因子的实测研究.Guo等[19]基于室内模拟燃烧实验,得到6种树枝明烧和闷烧排放PM2.5中10种水溶性离子的排放因子. Ozgen等[20]模拟两种木柴在壁炉和木柴炉中的燃烧,得到不同木柴/炉具组合排放超细颗粒物(PM0.1)中水溶性离子的排放因子.刘亚男等[21]研究了秸秆、薪柴及民用煤燃烧排放0~2.5、2.5~10和10~100µm三个粒径段颗粒物中水溶性离子的排放因子.由于燃料种类、燃烧方式和采样方法的不同,现有排放因子存在较大差异,需要持续更新.
气溶胶化学组分的粒径分布影响着其环境、气候和健康效应.在计算硫酸盐气溶胶的直接辐射强迫时,Kiehl等[22]假定硫酸盐气溶胶的体积中值粒径为0.42µm.前人在开展空气质量模拟时,通常默认爱根核模态和积聚模态气溶胶的体积中值粒径分别为0.03和0.3µm[23].这些参数值是基于近源观测得到的大气气溶胶粒径分布的平均值,并非直接的源排放数据[24].Elleman等[25]结合文献中报道的以交通为主的城市地区、燃煤电厂和船舶排放的气溶胶粒径分布数据更新模型的默认粒径以改进模拟效果,发现更新参数前模拟的气溶胶数浓度低估1~2个数量级,而更新后的模拟浓度低估1个数量级,更接近观测结果.另外,颗粒物在人体的沉积位置和其对人体健康的危害与其粒径大小密切相关.PM10、PM2.5和PM0.1在肺泡区域的沉积率分别为1%、39%和43%,PM0.1还可扩散到循环系统并进入肝脏等器官[26-27].因而研究污染源排放颗粒物及其化学组分的粒径分布,对于更新和改进相应模型的参数设置,提高模拟结果的准确性以及评估污染物对人体健康的危害具有重要意义.当前,仅有少数研究关注了生物质燃烧排放水溶性离子的粒径分布.Zhang等[28]分析了木柴燃烧排放9个粒径段颗粒物中水溶性离子的分布.Park等[29]研究了秸秆和树木枝叶燃烧排放K+、Cl−、SO42−和草酸盐的粒径分布.Goetz等[30]使用气溶胶质谱研究了牛粪、木柴和农作物秸秆在南亚传统炉具中燃烧排放的有机物、Cl−和SO42−的粒径分布特征.
本研究基于实验室模拟燃烧和稀释通道采样系统,对6种木柴室内燃烧排放的14级粒径颗粒物进行采样,分析8种水溶性离子,获得分粒径水溶性离子排放因子,识别不同粒径段水溶性离子组成特征,计算得到不同离子的质量中值粒径,为分粒径组分清单构建、相关气候、空气质量模型参数设置等提供基础数据.
1 材料与方法
1.1 样品来源
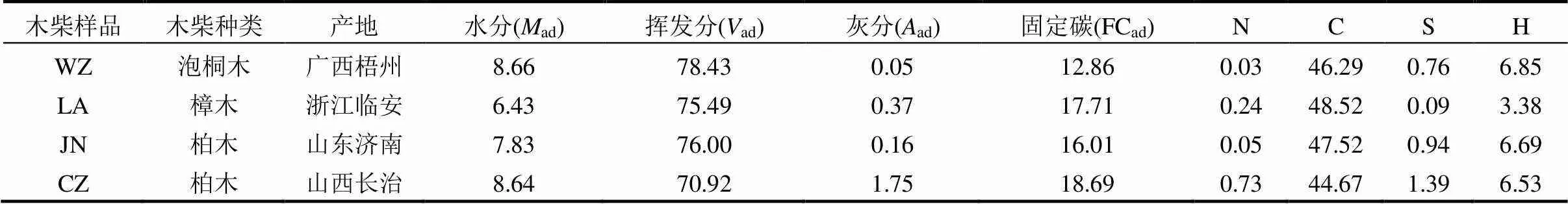
表1 木柴样品组分分析(%)
注:其余两种木柴: ZJ:柑橘木,湖北枝江; SY:松木,辽宁沈阳.
本研究从6个不同省市农户家中收集6种木柴,分别为柑橘木(ZJ)、泡桐木(WZ)、樟木(LA)、柏木(JN)、柏木(CZ)和松木(SY).其中4种木柴样品的组分分析见表1.实验所用炉具是从市场购买的常见民用炉具,高43cm,外径30cm,内径12cm.
1.2 燃烧实验
采用稀释通道采样系统采集烟气,模拟烟气进入大气后的稀释、冷却和凝结等过程[31].实验流程图见Cheng等[32]的研究.每种木柴分别进行燃烧实验.每次实验时,模拟农户炊事过程中木柴的使用量,用电子秤称取木柴4~5kg,分3次添加.木柴引燃后放入炉具并开始采样,采样持续整个燃烧过程,时长为2h左右.木柴燃烧至无明火时,采样结束.燃烧过程中保证充足的空气使燃料燃烧充分,以模拟农户炊事过程中木柴的燃烧方式.引燃木柴后,风机以恒定流量将排放的烟气抽入烟道,在距火苗1.5m处使用采样枪等速采样,采集部分烟气进入稀释通道系统.空压机产生的空气经过滤后进入稀释舱与烟气混合稀释,稀释倍数为30倍.待稀释烟气在停留舱混合均匀后进行滤膜采集.
1.3 样品采集
采用荷电低压撞击采样器采集烟气中的颗粒物,采样流量为10L/min,采集颗粒物的粒径分别为0.006~0.016, 0.016~0.030, 0.030~0.054, 0.054~0.094, 0.094~0.15, 0.15~0.25, 0.25~0.38, 0.38~0.60, 0.60~ 0.94, 0.94~1.6, 1.6~2.5, 2.5~3.6, 3.6~5.3和5.3~ 10µm,共14个粒径段[33].考虑超细颗粒物和亚微米颗粒物对人体健康的危害[34-35],本研究将14级颗粒物分为超细颗粒物、亚微米颗粒物、细颗粒物和粗颗粒物予以讨论.本研究中超细颗粒物(PM0.094)包括前4个粒径段,亚微米颗粒物(PM0.94)包括前9个粒径段,细颗粒物(PM2.5)包括前11个粒径段,粗颗粒物(PM2.5~10)包括2.5~3.6、3.6~5.3和5.3~10µm 3个粒径段.
