Different surgical combinations for neovascular glaucoma with and without vitreous hemorrhage
Abstract
•KEYWORDS:neovascular glaucoma; Conbercept; glaucoma drainage device implantation; vitrectomy; panretinal photocoagulation
INTRODUCTION
The most common causes of neovascular glaucoma (NVG) are diabetic retinopathy and central retinal vein occlusion in a clinic[1-2]. In NVG patients, the growth of new vessel causes peripheral anterior synechia that results in angle-closure and, finally, in elevated intraocular pressure (IOP)[3]. At present, vascular endothelial growth factor (VEGF) has been proven to play a critical role in neovascularization of NVG and other diseases. Anti-VEGF is able to effectively reduce neovascularization and vascular permeability for the purpose of eliminating anterior segment neovessels of NVG caused by ischemic ophthalmopathy and of effectively controlling IOP[1]. The long-term control of IOP for NVG depends on effectively filtered and sufficient pan-retinal photocoagulation laser (PRP). Trabeculectomy was adopted to control IOP, however, postoperative complications, such as shallow anterior chamber block and filtered opening proliferation and block, usually cause uncontrolled IOP. Until now, the implantation of EX-PRESS glaucoma drainage device has been superior to traditional trabeculectomy, because it produces fewer complications[4].
Recently, a new anti-VEGF agent,conbercept (Chengdu Kang Hong Biotech Co., Sichuan, Chengdu), has been shown to quickly regress choroidal neovascularization in age-related macular disease or pathological myopia[5-6].Some studies have suggested that intravitreal conbercept is an effective treatment for managing NVG that has fewer short-term postoperative complications[7],but the effect of conbercept on iris neovascularization is not fully known. This research aims to assess the clinical efficacy of intravitreal injection of conbercept combined with EX-PRESS glaucoma drainage device implantation and panretinal photocoagulation for NVG with and without vitreous hemorrhage (where the iridotrabecular touch is more than 180°).
SUBJECTS AND METHODS
PatientsIn this retrospective study, 39 eyes in 37 patients diagnosed with NVG with and without vitreous hemorrhage in the ophthalmology department of Xi’an No.1 Hospital between January 2016 and December 2017 were enrolled in this study. Participants were divided into two groups, based on whether they had vitreous hemorrhage. Twenty-one eyes in 20 patients without vitreous hemorrhage were in Group 1, and 18 eyes in 17 patients with vitreous hemorrhage were in Group 2. There were 21 male patients and 16 female patients aged 29-67 (average range: 49±7.9) years old (mean±SD). There were 23 patients with diabetic retinopathy, 12 patients with central retinal vein occlusion, and 2 patients with ocular ischemic syndrome. The inclusion criteria were that patients had angle-closure NVG (detected by ultrasonic biological microscope), and the patients’ general condition was good enough to tolerate surgery. Exclusion criteria included an open-angle or iridotrabecular touch less than 180°, synechiae closure, patients with a history of eye surgery, patients with other eye lesions or primary glaucoma, patients with significant complications during or after operation, and patients failing to follow up on time after the operation. This study adhered to the tenets of Declaration of Helsinki and obtained consent from Institutional Research Ethical Committee and patients.
Methods
IntravitrealconberceptinjectionThe intravitreal injection of conbercept was performed in the operating room. Topical anesthesia with eye drops of oxybuprocaine hydrochloride was given 10min before the intravitreal injection. Then the eyelids were opened with an eye speculum, and povidone iodine was used to disinfect the conjunctival sacs. Conbercept in the amount of 0.05 mL was injected into the vitreous cavity through the conjunctiva and sclera, 4 mm distant from the corneal limbus. The wound was pressed with a cotton swab for one minute after the needle was removed. After intravitreal injection, the operative eye was patched with tobramycin and dexamethasone ophthalmic ointment for one day. On the second day, the operative eye was examined through a slit-lamp microscope, and antibiotic eye drops continued to be administered for 3d.
