Direct contact moxibustion promotes apoptosis of gastric cancer cells in rats by regulating intestinal flora
PAN Lijia,MA Shuya,WEN Jing,ZHANG Xiaoqi,XING Haijiao,JIA Chunsheng
PAN Lijia,MA Shuya,WEN Jing,ZHANG Xiaoqi,XING Haijiao,JIA Chunsheng,College of Acupuncture and Moxibustion,Hebei University of Chinese Medicine,Shijiazhuang 050200,China
Abstract OBJECTIVE:To examine whether direct contact moxibustion (DCM) can prevent and treat gastric cancer(GC)by regulating intestinal flora in rats.METHODS:Male Wistar rats were randomly divided into normal group,normal+DCM control group,model group,and model+DCM group.Gastric cancer rats were induced by N-methyl-N-nitro-N-nitrosoguanidine (MNNG,20 mg/mL) by gavage.At the same time,the model rats and normal rats were given DCM at Zusanli (ST36),Weishu(BL21),and Zhongwan (CV12) for 16 weeks.After treatment,gastric tissues were collected to analyze the pathological changes and the apoptosis of gastric mucosa cells.In addition,the cecal stool was taken and analyzed by 16s rRNA sequencing.RESULTS:Gastric cancer-like pathological changes and different abundance of the intestinal flora were found in the model group.DCM promoted mucosa tissue apoptosis and regulated the abnormal changes of the intestinal microflora caused by MNNG;DCM also inhibited the growth of Ruminococcaceae and Prevotellaceae flora and promoted the growth of probiotic Akkermansia.Furthermore,DCM made the composition and abundance of intestinal microflora in the GC rats tending to the normal rats.CONCLUSION:DCM stimulating Zusanli (ST36),Weishu (BL21),and Zhongwan (CV12) promoted the apoptosis of gastric mucosa and delayed the progression of gastric cancer,possibly by decreasing Ruminococcaceae and Prevotellaceae bacteria(bacteria that produce short-chain fatty acids in the intestine) and promoting the growth of probiotic Akkermansia.
Keywords:moxibustion;methylnitronitrosoguanidine;stomach neoplasms;gastrointestinal microbiome
INTRODUCTION
Gastric cancer (GC) is the fifth most common cancer and the third leading cause of cancer-related death worlwide.1Although gastric cancer incidence and mortality rates continue to decline,this malignancy is still the main contributor to the global disability-adjusted life-year burden.2Surgery,radiotherapy and chemotherapy are still the commonly methods for the treatment of gastric cancer.3Yet,these therapies are associated with certain side effects,such as nausea and vomiting,4fatigue,5and neutropenia.6
Recent studies have shown the moxibustion,a vital part of acupuncture therapy,may alleviate cancer pain,7reduce leukopenia,8cancer-related fatigue,9and gastrointestinal reaction,10as well as improve body immunity11and patients'quality of life12after chemotherapy.
Direct contact moxibustion (DCM) refers to a traditional moxibustion therapy in which moxa of appropriate size is directly placed on the skin and ignited.Clinical studies have shown that DCM can reduce the neutrophil-lymphocyte ratio(NLR)and improve the quality of life in patients with advanced gastric cancer.13DCM could significantly reduce the application of opioids and increase T lymphocyte subsets (CD3+,CD4+,and CD4+/CD8+) with moderate and severe cancer pain.14Moreover,compared with mild moxibustion and ginger-separated moxibustion,DCM is more effective in improving blood routine,fatigue,insomnia,and pain.15
DCM can lead to scar formation after therapy.According to an ancient saying,"Huang Di Ming Tang Jiu Jing",16if the skin is scared after treatment,the patients will most probably recover from disease;otherwise,they will not.Modern researches suggested that direct moxibustion can cause aseptic suppuration of local tissues.Hence,the immune system is being challenged,which is helpful to increase the number of white blood cells and phagocytes and enhance the body's ability to resist diseases.Additionally,after infection and suppuration at acupoints,bacteria can produce endotoxins,which then stimulate the immune reaction.17A number of clinical studies have shown that scar moxibustion combined with Traditional Chinese and Western Medicine can regulate immune function in tumor patients and improve their quality of life.11,18,19
Intestinal flora is a complex micro-ecosystem in the human body.The imbalance of intestinal flora has a vital role in the occurrence and development of oral carcinoma,esophageal cancer,gastric cancer,gastric mucosa-associated lymphoid tissue (MALT) lymphoma,gastric diffuse large B cell lymphoma (DLBCL),colorectal cancer,pancreatic cancer,liver cancer,and gallbladder cancer,through different mechanisms,such as DNA damage,activation of carcinogenic pathways,production of carcinogenic metabolites,stimulation of chronic inflammation and suppression of anti-tumor immunity.20,21This study examined whether DCM can prevent and treat gastric cancer by regulating intestinal flora in rats.