采样滤膜为直径25mm的石英滤膜.采样前将石英滤膜在马弗炉中经过800℃,0.5h的烘烤,然后在25℃和40%相对湿度的超净实验室中平衡48h,称重后装入清洁膜盒后放入干燥皿备用.采样结束后,将滤膜从采样器中取出装入原膜盒,平衡称重后放入−20℃的冰箱中保存.
1.4 水溶性离子分析与质量控制
取整张滤膜剪碎于灭菌离心管中,加入5mL超纯水,摇匀后静置30min,超声振荡40min.用孔径为0.45 µm的水系滤膜过滤上层清液,使用TH-980H型(天虹,武汉)离子色谱仪对8种离子(Na+、NH4+、Mg2+、K+、Ca2+、Cl−、NO3−和SO42−)进行分析.阳离子使用Shodex YS-50分析柱,淋洗液为4.5mmol/L甲烷磺酸,流量为0.9mL/min.阴离子使用Shodex SI-90 4E分析柱,淋洗液为1.8mmol/L的Na2CO3和1.7mmol/L的NaHCO3,流量为1.15mL/ min.8种离子的仪器检出限均低于0.01 µg/mL.8种离子标准曲线的相关系数均大于0.999.
引渡本应是反腐败国际刑事司法协助最直接、最有效的方式,但由于实际操作上的障碍而难以发挥作用。我国目前已与多个国家缔结了双边引渡条约并且参加了不少含有引渡条款的多边国际公约,但遗憾的是,由于掺杂着战略关系和外交关系的考虑,伴随着司法观念、人权理念的冲突,我国与西方发达国家缔结的引渡条约数量偏少。此外,很多国家规定,他国就腐败犯罪事宜向其提出引渡等请求时,要受到双重犯罪原则、特定性原则、政治犯罪不引渡原则或死刑不引渡原则的限制,从而为引渡腐败分子带来了困难。因此,我国在积极缔结引渡条约的同时,要不断探索引渡的替代措施,利用多种途径实现对外逃腐败犯罪分子的有效惩处:
样品分析过程执行严格的质量控制.滤膜提取和淋洗液配置均使用电阻率为18.2MΩ·cm的超纯水.每天仪器开启,待基线稳定后测样.每次进样前先注射一针超纯水去除残留杂质,每分析10个样品抽取第1个样品重复分析,确保前后2次的测量误差在10%以内再进行后续分析.此外,测定空白滤膜样,从样品分析结果中扣除其平均值.
1.5 排放因子计算
将排放的污染物质量除以消耗的燃料质量来确定排放因子,表示为每千克消耗的干燃料排放的污染物.排放因子按公式(1)计算[36]:
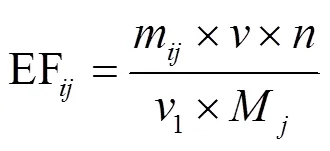
式中: EF为第种木柴燃烧后类水溶性离子的排放因子,mg/kg;m为第种木柴燃烧后类水溶性离子的质量,mg;为烟气流量,L/min;1为采样流量, L/min;为稀释倍数;M为第种木柴的消耗量,kg.
1.6 阴阳离子平衡
阴阳离子平衡用于检验生物质燃烧排放颗粒物的酸碱性,通过式(2)、(3)计算阴阳离子当量排放因子:
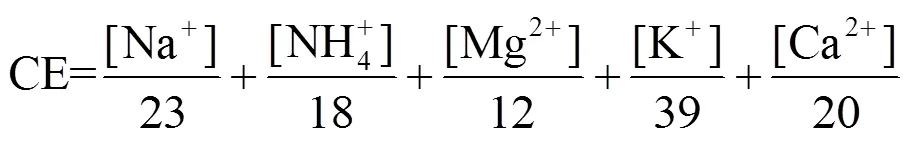
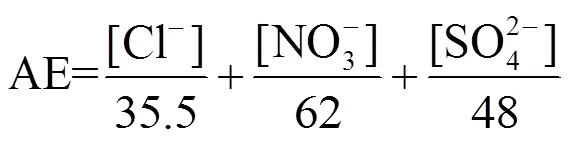
式中:CE为阳离子当量排放因子,AE为阴离子当量排放因子,[]为各水溶性离子排放因子,mg/kg.
2 结果与讨论
2.1 水溶性离子排放因子的粒径分布
如图1所示,Ca2+的排放因子呈双峰分布,粗、细粒子中均有峰值,在粗粒子2.5~3.6µm内有主峰,为0.16mg/kg;在细粒子0.25~0.38µm内有次峰,为0.14mg/kg.Na+和Mg2+的排放因子较小,在此不作讨论.其余离子呈单峰分布,且峰值均出现在亚微米粒径段内.NH4+、NO3−和SO42−的排放因子在0.25~ 0.38µm内出现峰值,分别为0.41、0.58和0.84mg/kg. K+和Cl−的排放因子在0.15~0.25µm内出现峰值,分别为0.89和0.99mg/kg.Park等[29]研究表明,秸秆和树木枝叶燃烧排放的K+、Cl−和SO42−呈单峰分布,除银杏叶和枫叶峰值分布在0.55~1.0µm外,其它生物质峰值均分布在0.32~0.55µm粒径段.Goetz等[30]采用AMS测得硬木和树枝在传统泥炉中燃烧排放PM1中的Cl−分别在133和123nm处出现峰值,与本研究结果具有可比性.由此可见,减少木柴燃烧可有效降低大气亚微米颗粒物中水溶性离子的含量.
