C9N4 as excellent dual electrocatalyst:A first principles study∗
Wei Xu(许伟),WenWu Xu(许文武),2,and Xiangmei Duan(段香梅),2,†
1School of Physical Science and Technology,Ningbo University,Ningbo 315211,China
2Laboratory of Clean Energy Storage and Conversion,Ningbo University,Ningbo 315211,China
Keywords:C9N4 nanosheets,dual electrocatalyst,hydrogen evolution reaction,oxygen evolution reaction
1.Introduction
The scarcity of fossil fuels and the serious environmental pollution caused by their combustion make it urgent to develop renewable and clean energy alternatives.[1]Hydrogen,a renewable and clean energy with low emission,is expected to be one of the most ideal energy substitutes for solving environmental pollution.[2]Recently,hydrogen production by electrocatalytic hydrolysis has received widespread attention.Whether the catalyst promotes the activity of hydrogen evolution reaction(HER)and oxygen evolution reaction(OER)is a key factor in the electrocatalytic water splitting process.[3]So far,Pt has been regarded as an excellent catalyst for HER in the thermochemistry model of Nφrskov.[4]Iridium oxide and ruthenium oxide are superior for the OER.[5]The high cost and low production hinder their extensive use.Currently,it is urgent to develop high performance,low cost,and suitable for widely used catalysts to promote the activity of HER and OER.
It has been known that adding heteroatoms and anchoring transition metals to carbon-based two-dimensional(2D)materials could significantly improve the catalytic activity of HER and OER.[6,7]In particular,single atom catalysts(SACs)on 2D sheets,with the coordination between anchored atoms and their surroundings,maximizes the performance and number of active sites.[8]For instance,Ni atom anchoring nitrogendoped graphene exhibits good OER performance.[9]The heterojunction composed of N-doped graphene and g-C3N4enhances HER activity,[10]and Mn atom anchored C2N sheet shows good dual functions of OER and HER.[7]In view of the fact that most carbon nitride monolayers are semiconductors,used as HER catalyst,it is necessary to increase their conductivity by doping or anchoring metal atoms,and building heterojunctions.2D materials composed of non-metallic elements with metallic characteristics will undoubtedly be ideal substitutes for precious metal materials.
Recently,a new type of carbon nitride porous nanosheet,C9N4,has been successfully predicted.[11]The good stability and unexpected conductivity of this flat structure open up a new way to explore non-metallic 2D materials.Based on the first principles calculations,we find that at certain hydrogen coverage,hydrogen atoms adsorbed on the 12-membered ring(H-tmr)and the 9-membered ring(H-nmr),show excellent property of the HER,even better than Pt.With the increase of the tensile strain applied to C9N4,the Gibbs free energy change(ΔGH∗)reduce gradually.Especially,under the strain of 2%–6%,H-tmr at the coverage of 2/3(tmr),the catalyst exhibits significant effect of HER.Further study shows that building heterojunctions,and/or anchoring Rh atom on C9N4can indeed stretch the lattice and enhance the catalytic capability of HER.In addition,applying a tensile strain within 2%to Rh@C9N4nanosheets,the HER activity is intentionally maintained or better.The heterojunctions composed by a latticematched 2D sheets and Rh@C9N4,can not only achieve extension,but also ensure that the value ofΔGH∗is better than that of Pt.With Rh atom as the active site,ΔGH∗and the overpotential of OER is−0.03 eV and 0.58 V,respectively.Therefore,Rh@C9N4has excellent electrocatalytic dual performance and provides a potential way for developing novel water splitting catalysts.