MATERIALS AND METHODS
Animal modeling and grouping
A total of 24 healthy male Wistar rats,weighing(180±20) g,were provided by Beijing Vital River Laboratory Animal Technology [license No.SCXK-(Jing)2016-0006 Beijing,China].All the animals were raised in the Hebei Key Laboratory of the Specific Effect of Acupuncture and Moxibustion.Animals had a free access to food and water.All animal studies were done in compliance with the regulations and guidelines of Hebei University of Chinese Medicine institutional animal care (batch No.DWLL2019007) and conducted according to the AAALAC and the IACUC guidelines.All rats were anesthetized by intraperitoneal injection of 10 g/L pentobarbital sodium (30 mg/kg).All measures were designed to minimize the suffering of experimental animals.
After 1 week of adaptive feeding,the rats were randomly divided into 4 groups (6 rats in each group):normal group,normal+DCM control group,model group,model+DCM group.The gastric cancer model was established by intragastric administration with N-methyl-N-nitro-N-nitrosoguanidine (MNNG,TCI,Tokyo,Japan).The model group and the model+DCM group were given a fresh configuration of 20 mg/mL MNNG (dissolved 1 g MNNG in distilled water of 50 mL),and the intragastric dose was 100 g/mL,once a day for a total of 16 weeks.22The normal group and the normal+DCM group were given the same dose of 100 g/mL saline (Shijiazhuang Siyao Co.,Ltd.,Shijiazhuang,China).
DCM intervention
The model was made by intragastric administration,and DCM treatment was given at the same time.After fixation,bilateral Zusanli (ST36),bilateral Weishu(BL21),and Zhongwan (CV12) were chosen.23The rats were treated with a self-made moxa cone (moxa were from Nanyang,Hanyi Trading Co.,Ltd.,Henan Province,40∶1)with a diameter of 3 mm and a height of 3 mm (Figure 1A,1B).One moxa cone was placed on every acupoint;the burning time of each moxa was 12-15 s.The treatment time of each rat,including fixed mice and fixed moxa,was approx.8 min.The rats were treated every 3 days to keep the acupoint skin in a scab state(Figure 1C)for 16 consecutive weeks.
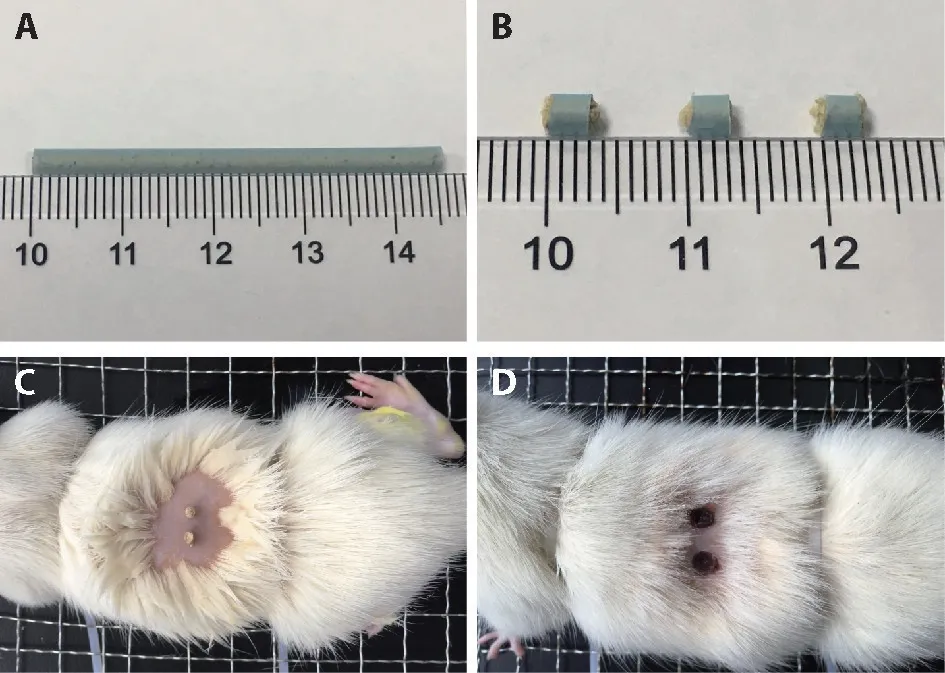
Figure 1 Direct contact moxibustion intervention
After treatment,rats were anesthetized by intraperitoneal injection of 10 g/L pentobarbital sodium(30 mg/kg)(Sigma,St.Louis,USA).Then,the gastric tissues of rats were preserved in 4% paraformaldehyde buffer(Servicebio,Wuhan,China).Afterward,the cecal contents were aseptically collected and stored in a refrigerator at-80 ℃for 16s rRNA sequencing.
Hemotoxylin and eosin(HE)staining
Upon collection,gastric tissue was fixed in 4%paraformaldehyde,dehydrated,embedded,cut into 4 µm sections,and placed on slide glass(Anhydrous ethanol,xylene,and neutral gum were all come from National Pharmaceutical Group Chemical Reagent Co.,Ltd.,Shanghai,China).Samples were then stained with HE(HE dye solution,Servicebio,Wuhan,China),mounted,and analyzed using a microscope (Leica DM400 B LED Germany).