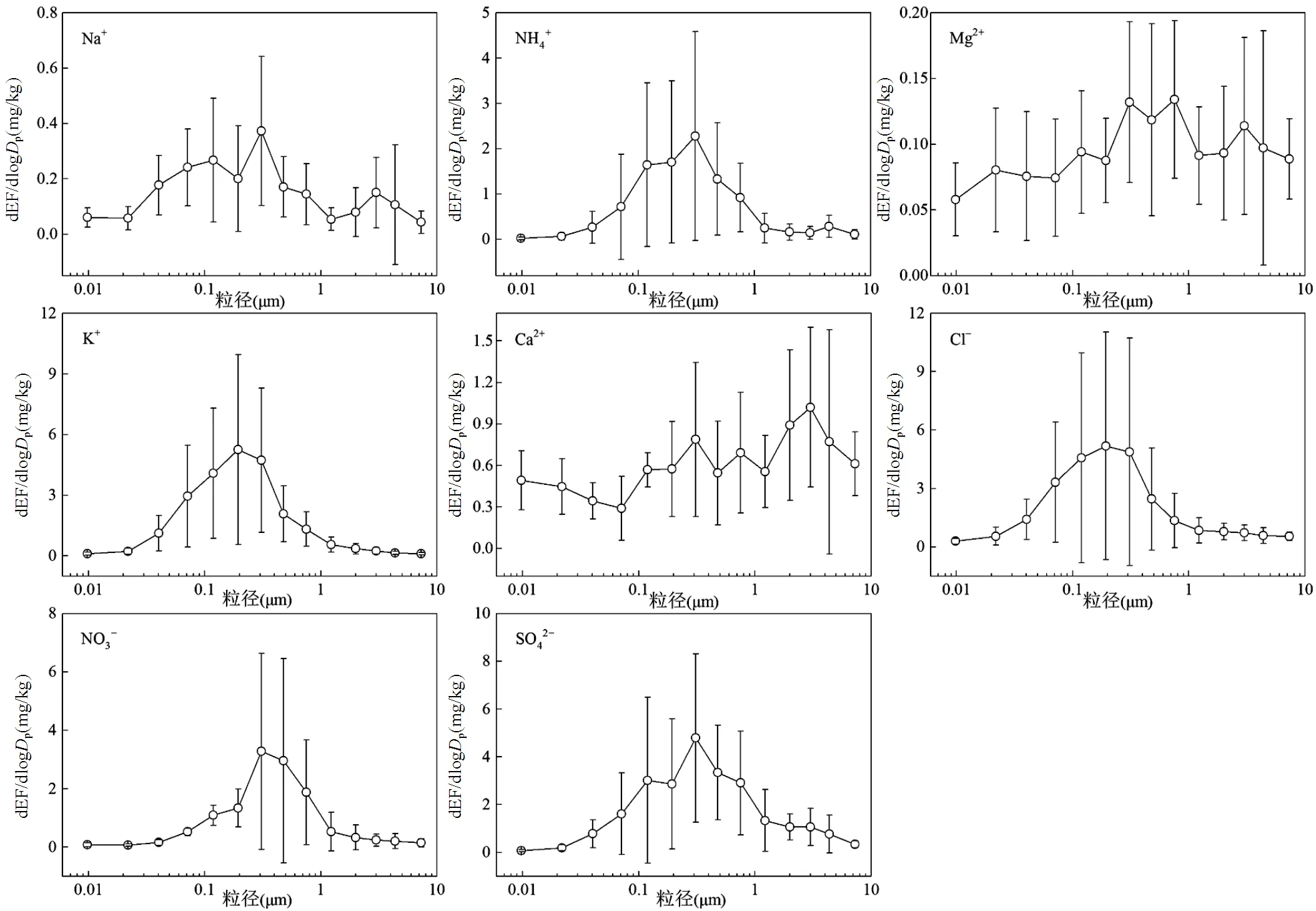
图1 室内木柴燃烧水溶性离子排放因子的粒径分布
EF为各水溶性离子的排放因子,mg/kg;p为各采样粒径段上限粒径和下限粒径的几何平均值,mm
2.2 分粒径颗粒物中水溶性离子的排放因子
如图2所示,6种木柴燃烧排放88.3%~96.9%的水溶性离子分布在细颗粒物中,79.5%~91.1%分布在亚微米颗粒物中.不同种类木柴的总水溶性离子排放因子相差2~10倍.PM0.094、PM0.94、PM2.5和PM10中水溶性离子的排放因子分别为1.04~ 9.33、5.00~ 48.87、5.46~52.00和6.14~53.68mg/kg.长治柏木中硫、氮和灰分的含量分别是济南柏木中的1.5、14.6和10.9倍,这可能是造成长治柏木燃烧排放总水溶性离子的排放因子是济南柏木燃烧排放的4倍的主要原因.长治柏木燃烧排放SO42−、NO3−、NH4+、Na+、Mg2+、K+、Ca2+和Cl−的排放因子分别是济南柏木燃烧排放的4.0、0.9、8.6、1.7、1.7、4.6、1.3和8.1倍,表明水溶性离子排放因子的差异与木柴的化学组成有关.Ozgen等[20]测得木柴燃烧排放PM0.1中水溶性离子的排放因子为28~ 67mg/kg,是本研究的3~64倍.Calvo等[37]实测得到木柴燃烧排放PM2.5中水溶性离子的排放因子为143.1~300.2mg/kg,是本研究的2.8~55倍.Guo等[19]直接采样得到PM2.5中水溶性离子的排放因子为425~1546mg/kg,是本研究的8.2~283倍.Alves等[38]的结果显示,金荆树燃烧烟尘中水溶性离子排放因子是本研究的1.5~259倍.
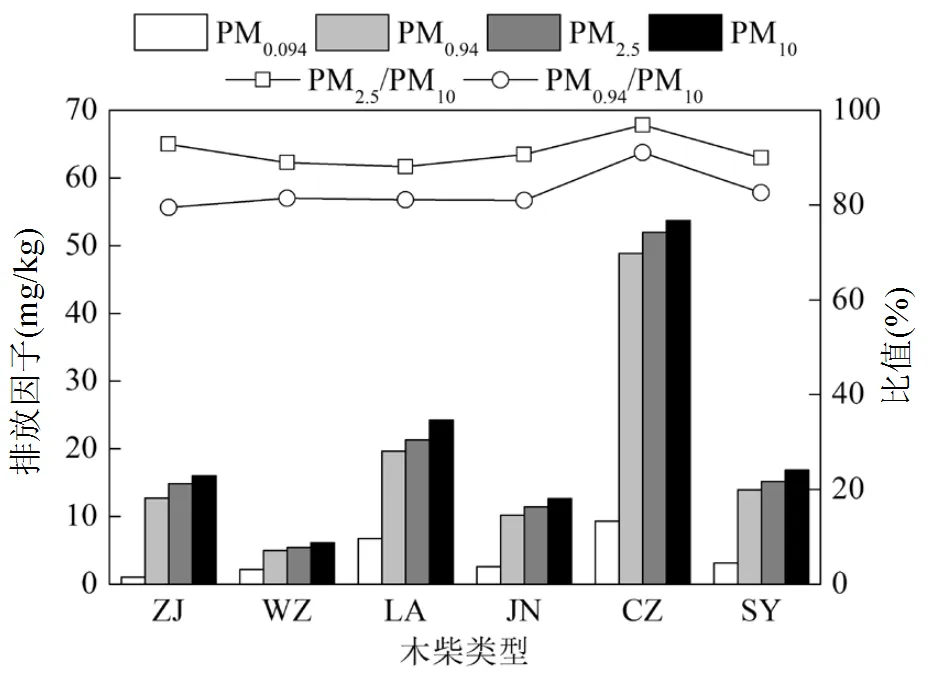
图2 不同产地木柴室内燃烧水溶性离子的排放因子
表2将本研究的排放因子与文献结果进行对比,发现不同研究所得水溶性离子排放因子的差异最大可达80倍.木柴燃烧排放PM10的阳离子中,K+的排放因子最大,为(4.7±3.6) mg/kg,分别为Na+、NH4+、Mg2+和Ca2+排放因子的11.7、2.5、23.5和2.8倍;阴离子中,Cl−和SO42−的排放因子较大,分别为(5.5±5.6)和(4.6±3.8) mg/kg,分别是NO3−排放因子的2.2和1.8倍.Ozgen等[20]的结果中Ca2+和Cl−的排放因子与本研究结果相似,K+、NO3−和SO42−排放因子分别比本研究PM0.094结果高8.9、14.5和36.8倍.Calvo等[37]结果中Na+的排放因子比本研究PM2.5结果高100~135倍,榉木其余离子的排放因子与本研究结果相似,其它两种木柴各离子的排放因子比本研究结果高2~36倍.Guo等[19]采用直接采样方法所得PM2.5中各离子的排放因子均高于本研究结果,高7.4~155倍.Sen等[39]的研究结果中各离子的排放因子高于本研究结果5~100倍.刘亚男等[21]所得木柴燃烧PM中K+的排放因子比本研究结果高11~27倍,Ca2+的排放因子比本研究结果高86~124倍.本研究所得排放因子数据偏低,可能是由于采样仪器和采样方法的不同导致的.木柴燃烧水溶性离子的排放因子受采样方法、采样仪器、燃料类型、燃烧条件和状态等影响较大,在应用于排放清单构建时,应尽可能涵盖不同学者的研究结果.