2.Computational method
All the computations are carried out by the spin-polarized density functional theory(DFT)using the projector augmented wave potentials which is operated in the Vienna ab initio Simulation Package(VASP).[12,13]A kinetic energy cutoff of 500 eV is used to set the plane-wave basis.The generalized gradient approximation contained in Perdew–Burke–Ernzerhof is employed to manage electron exchangecorrelation.[14]The empirical correction method D3 is used to describe a damped van der Waals correction.[15]The Brillouin zones are sampled with 5×5×1 Monkhorst–Pack meshes,and the van der Waals interaction based on Grimme’s scheme(D3)is employed.A vacuum space of 20˚A along the z direction was added to avoid the artificial interaction between the periodic images.The convergence criterion for energy is set to be 10−5eV,and the Hellmann–Feynman force of the atoms is less than 10−2eV·˚A−1.The climbing-image nudged elastic band(CI-NEB)method is applied to obtain the diffusion barrier of HER and OER among the nearest stable sites.[16]Bader’s charge population analysis is applied to calculate the atomic charge and electron transfer in different systems.[17]
The interaction between C9N4and hybrid can be judged by the interfacial binding energy,Eb,which is defined as



whereΔEH∗is the adsorption energy of one H atom on C9N4,and equals
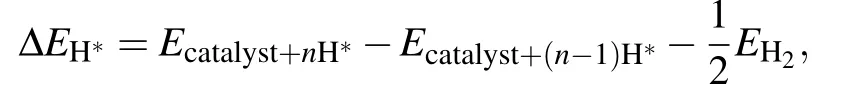
ΔEZPEandΔSHrepresent the change of the zero-point energy and the entropy between the adsorbed state and gas phase,respectively.T is the temperature,and TΔSHis a constant value and taken as−0.20 eV.[4,20,21]ΔEZPEis obtained by[20]
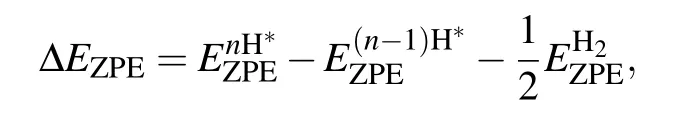
The OER follows four elementary paths at the standard conditions of pH=0,and U=0 V,[7,22]and the free energy changeΔG of each path can be obtained according to the literature.[22]The overpotentialηof OER(ηOER),an important criterion of the paths,is defined as the maximum of the fourΔG divide by e,then subtract oxygen reduction potential 1.23 V.[23]
3.Results and discussion
The optimized unit cell of C9N4monolayer is a porous atomic lattice of 9-and 12-membered rings formed with C and N atoms.The lattice constant of C9N4is computed to be 6.87˚A.The C–N bond length that connects the pentagon and makes up the pentagon is 1.30˚A,and 1.34˚A,respectively,and the C–C bond lengths of 9-and 12-membered rings are 1.56˚A and 1.45˚A,respectively,which are exactly the same with the corresponding values in the literature.[11]We calculateΔGH∗by adding one hydrogen atom after another to the sheet.The diffusion energy barrier of H atom from one adsorption point to the adjacent one is 1.04 eV,indicating that H is trapped and hard to move.We consider all the possibilities of H adsorbed on the 9-and 12-membered rings at different coverages.
For a single H atom adsorbed on C9N4,there are 3 equivalent sites to bind with N atoms in each cavity.The H-coverage,θ,is denoted as n/3(n=1,2,3).As listed in the left three columns of Table 1,when one H atom is adsorbed on the 12-membered rings(tmr)or the 9-membered rings(nmr),θis 1/3,respectively.The coverage of 1/3(nmr)shows a good hydrogen evolution performance,with a moderate and negative Eaand close to zeroΔG.However,for the coverage of 3/3(tmr)and 2/3(nmr),the positive Eaindicates that the adsorption of H atom is endothermic(will not be considered further).For the adsorption of H atoms on the 2×1 supercell,when θis 1/3(tmr)[i.e.,2/6(tmr)in the table]and 1/6(nmr),the HER performance is excellent.As the supercell increased to be 2×2,forθ=1/6[2/12(tmr)],the hydrogen evolution performance is also acceptable.In order to find a strategy to improve the HER activity of C9N4,we mainly focus on the case ofθ=2/3(tmr),with weak adsorption(−0.07 eV)and poor HER performance(ΔG=0.33 eV).Figure 1 shows their optimal structures with good hydrogen evolution performance.As illustrated in Fig.2(a),the cases where the H coverage is less than 2/3(tmr)show good hydrogen evolution performance,and some are even better than Pt.[4]Particularly,for two hydrogen atoms adsorbed on the 12-membered ring(2H-tmr)at 2/6(tmr),ΔGH∗is close to−0.02 eV,showing the excellent activity of HER.The catalytic performance of C9N4can be improved by moderating the coverage of hydrogen.