TUNEL assay
Apoptosis of histiocytes was observed by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-biotin nick-end labeling(TUNEL) method.After the tissue sections were dewaxed with xylene and hydrated with gradient ethanol,a histochemical pen was utilized to draw a circle around the tissue (to prevent the flow of liquid),and the protease K (20 µg/mL,Servicebio Company,Wuhan,China) was added to cover the tissue.After incubating at 37 ℃for 25 min,the slides were washed in PBS (pH7.4) for 3 times,the tissues were covered with membrane breaking solution (Servicebio Company,Wuhan,China) in the circle for 20 min at room temperature and then washed with PBS 3 times.The TUNEL reaction mixture (TUNEL Kit,Roche,Basel,Switzerland) was prepared according to the following procedures:(a) reagent 1 (TdT) and reagent 2 (dUTP)were mixed at 1∶9 and used to cover the tissue;(b)the tissue was then incubated in 37 ℃incubator for 2 h and mixed with goat serum(Servicebio Company,Wuhan,China);(c)tissue was incubated at room temperature for 30 min;endogenous peroxidase was blocked with a 3%hydrogen peroxide solution,which was later incubated for 15 min at room temperature without light;(d)the tissue was incubated with horseradish peroxidase in a 37 ℃incubator for 30 min.The slices were then rinsed with PBS;the freshly prepared DAB chromogenic solution was dripped into the circle (Servicebio Company,Wuhan,China).The samples were observed under a microscope,and the time for the color development was controlled (the brown color indicated a positive result).The slices were rinsed with tap water to stop coloring.After hematoxylin re-staining,the slices were dehydrated and transparent in gradient alcohol and xylene and sealed with neutral gum.According to the microscopic observation,the brown granules in the nucleus are positive cells,which are apoptotic cells.
Five unrepeated visual fields were randomly observed at 40 × magnification;photographs were taken with a Leica DM400 Microscope Camera,the number of positive cells calculated by Image-ProPlus software.The apoptosis index was defined as follows:the number of positively stained cells counted in five fields divided by the total number of cells in these five visual fields.24
16SrRNA sequencing
The total bacterial genomic DNA was extracted from fecal samples by QIAamp DNA Mini Kit (Qiagen,Hilden,Germany).The V3-V4 region of the 16SrRNA gene of total fecal DNA was amplified by universal primers 319F and 806R:319F:5'ACTCCTACGGGAGGCAGCAG 3' and 806R:5'GGACTACHVGGGTWTCTAAT 3'.25The products were then purified,quantified,and homogenized to form a sequencing library.The constructed library was tested for quality.The high-throughput sequencing was carried out by using the Illumina HiSeq 2500(Illumina,USA).The similarity of the clustering sequences was 97%,and 0.005% of all sequences were used as a threshold to filter OTU.Based on the Silva(Release132,http://www.arbsilva.de) taxonomic database,the OTU obtained was taxonomically annotated.
Data analysis
The data were processed using SPSS v18.0 software(IBM Corp.Armonk,NY,USA).The apoptosis index was expressed as the mean±standard deviation(±s).If the data were normally distributed,the least significant difference analysis was used;if the data were non-normally distributed,the Kruskal-Wallis monument was applied;if there were significant differences,the Mann-WhitneyUtest was used for pairwise comparison.P<0.05was consideredstatisticallysignificant.
Microbial data analysis:according to the sequencing results,the species diversity was analyzed at the OUT level.QIIME software was used for Beta diversity analysis,including principal coordinates analysis(PCoA),principal component analysis (PCA),hierarchical clustering with an unweighted pair-group method with arithmetic mean (UPGMA).At the genus level,species abundance in TOP20 was selected for taxonomic annotation and difference analysis,and the Wilcox test was used for between-groups comparison.The linear discriminant analysis (LDA)effect size (LEfSe) method was used to identify taxa with relative differential abundance in each experimental group,and nonparametric Kruskal-Wallis and rank checks were used for each set of data in this section analysis.The α value of the test was set to <0.05.Then,the LDA was used to estimate the influence of the abundance of each component (species)on the difference effect,and the threshold of the logarithmic LDA score of the distinguishing feature was set to >4.0.
RESULTS
Effect of DCM on the gastric mucosa in GC rats
The pathological changes caused by the intragastric administration of MNNG mainly occurred in the forestomach.As is shown in Figure 2,the layers of squamous epithelium,submucosa,and muscularis mucosa in the normal group and normal+DCM group were clear.In those groups,no hyperplasia and keratosis were observed (Figure 2A,2B),suggesting that DCM does not affect normal rats' gastric mucosa.Nonetheless,the gastric mucosa and cell polarity in the model group were disordered.Additionally,the squamous epithelium was destroyed,squamous epithelial cells proliferated and keratinized,which also spread down to the muscle layer (Figure 2C),thus suggesting squamous cell carcinoma-like pathological changes.26In the model+DCM group,epithelial papillary hyperplasia and papilloma were observed,but the squamous epithelium did not invade the submucosa (Figure 2D).It is suggested that DCM can alleviate the pathological progression and prevent further carcinogenesis of GC rats induced by MNNG.