表2 与文献中排放因子的对比(mg/kg)
注:–为未分析.除文献[19]为直接采样外,其它均为稀释采样.文献[20]中稀释倍数为90~150倍,文献[39]中稀释倍数为40~60倍.
2.3 水溶性离子比值的粒径分布
木柴燃烧排放颗粒物中K+/Cl−、K+/NO3−、K+/SO42−和SO42−/NO3−比值随颗粒物粒径的变化见图3.本研究中K+/Cl−比值在0.054~0.94µm粒径段表现出高值,变化范围为0.85~1.32;在<0.054µm和>0.94µm粒径段表现出低值,变化范围为0.20~0.66.K+/NO3−比值在0.054~0.94µm粒径段表现出低值,范围为2.52~8.10;在<0.054µm和>0.94µm粒径段表现出高值,范围为10.85~41.76.K+/SO42−比值在0.38~2.5µm粒径段表现出低值,范围为0.46~0.75;在<0.38µm和>2.5µm粒径段表现出高值,范围为0.87~3.49,可能是因为木柴燃烧排放的NO3−主要分布在亚微米颗粒物中,排放的SO42−主要分布在亚微米颗粒物和细颗粒物中.SO42−/NO3−比值在0.030~0.94µm粒径段表现出低值,范围为2.20~8.85;在<0.030µm和>0.94µm粒径段表现出高值,范围为18.57~137.94.
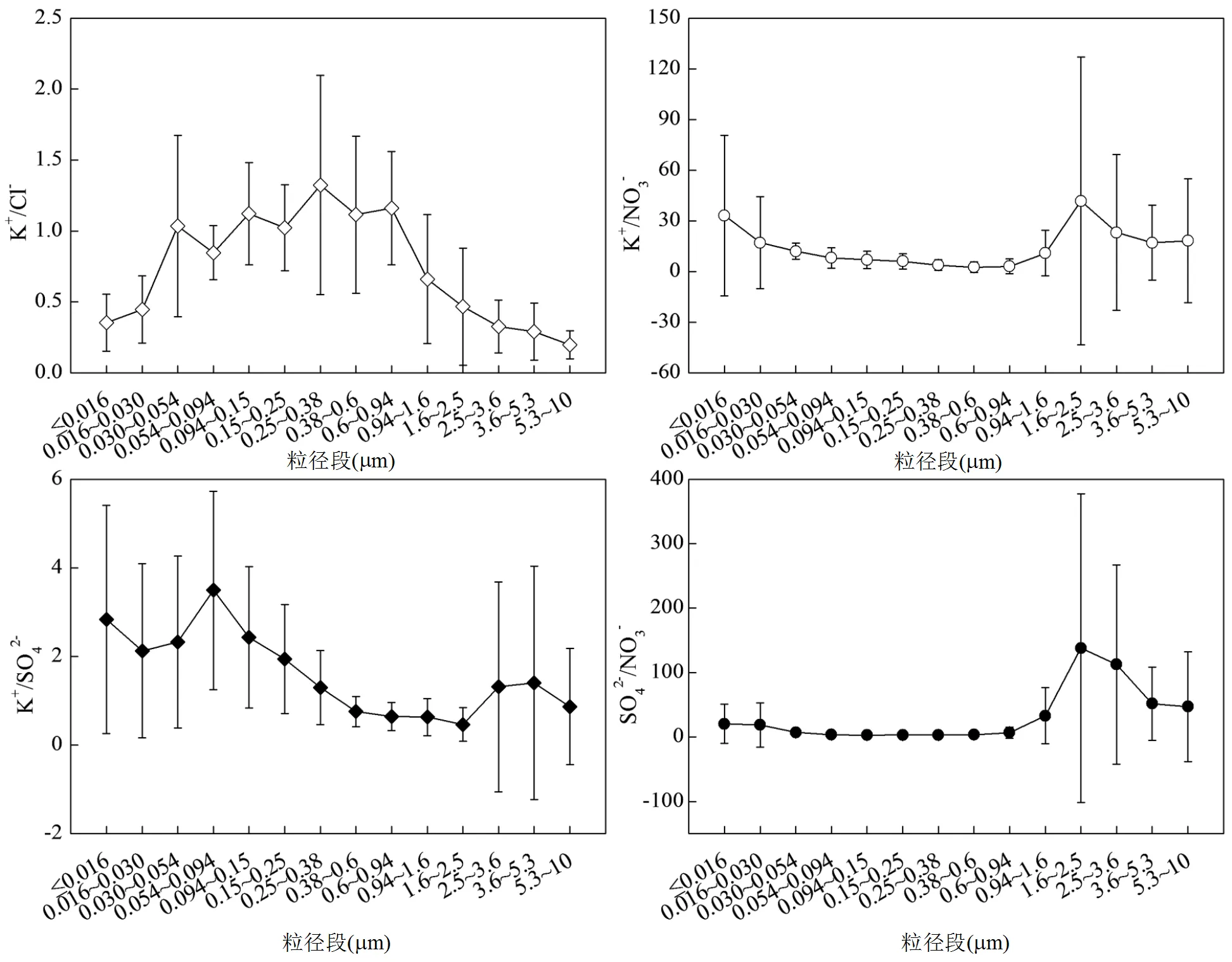
图3 室内木柴燃烧排放分级颗粒物中K+/Cl−、K+/NO3−、K+/SO42−和SO42−/NO3−比值
有研究表明,K+/Cl−和K+/SO42−比值可用于识别不同种类生物质燃烧源对大气颗粒物的贡献,分粒径比值还可应用于分粒径源解析研究中[29,40].K+/ Cl−、K+/NO3−、K+/SO42−和SO42−/NO3−比值还可用于判断生物质燃烧排放气溶胶在大气中的传输老化程度.在生物质燃烧排放气溶胶传输过程中,KCl与SO2和NO发生非均相反应转化为K2SO4和KNO3,并生成气态HCl,导致老化气溶胶中Cl−含量降低,SO42−和NO3−的含量升高,K+/Cl−比值增加, K+/SO42−和K+/NO3−比值降低[41-42].本研究中木柴燃烧排放的PM0.94中的K+/Cl−比值为1.02,表明烟气中K+主要与Cl−结合.而受生物质气溶胶影响的大气PM1中的K+/Cl−比值为1.60[1],该比值大于1表明Cl−的损失以及SO42−和NO3−的生成.前人研究表明受生物质燃烧排放气溶胶影响的大气PM1.1、PM1.1~2.1和PM2.1~10中K+/NO3−比值分别为0.50、0.30和0.32, K+/SO42−比值分别为0.28、0.15和0.07[1],各粒径段比值均低于本研究中的相应初始比值.3个粒径段的K+/NO3−初始排放比值分别是老化后相应比值的9.1、36.2和51.8倍,K+/SO42−初始排放比值分别是老化后该比值的5.3、3.5和16.2倍.