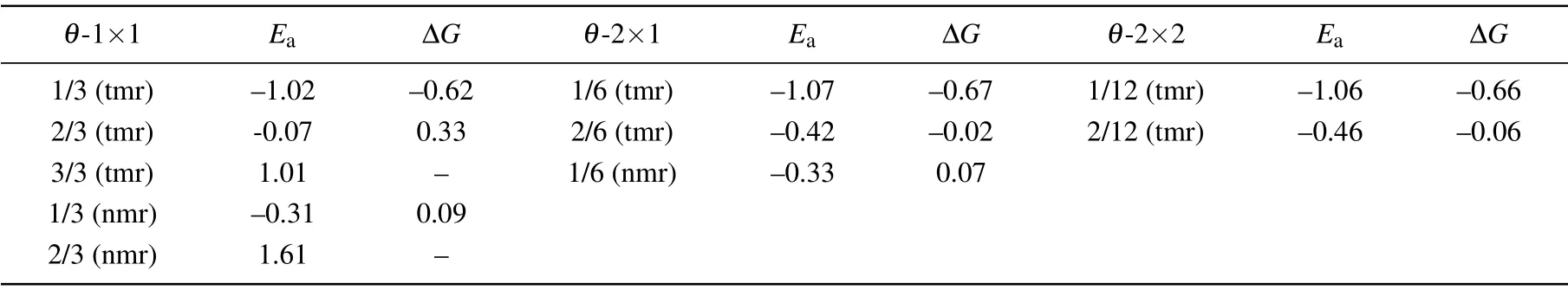
Table 1.Ea(in unit eV),ΔG(in unit eV)of all the possibilities of H adsorbed on the 9-and 12-membered rings at different coverages.
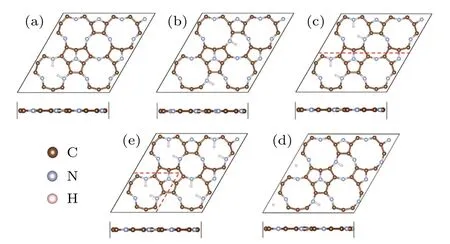
Fig.1.The structure of hydrogen atoms absorbed at different hydrogen coverages(a)–(e):θ=2/12,1/6,2/6,1/3,2/3.Panels(a),(c),and(e)represent H adsorbed on the 12-membered ring(tmr).Panel(b)and(d)stand for H on the 9-membered ring(nmr).
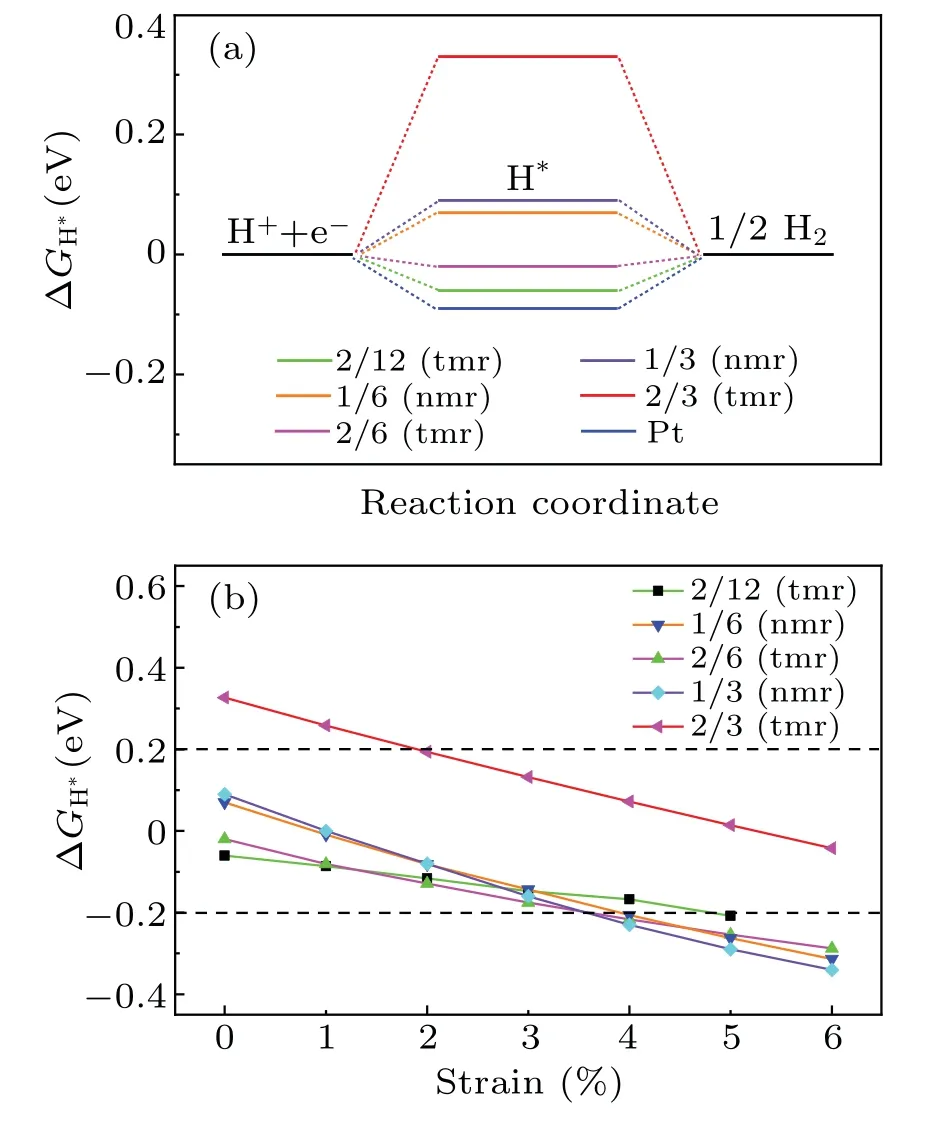
Fig.2.(a)ΔGH∗at different hydrogen coverages.ΔGH∗of Pt was included for comparison.(b)ΔGH∗as a function of the tensile strain.