The positive cells were expressed in all the four groups,but the expression in the model group and model +DCM group was significantly higher than that in the normal group and normal +DCM group (P <0.05,model group,model +DCM group vs normal group and normal +DCM group),and the staining in the model +DCM group was stronger than that in the model group(P<0.05).
Effect of DCM on the gastric mucosa apoptosis in GC rats
The detection of gastric mucosa apoptosis by TUNEL assay.The positive cells were expressed in all four groups (Figure 3).The difference in the apoptosis index between the four groups was statistically significant (Kruskal-WallisH=13.681P=0.003).As shown in Figure 4,there was no significant difference between the normal group and the normal +DCM group (Mann-Whitney'sUtest,P=0.773).The apoptosis index in the gastric mucosa was significantly increased in the model group compared with the normal group and the normal+DCM group(Mann-Whitney'sUtest,P=0.033),the model +DCM group was also significantly higher than that in the normal group and the normal+DCM group(Mann-Whitney'sUtest,P=0.011).Compared with the model group,there was a significant increase in the model+DCM.(Mann-Whitney'sUtest,P=0.037)(Figure 4).The analysis above indicated that DCM promoted apoptosis of gastric mucosal tissue in rats with gastric cancer.

Figure 2 Gastric mucosa of rats htoxylin-eosin (HE) staining in each group(×200)
Effect of DCM on intestinal flora in GC rats
The diversity analysis,including PCoA,PCA,cluster analysis,are shown in Figure 5.Diversity analysis results showed differences in intestinal flora between groups.As shown in Figure 5A,the plane distribution areas of the four groups overlapped,and the demarcation was not clear.The PC1 analysis implied a farther distance between the model+DCM group and the normal group,the normal+DCM group,and the model group,respectively.However,the distance between the normal group,the normal +DCM group,and the model group were closer(Figure 5B).

Figure 3 Gastric mucosa of rats by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate(dUTP)-biotin nick-end labeling (TUNEL) assaying in each group(×400)
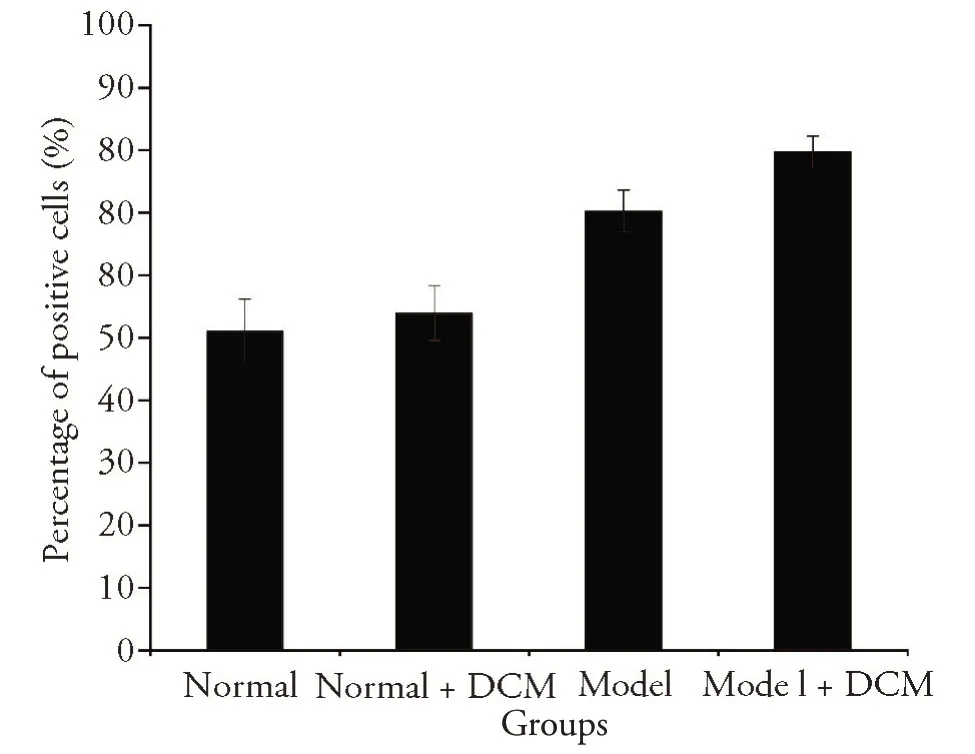
Figure 4 The effect of DCM on the apoptosis index of gastric mucosa in rats with gastric cancer by TUNEL
The PC2 analysis results showed that the distance between the model+DCM group and the normal group was closer than the distance between the other groups (Figure 5D).The UPGMA was applied to cluster the samples in order to judge the similarity of species composition among the samples.In Figure 5C,the species composition of the model+DCM group was similar to that of the normal group,and the composition of the normal+DCM group and model group were similar.The results showed that the species composition of microflora changed after the administration of MNNG,the intervention of DCM changed the species of GC rats,and the changes were close to normal rats.The composition of microflora was also changed after DCM on normal rats,which was close to that of model rats.