这些结果均表明生物质气溶胶传输过程中发生了非均相反应导致Cl−、SO42−和NO3−的含量发生变化.本研究中木柴燃烧排放的PM0.94、PM0.94~2.5和PM2.5~10中SO42−/NO3−比值分别为3.25、41.86和56.19.前人研究表明受生物质燃烧排放气溶胶影响的大气PM1.1、PM1.1~2.1和PM2.1~10中SO42−/NO3−比值分别为1.78、2.19和4.42[1],远低于本研究所得的相应初始排放值.3个粒径段初始排放比值是老化后该比值的1.8、19.1和12.7倍,这可能是由于在生物质气溶胶传输过程中NO3−的生成速率大于SO42−的生成速率导致的[43].Akagi等[43]也发现生物质烟气排放约4.5h后的顺风烟羽中的SO42−/NO3−比值降低,由初始值0.037降为0.017.因此,作为研究生物质燃烧排放气溶胶大气传输转化的基础,这些比值的初始排放值在后续研究中需要被关注.
2.4 阴阳离子比值的粒径分布
气溶胶的酸度是颗粒物的重要化学性质之一,可影响二次有机气溶胶的形成和多种大气化学反应,对人体健康、环境和气候有重要意义[44-45].研究源排放气溶胶酸度的粒径分布可以为研究排放源对气溶胶酸度的贡献和区域酸沉降提供参考[46].
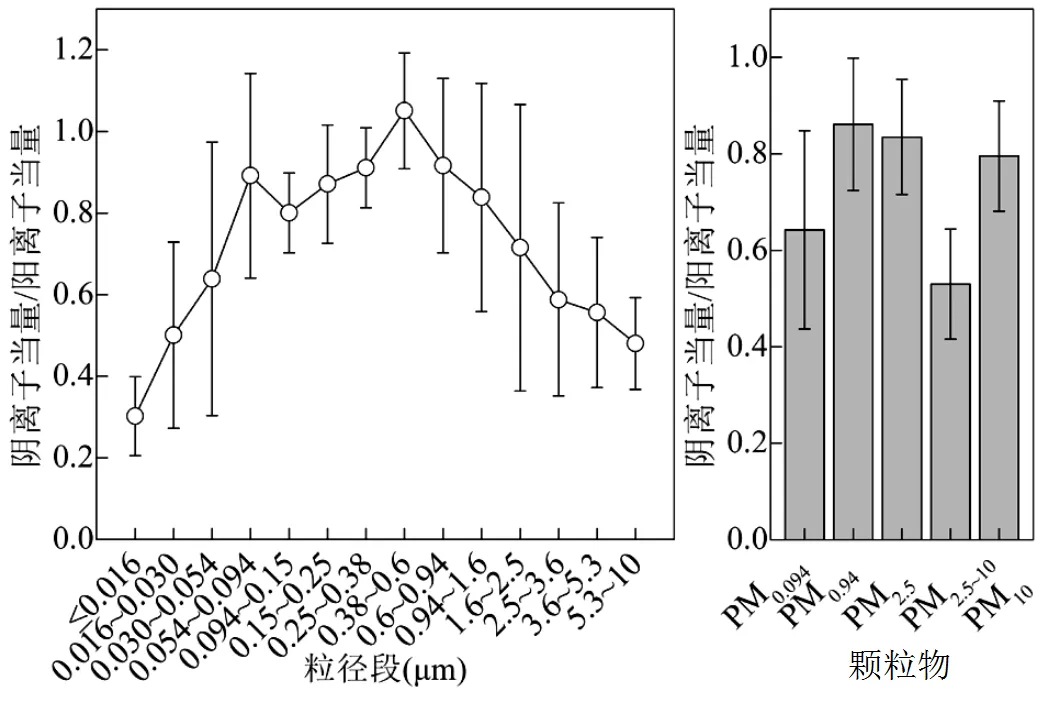
图4 室内木柴燃烧排放分级颗物中阴阳离子当量比值
图4为木柴燃烧排放颗粒物中阴阳离子当量排放因子比值随粒径的变化.PM10中的比值为0.80± 0.11,除0.38~0.60µm粒径段内该比值为1.05±0.14外,其它各粒径段比值变化范围为0.30~0.92,均小于1,表明阳离子含量高于阴离子含量,可能与阴离子未完全检测有关,如CO32−、HCO3−以及有机阴离子如草酸根等[47-48].颗粒物中阴阳离子当量排放因子比值在£0.016µm粒径段内最小,比值为0.30±0.10,随着颗粒物粒径的增加,该比值先增大再减小,在0.38~0.6µm粒径段内比值最大,为1.05±0.14,最大值是最小值的3.5倍.PM0.094、PM0.94、PM2.5和PM2.5~10中的阴阳离子当量比值分别为0.64±0.21、0.86± 0.14、0.84±0.12和0.53±0.11,表明亚微米颗粒物和细颗粒物的酸度高于超细颗粒物和粗颗粒物的酸度.PM0.94和PM2.5的酸度较大是因为硫酸盐和硝酸盐主要集中在PM0.94和PM2.5中.PM2.5~10的酸度较小可能是粗颗粒物中存在未检测的CO32−和HCO3−与粗颗粒物中的Ca2+和Mg2+结合有关.由此可见,木柴燃烧排放不同粒径段颗粒物的酸度存在差异,其排放后在大气中的化学过程及潜在环境效应也会有差异,需进一步深入研究.