EX-PRESSglaucomadrainagedeviceimplantationPatients without vitreous hemorrhage (Group 1) received an implantation of an EX-PRESS glaucoma drainage device (P50, Alcon In, USA) on the 4d after intravitreal injection. Posterior bulbar nerve block anesthesia was carried out. The preoperative preparation was the same as for the intravitreal injection. A conjunctival fornix-based incision was completed at 12∶00, and the sclera surface was cauterized for hemostasis. A 4 mm×3 mm trapezoidal scleral flap was completed and punctured into the anterior chamber with a number 25G syringe at the black and white junction of the sclera. After the needle was removed, the glaucoma drainage device was implanted through the puncture mouth. The handle was rotated to set the drain entrance towards the inner surface of the cornea. The drainage device was adjusted to ensure that it did not contact the internal surface of the cornea and iris. The anterior chamber depth of the operative eye was observed, and the scleral flap and conjunctiva were sutured with a 10-0 suture. Finally, the operative eye was patched with tobramycin and dexamethasone ophthalmic ointment.
PanretinalphotocoagulationPanretinal photocoagulation was performed two weeks after EX-PRESS implantation in cases without vitreous hemorrhage (Group 1). A multi-wavelength krypton yellow laser was selected; the spot diameter was 200-500 μm, and the exposure time was 0.1-0.2s. There were four laser sites in total (above the optic disc, below the optic disc, about one optic disc diameter on the nasal side of the optic disc, and outside the temporal vascular arch). There was one four-quadrant photocoagulation, and then there were three more in a subsequent period of 4wk. There were about 2000 total photocoagulation points. The patients were treated with nonsteroidal ophthalmic eye drops after the operation.
The23GvitrectomyandpanretinalphotocoagulationCases with vitreous hemorrhage (Group 2) received vitrectomy and panretinal photocoagulation. Posterior bulbar nerve block anesthesia was carried out. The preoperative preparation was the same as for the vitreous injection. Three 23G channels were built in the pars plana, and total vitrectomy was performed. The proliferating vascular membrane was removed, and panretinal photocoagulation was performed during the operation. Either disinfected air or silicon oil filled in the vitreous cavity according to the patient’s condition at the end of the operation.
ObservationandFollowupVisitThe observation indicators included preoperative best corrected visual acuity (BCVA), iris neovascularization, and preoperative IOP. Postoperative BCVA was recorded at 6mo, as was the postoperative IOP at 1d, 1wk, 1, 3, and 6mo. Recurrence of iris neovascularization and the incidence of postoperative complications were recorded. The fundus change was observed, and an fluoresence fundus angiography (FFA) examination was carried out one month after the panretinal photocoagulation. In cases where there were some nonperfusion areas in the retina, retinal photocoagulation was performed.
StatisticalAnalysisSPSS 21.0 software was used for statistical analysis. Mean±standard deviation (SD) indicates research data. The BCVA before and after the operation in the same group was inspected using a Kruskal-Wallis test. The IOP of the same group before and after operation was analyzed by one-way ANOVA followed by a LSDt-test;P<0.05 was considered statistically significant.
RESULTS
BestCorrectedVisualAcuityThe BCVA of 21 eyes in 20 patients without vitreous hemorrhage in Group 1 at the final follow up visit (6mo) was categorized into no light perception (NLP) in four cases (19.0%), light perception (LP) in three cases (14.3%), hand move (HM) in two cases (9.5%), count finger (CF) in three cases (14.3%), 0.01-0.04 in seven cases (33.3%), 0.05-0.25 in one case (4.8%), and above 0.3 in one case (4.8%). There was no significant difference between pre- and postoperative BCVA in 6mo (P=0.727) (Table 1).
The BCVA of 18 eyes in 17 patients with vitreous hemorrhage in Group 2 at the final follow up visit (6mo) was categorized into NLP in three cases (16.7%), LP in one case (5.6%), HM in two cases (11.1%), FC in two cases (11.1%), 0.01-0.04 in four cases (22.2%), 0.05-0.25 in four cases (22.2%), and above 0.3 in two cases (11.1%). There was significant difference between pre- and postoperative BCVA in 6mo (P=0.018) (Table 2).