At the genus level,species abundance in TOP20 was selected for taxonomic annotation and difference analysis(Figure 6A),and the Wilcox test was used for inter-group analysis (Figure 6C).There were significant differences in Akkermansia,Ruminococcaceae_ UCG-005,Ruminococcaceae_NK4A214_group,[Eubacterium]_coprostanoligenes_group,Blautia,and Prevotellaceae_UCG-003 among groups (Figure 6C).In the model group,Prevotellaceae_UCG-003 (4.99%) and Ruminococcaceae_UCG-005 (14.7%) significantly increased compared to other groups(P<0.05).The obvious increase in Akkermansia (27.09%) was observed in the model+DCM group,while the content of[Eubacterium]_coprostanoligenes_group (5.55%) increased dramatically in the normal+DCM group.Blautia(2.63%,2.62%)(P<0.05)increased in both the model group and the model+DCM group,while Ruminococcaceae_NK4A214_group was reduced in the normal+DCM and model+DCM groups (5.61%,2.96%) (P<0.05).The heat map results showed similar composition and abundance of intestinal flora in the model+DCM group and the normal group (Figure 6B),thus suggesting that DCM can significantly adjust this abnormal change.The DCM also changed the intestinal flora of normal rats.
The LEfSe method was used to determine the microbial taxa with rich differences between experimental groups.There were significant differences in 13 floras among the four groups.As shown in Figure 7,the higher abundance microbial taxa in normal group were cultured_bacterium_g_Ruminococcaceae_NK4A214_group,g__Ruminococcaceae_NK4A-214_group,f__Peptococcaceae,g__uncultured_bacterium_f_Peptococcaceae,s__uncultured_bacterium_f_ Peptococcaceae;in the normal +DCM group were uncultured_bacterium_g__E[..]ium__coprostanoligenes_group,g_Eubacterium__coprostanoligenes_group;in model group were s__uncultured_bacterium_g_Ruminococcaceae_UCG_005,g__Ruminococcaceae_UCG_005,s__uncultured_bacterium_g_Prevotellaceae_ UCG_003,g__Prevotellaceae_UCG_003;in the model+DCM group were p__Verrucomicrobia,c__Verrucomicrobiae,f__Akkermansiaceae,g__Akkermansia,s__uncultured_bacterium_g_Akkermansia,o__Verrucomicrobiales.LEfSe analysis further explained the enrichment level and variation of the four groups,and the results showed that DCM changed the types and abundance of intestinal flora in normal rats and model rats.