2.5 水溶性离子的质量中值粒径
质量中值粒径(MMD)常被用于人体健康风险评估[49]和气溶胶及其化学组分的气候、空气质量效应模拟[22-23].本研究得到的木柴燃烧排放水溶性离子的质量中值粒径见表3.木柴燃烧排放总水溶性离子的质量中值粒径为(0.30±0.07)µm,各离子的质量中值粒径范围为0.24~0.44µm,均在亚微米粒径段,表明木柴燃烧排放的水溶性离子超过50%可以在人体肺泡区域沉积,需要提高对木柴燃烧导致的室内空气污染及其带来的人体健康效应的重视.Kiehl等[22]计算硫酸盐气溶胶的辐射强迫时,假定其体积中值粒径为0.42µm,并根据Whitby[24]的方法转化成质量中值粒径为0.55µm,高于本研究中SO42−的质量中值粒径((0.38±0.05)µm).Boucher等[50]发现当假定硫酸盐体积中值粒径从0.2µm增加到0.4µm时,模拟的辐射强迫增加约20%.因此,Kiehl等[22]的计算会高估木柴燃烧排放硫酸盐的辐射强迫.严沁等[51]实测得到块煤明烧、蜂窝煤明烧和闷烧排放SO42−的质量中值粒径分别为0.89、0.79和0.38µm.现有模型中气溶胶化学组分的质量中值粒径参数的假设值与实际观测值存在较大偏差,使得模拟结果存在较大不确定性.目前此类实测研究仍鲜见报道,需予以重视和持续更新.

表3 室内木柴燃烧排放水溶性离子的质量中值粒径(µm)
3 结论
3.1 木柴燃烧Ca2+的排放因子呈双峰分布,在0.25~0.38和2.5~3.6µm粒径段出现峰值,分别为0.14和0.16mg/kg.其余离子的排放因子呈单峰分布, NH4+、NO3−和SO42−的排放因子在0.25~0.38µm内出现峰值,分别为0.41、0.58和0.84mg/kg. K+和Cl−的排放因子在0.15~0.25µm内出现峰值,分别为0.89和0.99mg/kg.
3.2 室内木柴燃烧排放的PM0.094、PM0.94、PM2.5和PM10中水溶性离子的排放因子分别为1.04~ 9.33、5.00~48.87、5.46~52.00和6.14~53.68mg/kg.
3.3 木柴燃烧排放水溶性离子的K+/Cl−、K+/NO3−、K+/SO42−和SO42−/NO3−比值有明显的粒径分布变化,在应用于源解析和研究生物质燃烧排放气溶胶传输老化时需注意.
3.4 木柴燃烧排放各粒径段阴阳离子当量比值变化范围为0.30~1.05,PM0.094、PM0.94、PM2.5和PM2.5~10中的当量比值分别为0.64±0.21、0.86±0.14、0.84± 0.12和0.53±0.11,亚微米颗粒物和细颗粒物的酸度大于超细颗粒物和粗颗粒物的酸度.
3.5 木柴燃烧排放总水溶性离子的质量中值粒径为(0.30±0.07)µm,各离子的质量中值粒径范围为0.24~0.44µm,均在亚微米粒径段.需要通过实测来更新模型的参数设置以改进模拟结果.
[1] Singh N, Banerjee T, Raju M P, et al. Aerosol chemistry, transport, and climatic implications during extreme biomass burning emissions over the Indo-Gangetic Plain [J]. Atmospheric Chemistry and Physics, 2018,18(19):14197-14215.
[2] Cheng Z, Wang S, Fu X, et al. Impact of biomass burning on haze pollution in the Yangtze River delta, China: A case study in summer 2011 [J]. Atmospheric Chemistry and Physics, 2014,14(9):4573-4585.
[3] 朱佳雷,王体健,邢 莉,等.江苏省一次重霾污染天气的特征和机理分析 [J]. 中国环境科学, 2011,31(12):1943-1950. Zhu J L, Wang T J, Xing L, et al. Analysis on the characteristics and mechanism of a heavy haze episode in Jiangsu Province [J]. China Environmental Science, 2011,31(12):1943-1950.
[4] Zhang X, Hecobian A, Meng Z, et al. Biomass burning impact on PM2.5over the southeastern US during 2007: integrating chemically speciated FRM filter measurements, MODIS fire counts and PMF analysis [J]. Atmospheric Chemistry and Physics, 2010,10(14):6839-6853.
[5] Sana A, Kafando B, Dramaix M, et al. Household energy choice for domestic cooking: distribution and factors influencing cooking fuel preference in Ouagadougou [J]. Environmental Science and Pollution Research, 2020,27(15):18902-18910.
[6] Duan X L, Jiang Y, Wang B B, et al. Household fuel use for cooking and heating in China: Results from the first Chinese Environmental Exposure-Related Human Activity Patterns Survey (CEERHAPS) [J]. Applied Energy, 2014,136:692-703.
[7] World Health Organization (WHO). Global Health Observatory (GHO) data: Mortality from household air pollution [EB/OL]. https:// www.who.int/gho/phe/indoor_air_pollution/burden/en/, 2020-9.
[8] Liu T N, Zhuang G S, Huang K, et al. A typical formation mechanism of heavy haze-fog induced by coal combustion in an inland city in North-Western China [J]. Aerosol and Air Quality Research, 2017, 17(1):98-107.
[9] Wang X, Jacob D J, Eastham S D, et al. The role of chlorine in global tropospheric chemistry [J]. Atmospheric Chemistry and Physics, 2019,19(6):3981-4003.