Table 1 Preoperative and postoperative BCVA at final follow up visit in unmerged vitreous hemorrhage group (Group 1)
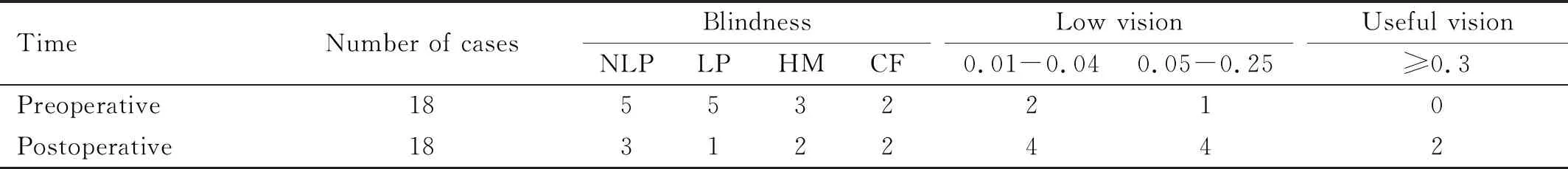
Table 2 Preoperative and postoperative BCVA at final follow up visit in merged vitreous hemorrhage group (Group 2)
PreoperativeandPostoperativeIOPThe postoperative IOP of patients without vitreous hemorrhage in Group 1 was 20.5±4.3 mmHg (1d), 19.6±3.8 mmHg (1wk), 20.1±3.7 mmHg (1mo), 19.9±4.2 mmHg (3mo), and 19.3±2.9 mmHg (6mo). The postoperative IOP at each time point was statistically different from the preoperative IOP (P<0.05), and the comparative difference of postoperative IOP at each time point is of no statistical significance (P>0.05). The postoperative IOP of patients with vitreous hemorrhage in Group 2 was 22.3±3.7 mmHg (1d), 20.6±2.8 mmHg (1wk), 20.4±3.8 mmHg (1mo), 18.9±4.1 mmHg (3mo), and 19.3±3.4 mmHg (6mo). The postoperative IOP of every time point was significantly lower than the preoperative IOP in Group 2 (P<0.05).
RecurrenceofIrisNeovascularizationThe success rate of surgery was 90.5% in Group 1 and two eyes (9.5%) without vitreous hemorrhage (Group 1) experienced postoperative recurrence of iris neovascularization. The success rate of surgery was 94.4% in Group 2 and postoperative recurrence of iris neovascularization was observed in one eye (5.6%) in the vitreous hemorrhage group (Group 2) at 3mo postoperative. The iris neovascularization disappeared within one week after all three eyes received another intravitreal injection of 0.05 mL conbercept.
PostoperativeComplicationsIn Group 1, two eyes in two patients had anterior chamber hemorrhage, and one eye in one patient had vitreous hemorrhage, which was absorbed after symptomatic treatment. The retinal nonperfusion area of three eyes in three cases in Group 1 and the retinal nonperfusion area of two eyes in two cases in Group 2 were found by FFA one month after the panretinal photocoagulation and the supplemental photocoagulations of retinal nonperfusion areas were performed. In the 6mo follow up, the IOP of three eyes in three cases in Group 1 were higher than 21 mmHg; meanwhile, the IOP of two eyes in two cases in Group 2 were higher than 21 mmHg. Three kinds of IOP-lowering medications were given. Among them, the IOP of three cases (three eyes) was controlled within 21 mmHg, and that of two cases (two eyes) was not controlled within 21 mmHg.
DISCUSSION
NVG is a refractory glaucoma caused by long-term ischemia, hypoxia, or inflammation of the retina, which causes the increased concentration of VEGF. VEGF can induce the formation of neovascularization or neovascular membrane on the iris surface and the anterior chamber angle, on the trabecular meshwork occlusion, and on the peripheral anterior synechiae, which then results in an increase in IOP. The high IOP, ocular ischemia, and hypoxia further damage the functions of the optic nerve and retina[3]. Especially when combined with vitreous hemorrhage, this is often very difficult for ophthalmologists to deal with. Common causes of NVG include central retinal vein occlusion, proliferative diabetic retinopathy, and ocular ischemic syndrome. Traditional therapy is often ineffective for NVG. The IOP of NVG has been hard to control through a single medical treatment. The long-term clinical effect of a conventional trabecular filtering operation and drainage valve implantation has usually failed, because neovascularization and occlusion in the filtrating canal occurred after the operation. Inaccurate cyclocryotherapy or cyclophotocoagulation usually resulted in eye atrophy or in uncontrolled IOP, which eventually led to the loss of visual function.