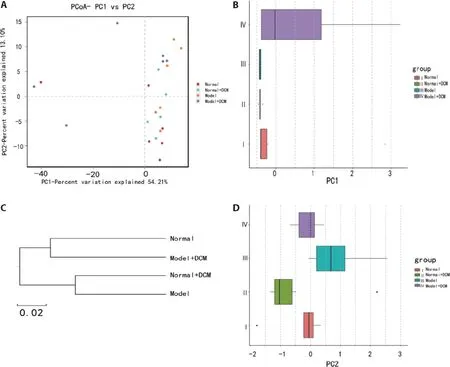
Figure 5 Effects of direct contact moxibustion(DCM)on the diversity of intestinal flora
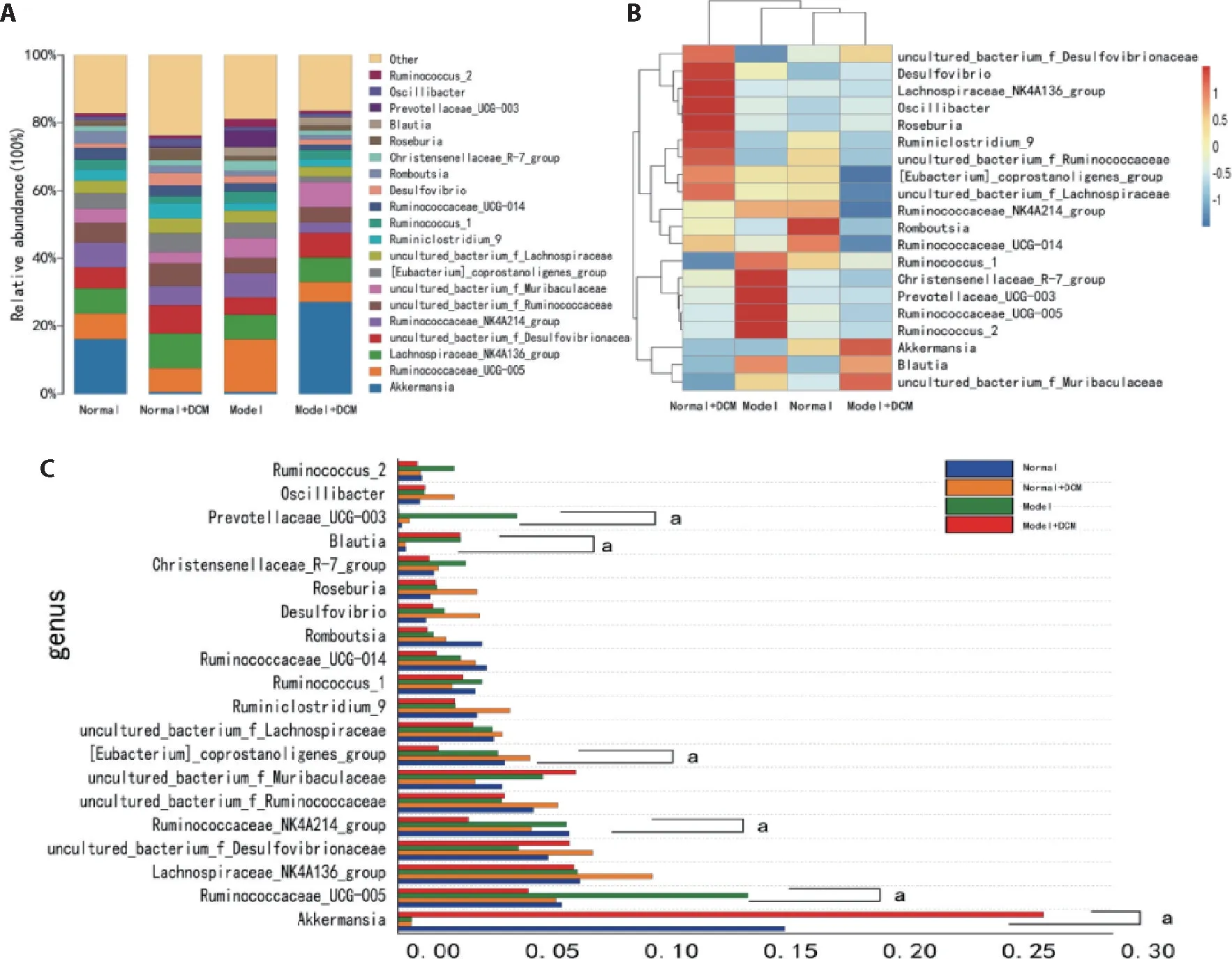
Figure 6 Effects of direct contact moxibustion on the composition of intestinal flora
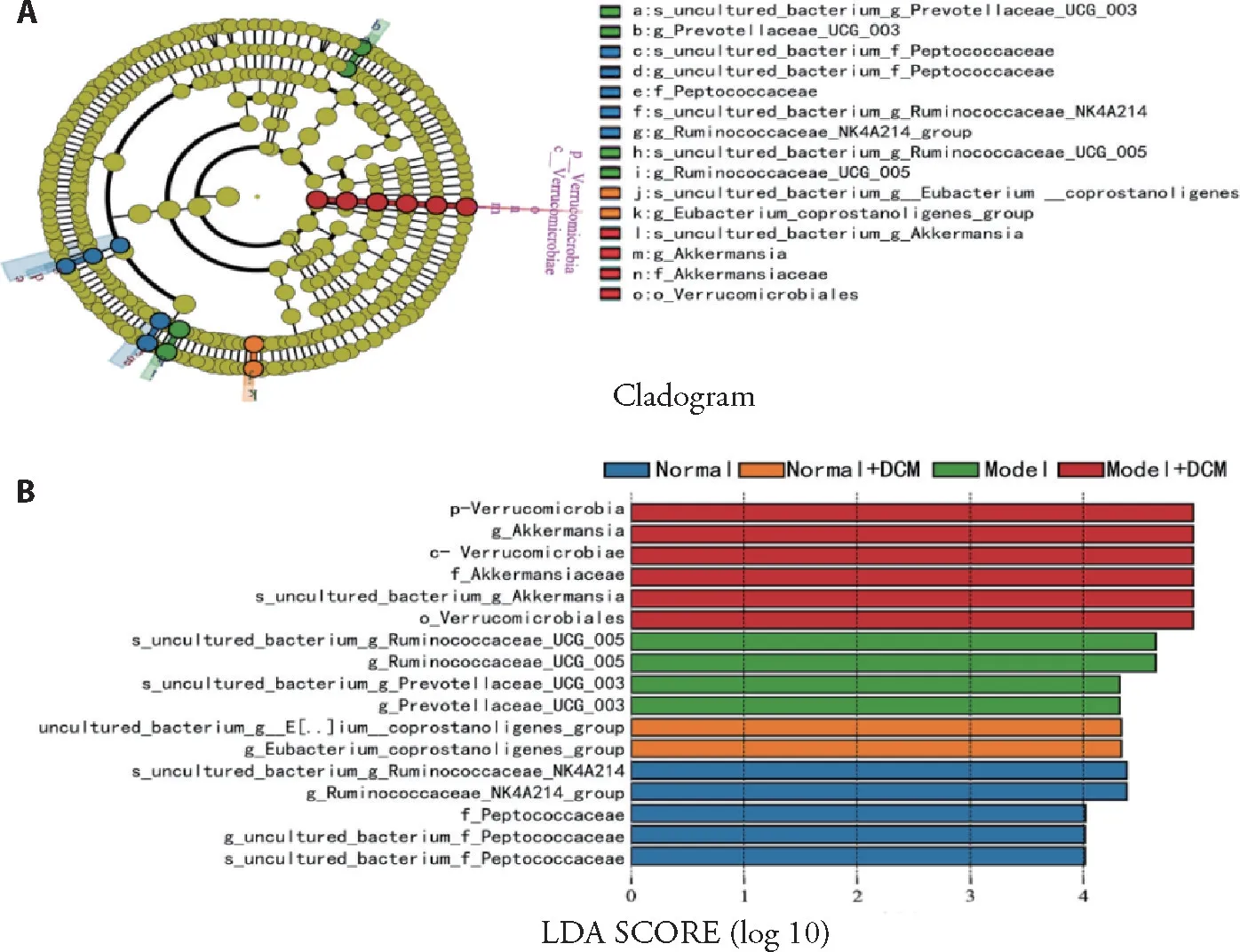
Figure 7 Composition characteristics of microbial community analyzed by LEFSe
DISCUSSION
DCM is a Traditional Chinese Medicine therapy for tumor treatment
In ancient times,moxibustion is a Traditional Chinese Medicine technique that promotes healing and is used to treat various diseases,including cancer.27Zusanli(ST36)is the sea point of the stomach meridian,which is commonly used to treat fatigue and health care.Zhongwan (CV12) is the influential point of Fu-organs,and the front-Mu point of the stomach and the Weishu(BL21)is the back-Shu point.These points are also commonly used points of acupuncture in regulating the immune function of patients with cancer.28The combined use of these points strengthens the body resistance,harmonizesQiand blood,and tonifies the spleen and stomach.
DCM can alleviate the pathological progress of gastric cancer and promote tissue cell apoptosis
N-methyl-N-nitro-N-nitrosoguanidine has been commonly used to establish squamous cell carcinoma model of forestomach.22,29In this study,our model group showed squamous cell proliferation and keratosis;cells proliferated downward into the muscular layer.In the model+DCM group,upper skin papillary hyperplasia and papilloma were observed,but the squamous epithelium did not invade the submucosa,which suggested that DCM can alleviate the pathological progression and prevent further carcinogenesis of GC rats induced by MNNG.