[10] Ma H M, Li J, Wan C, et al. Inflammation response of water-soluble fractions in atmospheric fine particulates: a seasonal observation in 10large Chinese cities [J]. Environmental Science & Technology, 2019,53(7):3782-3790.
[11] 洪 也,张 莹,马雁军,等.沈阳市PM2.5离子成分对呼吸疾病门诊数影响研究 [J]. 中国环境科学, 2018,38(12):4697-4705. Hong Y, Zhang Y, Ma Y J, et al. Effect of the association between PM2.5and its water-soluble ions and hospital outpatient visits for respiratory diseases in Shenyang City [J]. China Environmental Science, 2018,38(12):4697-4705.
[12] Niu Y, Chen R J, Xia Y J, et al. Fine particulate matter constituents and stress hormones in the hypothalamus-pituitary-adrenal axis [J]. Environment International, 2018,119:186-192.
[13] Souri A H, Choi Y, Jeon W, et al. Quantifying the impact of biomass burning emissions on major inorganic aerosols and their precursors in the US [J]. Journal of Geophysical Research: Atmospheres, 2017, 122(21):12020-12041.
[14] Cui M, Chen Y J, Zheng M, et al. Emissions and characteristics of particulate matter from rainforest burning in the Southeast Asia [J]. Atmospheric Environment, 2018,191:194-204.
[15] Fu X, Wang T, Wang S X, et al. Anthropogenic emissions of hydrogen chloride and fine particulate chloride in China [J]. Environmental Science & Technology, 2018,52(3):1644-1654.
[16] Liu L, Kong S F, Zhang Y X, et al. Morphology, composition, and mixing state of primary particles from combustion sources - crop residue, wood, and solid waste [J]. Scientific Reports, 2017,7(1):5047.
[17] 张永运,王宏青,肖 浩,等.东北亚冬季PM2.5水溶性离子空间分布特征及来源 [J]. 中国环境科学, 2019,39(6):2291-2298. Zhang Y Y, Wang H Q, Xiao H, et al. Winter spatial distribution and source apportionment of water-soluble ions in PM2.5, Northeast Asia [J]. China Environmental Science, 2019,39(6):2291-2298.
[18] 郭振东,朱 彬,王红磊,等.长江三角洲霾天气PM2.5中水溶性离子特征及来源解析 [J]. 中国环境科学, 2019,39(3):928-938. Guo Z D, Zhu B, Wang H L, et al. Characteristics and source analysis of water-soluble ions in PM2.5in the haze weather over in Yangte River Delta [J]. China Environmental Science, 2019,39(3):928-938.
[19] Guo F T, Ju Y H, Wang G Y, et al. Inorganic chemical composition of PM2.5emissions from the combustion of six main tree species in subtropical China [J]. Atmospheric Environment, 2018,189:107-115.
[20] Ozgen S, Becagli S, Bernardoni V, et al. Analysis of the chemical composition of ultrafine particles from two domestic solid biomass fired room heaters under simulated real-world use [J]. Atmospheric Environment, 2017,150:87-97.
[21] 刘亚男,钟连红,韩力慧,等.民用燃料烟气中气态污染物及水溶性无机离子的排放 [J]. 中国环境科学, 2019,39(8):3225-3232. Liu Y N, Zhong L H, Han L H, et al. Emission of gaseous pollutants and water-soluble inorganic ions from civil fuel flue gas [J]. China Environmental Science, 2019,39(8):3225-3232.
[22] Kiehl T J, Briegleb P B. The relative roles of sulfate aerosols and greenhouse gases in climate forcing [J]. Science, 1993,260(5106):311-314.
[23] Binkowski F S, Roselle S J. Models-3community multiscale air quality (CMAQ) model aerosol component-1. Model description [J]. Journal of Geophysical Research: Atmospheres, 2003,108(D6):4183.
[24] Whitby K T. The physical characteristics of sulfur aerosols [J]. Atmospheric Environment, 1978,12(1-3):135-159.
[25] Elleman R A, Covert D S. Aerosol size distribution modeling with the community multiscale air quality modeling system in the Pacific Northwest: 3. Size distribution of particles emitted into a mesoscale model [J]. Journal of Geophysical Research: Atmospheres, 2010, 115(D3):D03204.
[26] Madureira J, Slezakova K, Silva A I, et al. Assessment of indoor air exposure at residential homes: Inhalation dose and lung deposition of PM10, PM2.5and ultrafine particles among newborn children and their mothers [J]. Science of the Total Environment, 2020,717:137293.
[27] Nemmar A, Hoet P H M, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans [J]. Circulation, 2002, 105(4):411-414.
[28] Zhang H F, Wang S X, Hao J M, et al. Chemical and size characterization of particles emitted from the burning of coal and wood in rural households in Guizhou, China [J]. Atmospheric Environment, 2012,51:94-99.
[29] Park S S, Sim S Y, Bae M S, et al. Size distribution of water-soluble components in particulate matter emitted from biomass burning [J]. Atmospheric Environment, 2013,73:62-72.
[30] Goetz J D, Giordano M R, Stockwell C E, et al. Speciated online PM1from South Asian combustion sources-part 1: fuel-based emission factors and size distributions [J]. Atmospheric Chemistry and Physics, 2018,18(19):14653-14679.
[31] Lipsky E M, Robinson A L. Design and evaluation of a portable dilution sampling system for measuring fine particle emissions from combustion systems [J]. Aerosol Science and Technology, 2005,39(6):542-553.
[32] Cheng Y, Kong S F, Yan Q, et al. Size-segregated emission factors and health risks of PAHs from residential coal flaming/smoldering combustion [J]. Environmental Science and Pollution Research, 2019, 26(31):31793-31803.
[33] Liu X, Kong S F, Yan Q, et al. Size-segregated carbonaceous aerosols emission from typical vehicles and potential depositions in the human respiratory system [J]. Environmental Pollution, 2020,264:114705.
[34] Li N, Sioutas C, Cho A, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage [J]. Environmental Health Perspectives, 2003,111(4):455-460.
[35] Chalupa D C, Morrow P E, Oberdorster G, et al. Ultrafine particle deposition in subjects with asthma [J]. Environmental Health Perspectives, 2004,112(8):879-882.
[36] Chen Y J, Sheng G Y, Bi X H, et al. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China [J]. Environmental Science & Technology, 2005, 39(6):1861-1867.