A great deal of the literature has confirmed that VEGF plays a key role in the stimulation of neovascularization of NVG and other diseases. VEGF is released into the vitreous body and anterior chamber, which promotes the growth of the neovascular membrane of the iris and chamber angle, leading to anterior synechia of the iris and angle-closure and to increased IOP, which seriously impairs visual function[8]. Anti-VEGF treatment can effectively reduce the activity of neovascularization and the permeability of blood vessels, eliminate the anterior segment neovascularization of NVG caused by ischemic ophthalmopathy disappearance, and effectively assist in the control of IOP[9-10]. The approval of the first anti-VEGF agents for the treatment of neovascular age-related macular degeneration one decade ago marked the beginning of a new era in the management of several sight-threatening retinal diseases. Since then, emerging evidence has demonstrated the utility of anti-VEGF agents for an adjuvant treatment of other ocular diseases characterized by elevated VEGF levels, such as NVG[11]. Conbercept is a new anti-VEGF fusion protein and has significant effects on neovascularization, which has been adopted in the treatment of age-related macular degeneration, and other choroidal neovascularization of ophthalmic diseases[12]. It has been reported that pretreatment with intravitreal injections of conbercept and ranibizumab reduced the incidence of NVG after vitrectomy based on the 24mo follow up data[13].
The duration of the effect of anti-VEGF treatment is only usually 4-6wk, although anti-VEGF treatment can effectively reduce neovascularization of the ocular anterior segment of NVG[14]. In the past, most filtering methods we used involved a trabecular filtering operation, which resulted in many complications, such as a shallow anterior chamber, hyperplasia, and reocclusion in the filtrating canal. It has currently been proven that the filtering effect of an EX-PRESS glaucoma drainage device was better than that of the traditional trabecular filtering operation, with fewer complications[15-16]. Effective PRP can reduce the oxygen consumption of the retina, the nonperfusion area of the retinal vessels, and the production of neovascularization factors due to ischemia and hypoxia, and it can fundamentally prevent the growth of retinal neovascularization and the regeneration of neovascularization in the anterior chamber angle[17]. Direct laser photocoagulation of neovascularization can make the neovascularization shrink or disappear[18]. Some researcher has reported that combined treatment of IVR, PRP, and subsequent 5-fluorouracil augmented trabeculectomy is demonstrated to be a possible new paradigm for the management of advanced NVG with angle-closure and intractable elevation of IOP[19-20]. The trabeculectomy with adjunctive mitomycin C (MMC) is one of the most popular glaucoma surgeries worldwide. Thisltration surgery results in a better IOP reduction and has better lasting long-term results compared with other glaucoma surgeries. However, patients’ visual acuity was signicantly reduced after MMC trabeculectomyin[21]. In this study, we performed an intravitreal injection of conbercept to encourage the iris neovascularization to disappear and then implanted the EX-PRESS glaucoma drainage device to control the IOP. When the refracting media was clear, full panretinal photocoagulation was performed two weeks after the operation. When there was vitreous hemorrhage, vitrectomy was carried out to restore the transparency of the refracting media, and full PRP was performed; meanwhile, the EX-PRESS glaucoma drainage device was implanted. During six months of follow up visits, the IOP of enrolled patients after the operation decreased compared to preoperation. Although there was no significant improvement in the BCVA in the unmerged vitreous hemorrhage group, it had a positive clinical significance for the protection of the existing visual function and the alleviation of the patients’ pain. The BCVA of the last follow up visit in the merged vitreous hemorrhage group had improvement compared to preoperation.
In conclusion, conbercept, combined with EX-PRESS implantation and panretinal photocoagulation, is effective in the treatment of NVG, where the iridotrabecular touch is more than 180°.