The histological evolution of gastric cancer includes superficial gastritis,followed by atrophic gastritis,intestinal metaplasia,and finally,dysplasia-gastric cancer.Before the occurrence of intestinal metaplasia in the gastric mucosa,apoptosis index and the cell proliferation index increased,and there was a positive correlation between cell apoptosis and cell proliferation.30In the stage of intestinal metaplasia-dysplasia-gastric cancer,the apoptosis index gradually decreases,while cell proliferation is still increased,and cell apoptosis is negatively correlated with cell proliferation.30In this study,the pathological process of the model+DCM group was in the stage of intestinal metaplasia and dysplasia.Compared with the model group,the pathological process was significantly relieved.The apoptosis of the model+DCM group was greater than that of the model group,indicating that DCM can delay gastric cancer rats'development and promote apoptosis of tissue cells.
The intestinal flora as the starting point for the study of the effective mechanism of DCM from the holistic approach
According to modern medical theory,the mechanism of moxibustion is a "comprehensive effect" produced by the physical and chemical factors of moxibustion combustion,combined with the special functions of acupoints and the meridians.Moxibustion can adjust the body's neuro-endocrine-immune network and exert a comprehensive regulatory role in disease prevention and treatment.17As the holistic view is also a vital principle of acupuncture treatment in Traditional Chinese Medicine,it is necessary to study DCM action mechanism from different approaches.The intestinal flora exerts a vital role in human health and disease through the interaction with the host immune cell group,from gastrointestinal inflammation and metabolic diseases to nervous,cardiovascular,and respiratory diseases.It is relevant to the disorder of the composition and function of intestinal flora.31Hence,we studied the intestinal flora to investigate the effective mechanism of DCM from the holistic point of view.