[37] Calvo A I, Martins V, Nunes T, et al. Residential wood combustion in two domestic devices: Relationship of different parameters throughout the combustion cycle [J]. Atmospheric Environment, 2015,116:72-82.
[38] Alves C, Gonçalves C, Fernandes A P, et al. Fireplace and woodstove fine particle emissions from combustion of western Mediterranean wood types [J]. Atmospheric Research, 2011,101(3):692-700.
[39] Sen A, Mandal T K, Sharma S K, et al. Chemical properties of emission from biomass fuels used in the rural sector of the western region of India [J]. Atmospheric Environment, 2014,99:411-424.
[40] Chantara S, Thepnuan D, Wiriya W, et al. Emissions of pollutant gases, fine particulate matters and their significant tracers from biomass burning in an open-system combustion chamber [J]. Chemosphere, 2019,224:407-416.
[41] Diapouli E, Popovicheva O, Kistler M, et al. Physicochemical characterization of aged biomass burning aerosol after long-range transport to Greece from large scale wildfires in Russia and surrounding regions, Summer 2010 [J]. Atmospheric Environment, 2014,96:393-404.
[42] Li J, Posfai M, Hobbs P V, et al. Individual aerosol particles from biomass burning in southern Africa: 2,Compositions and aging of inorganic particles [J]. Journal of Geophysical Research: Atmospheres, 2003,108(D13):8484.
[43] Akagi S K, Craven J S, Taylor J W, et al. Evolution of trace gases and particles emitted by a chaparral fire in California [J]. Atmospheric Chemistry and Physics, 2012,12(3):1397-1421.
[44] Zhang Q, Jimenez J L, Worsnop D R, et al. A case study of urban particle acidity and its influence on secondary organic aerosol [J]. Environmental Science & Technology, 2007,41(9):3213-3219.
[45] Pye H O T, Nenes A, Alexander B, et al. The acidity of atmospheric particles and clouds [J]. Atmospheric Chemistry and Physics, 2020, 20(8):4809-4888.
[46] Shi G L, Xu J, Peng X, et al. pH of aerosols in a polluted atmosphere: Source contributions to highly acidic aerosol [J]. Environmental Science & Technology, 2017,51(8):4289-4296.
[47] Andreae M O, Browell E V, Garstang M, et al. Biomass-burning emissions and associated haze layers over Amazonia [J]. Journal of Geophysical Research: Atmospheres, 1988,93(D2):1509-1527.
[48] Thepnuan D, Chantara S, Lee C T, et al. Molecular markers for biomass burning associated with the characterization of PM2.5and component sources during dry season haze episodes in Upper South East Asia [J]. Science of the Total Environment, 2019,658:708-722.
[49] 杨国威,孔少飞,郑淑睿,等.民用燃煤排放分级颗粒物中碳组分排放因子 [J]. 环境科学, 2018,39(8):3524-3534. Yang G W, Kong S F, Zheng S R, et al. Size-resolved emission factors of carbonaceous particles from domestic coal combustion in China [J]. Environmental Science, 2018,39(8):3524-3534.
[50] Boucher O, Anderson T L. General circulation model assessment of the sensitivity of direct climate forcing by anthropogenic sulfate aerosols to aerosol size and chemistry [J]. Journal of Geophysical Research: Atmospheres, 1995,100(D12):26117-26134.
[51] 严 沁,孔少飞,刘海彪,等.中国民用燃煤排放细颗粒物中水溶性离子清单及减排启示 [J]. 中国环境科学, 2017,37(10):3708-3721. Yan Q, Kong S F, Liu H B, et al. Emission inventory of water soluble ions in fine particles from residential coal burning in China and implication for emission reduction [J]. China Environmental Science, 2017,37(10):3708-3721.
Size distribution of water-soluble ions in particles emitted from domestic firewood burning.
FAN Ze-wei1, KONG Shao-fei1*, YAN Qin1, ZHENG Shu-rui1, ZHENG Huang1, YAO Li-quan1, WU Jian1, ZHANG Ying1, NIU Zhen-zhen1, WU Fang-qi1, CHENG Yi1, ZENG Xin1, QIN Si1, LIU Xi1, YAN Ying-ying1, QI Shi-hua1,2
(1.School of Environmental Studies, China University of Geosciences, Wuhan 430074, China;2.State Key Laboratory of Biogeology and Environmental Geology, China University of Geosciences, Wuhan 430074, China)., 2021,41(5):2064~2072
Six kinds of domestic firewood were burned in a laboratory. The emitted particles in fourteen sizes were collected using a dilution sampling system and an electrical low-pressure impactor. The emission characteristic and size-resolved emission factors (EFs) of eight types of water-soluble ions were identified. Results showed that the EFs of Ca2+exhibited a bimodal size distribution, with peaks of 0.14 and 0.16mg/kg for particles within 0.25~0.38 and 2.5~3.6µm, respectively. The EFs of other ions were characterized by a unimodal size distribution. The EFs of NH4+, NO3−and SO42−peaked at 0.25~0.38µm, with peaks of 0.41, 0.58 and 0.84mg/kg, respectively. The EFs of K+and Cl−exhibited highest values at 0.15~0.25µm of 0.89 and 0.99mg/kg, respectively. The mass median diameters of total water-soluble ions from firewood burning were (0.30±0.07)µm, and those of individual ions ranged in 0.24~0.44µm. The EFs of water-soluble ions in PM0.094, PM0.94, PM2.5and PM10were 1.04~9.33, 5.00~48.87, 5.46~52.00 and 6.14~53.68mg/kg, respectively. The ratios of K+/Cl−, K+/NO3−, K+/SO42−and SO42−/NO3−in particles emitted from firewood burning varied with particle size. Their primary emission values should be emphasized when they were used in source apportionment and smoke aging researches. The anion/cation equivalent ratios of PM10from firewood burning were 0.80±0.11. The acidity of PM0.94and PM2.5were higher than those of PM0.094and PM2.5~10. This study is significant to establish emission inventory of size-resolved water-soluble ions, update and improve the parameter settings of corresponding climate and air quality models, and identify the evolution mechanisms of smokes during transport and aging.
domestic firewood burning;dilution tunnel;water-soluble ions;size distribution;emission factors
X51
A
1000-6923(2021)05-2064-09
樊泽薇(1998-),女,河北邢台人,中国地质大学(武汉)硕士研究生,主要研究方向为民用燃料燃烧排放污染物清单构建.
2020-10-09
国家重点研发计划(2017YFC0212602;2016YFA0602002);国家自然科学基金资助项目(41830965;42077202)
* 责任作者, 教授, kongshaofei@cug.edu.cn