In previous studies,a large number of studies have confirmed that there is a relationship between microorganisms and tumor prevention,occurrence,development,and treatment,and flora imbalance leads to cancer susceptibility in a variety of ways.32On the one hand,the imbalance of microorganisms in the stomach can induce inflammation and increase the release of growth factors and vascular growth factors and stimulate the production of cytokines and chemokines and promote the occurrence and growth of tumors.33On the other hand,intestinal microbes can also impact the therapeutic effect of chemotherapy drugs by directly metabolizing drugs or regulating the host immune response through their metabolites.34,35The final result may be to enhance the drug's efficacy,weaken or resist the anticancer effect of the drug,and mediate the toxic and side effects of the drug.36
Effect of DCM on intestinal flora in rats with gastric cancer
Helicobacter pylori,which can induce chronic gastritis,are associated with more than 90% of (GC) cases of gastric cancer.However,other bacteria have also been associated with gastric cancer development.37Yet,it remains unclear whether these microbial changes are the driving factors of malignant lesions or the result of histological progress accompanied by the cascade of precancerous lesions.38Wanget al's research39suggested that the structure of microbial communities was phylogenetically diversified in patients with gastric cancer,and at the phylum level,Proteus,Bacteroides,Clostridium,and actinomycetes were the dominant bacteria;at the genus level,lactic acid bacteria,Shigella,nitro spirochetes,fungi Burkholderia,and uncultured spirochetes were enriched.While,there are few reports on the characteristics of the intestinal flora of rats with gastric cancer induced by MNNG.Our results revealed that the abundance of Ruminococcaceae_UCG_005,g__Prevotellaceae_UCG_003,and Blautia was relatively increased,and a decrease in Akkermansia in rats treated with MNNG,which was different from that observed in the model+DCM group and normal group.These data suggested that DCM can improve the changes of intestinal flora in rats with gastric cancer induced by MNNG.
Possible mechanism of DCM in the prevention and treatment of gastric cancer by improving intestinal flora
Castaño-Rodríguezet alin 2017 proposed three possible pathways of gastric cancer caused by related bacteria:(a)overgrowth of oral bacteria that promote inflammation in the stomach;(b)excessive abundance of bacteria capable of secreting lactic acid in the stomach;(c)enrichment of short-chain fatty acid (SCFA) production pathways in the stomach.40Previous studies have shown that the increase of bacterial SCFA level can induce abnormal proliferation and transformation of colonic epithelial cells(butyrate induced abnormal proliferation and transformation of colonic epithelial cells).41Both Ruminococcaceae and Prevotellaceae can produce short-chain fatty acids by catabolism.42,43Therefore,it is speculated that the mechanism of moxibustion scar treatment of gastric cancer may be related to reduced accumulation of SCFA in the intestine and inhibited abnormal proliferation and transformation of epithelial cells.
Akkermansia is a kind of bacteria that can degrade mucin in the intestine.It belongs to the verrucous microflora,which is closely related to host metabolism and immune reaction.It is negatively related to obesity,diabetes,inflammation,and metabolic disorder,and is a potential probiotic that indicates potential anti-inflammatory response,strengthens intestinal barrier function,and regulates intestinal resident flora through growth factors released from mucin degradation.44A previous study has purified the hypothetical protein Amuc-1434 from Akkermansia muciniphila.This protein can activate the apoptosis pathway by up-regulating the expression of TRAIL and inhibit the proliferation of colorectal cancer cell LS174T.45Moreover,oral administration of Ackermann can enhance the anti-tumor effect of PD-1 inhibitors and make immunotherapy more effective.34Akkermansia is also one of the important probiotics in cancer immunotherapy combined with microbiome transplantation.46In this study,the abundance of Akkermansia in the model group was significantly lower than that in the normal group and model+DCM group,which indicated that DCM can improve the intestinal flora of gastric cancer rats induced by MNNG and promote the growth of probiotic Akkermansia.
In this study,we also observed changes in the intestinal flora of the rats in the normal+DCM group,which was more similar to that of the model group after the diversity analysis.It is speculated that the change was related to the stress state caused by long-term DCM.Under stress,the body can release stress hormones,such as dopamine,norepinephrine,epinephrine,and cortisol,which can change the composition of intestinal microorganisms during stress response.47Eubacterium__coprostanoligenes is the marker bacteria in the normal+DCM group.Studies have confirmed that Eubacterium__coprostanoligenes is related to anxiety disorder and psychosocial stress.48,49
In conclusion,DCM stimulating Zusanli(ST36),Weishu (BL21),and Zhongwan (CV12) promotes the apoptosis of gastric mucosa and delays the progression of gastric cancer,possibly by decreasing Ruminococcaceae and Prevotellaceae bacteria (bacteria that produce short-chain fatty acids in the intestine) and promoting the growth of probiotic Akkermansia.
 Journal of Traditional Chinese Medicine2021年6期
Journal of Traditional Chinese Medicine2021年6期
- Journal of Traditional Chinese Medicine的其它文章
- In vivo anti-diarrheal activity of jujube honey on castor oil-induced diarrhea in mice
- Ethanolic extract of Puhuang (Pollen Typhae) modulates lipopolysaccharide-induced inflammatory response through inducible nitric oxide synthase/cyclooxygenase-2 signaling in RAW 264.7 macrophages
- Target prediction and activity verification for the antidepressant action of Huangqin(Radix Scutellariae Baicalensis)
- Pingchuan formula (平喘方) improves allergic asthma in mice through inhibiting nuclear factor-kappa B/mitogen-activated protein kinase signaling pathway
- Can Fig and Olive Ameliorate the toxicity Induced by 2-nitropropane in some organs of mice? role of inflammatory versus anti-inflammatory genes
- Efficacy of Renshen(Radix Ginseng)plus Fuzi(Radix Aconiti Lateralis Preparata)on myocardial infarction by enhancing autophagy in rats