Strain engineering has been proved to be an effective method for tuning the HER properties of 2D materials.[24,25]For the cases plotted in Fig.2(a),ΔGH∗under tensile strain are shown in Fig.2(b).Within the tensile strain of 6%,the Gibbs free energy change decreases with the increase of strain.For the case of H atoms adsorbed on the 12-membered ring at θ=2/3(tmr),the HER performance,the worst without strain(see Fig.2(a)),is significantly improved at a strain range of 2%and 6%.
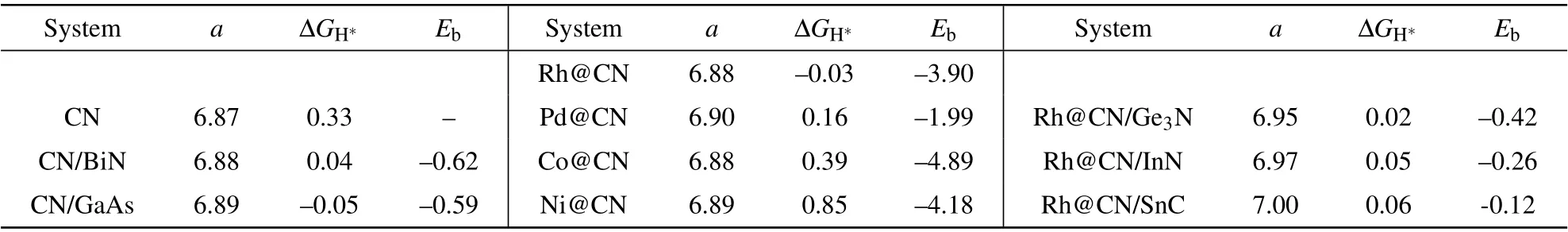
Table 2.The optimized lattice constant a(in unit˚A),ΔGH∗(in unit eV),and Eb(in unit eV)for C9N4(denoted as CN)and the hybridizations.

Fig.3.The density of states for the junctions(a)C9N4/BiN,(b)C9N4/GaAs.The Fermi level is set at 0 eV.
Anchoring suitable transition metals(TM)on the allotrope of C9N4has achieved remarkable effect on modulating the HER activity.[30]We anchor metal atom Co,Ni,Rh,and Pd to the 9-and 12-membered rings of C9N4nanosheet,respectively.The tests show that the 9-membered ring is too small to load TM atoms intact.We therefore focus on putting the TM atom inside and outside the 12-membered ring,respectively.The configuration with a TM atom outside the cavity,bound to the three N atoms,is the most stable.As listed in Table 2,the lattice of C9N4is indeed enlarged in the presence of TM atoms.TheΔGH∗of H adsorbed on the Rh atom is as low as−0.03 eV,thus Rh@C9N4is an outstanding catalyst for HER.To check whether a single metal atom prefers to be separated or aggregated on C9N4,the ratio of the binding energy to the bulk cohesive energy(Eb/Ecoh)is calculated.Generally,Eb/Ecohgreater than 0.5 indicates that the single atoms tend to be separated on the substrate,instead of gathering to clusters.[31,32]Take Rh@C9N4as an example,the binding energy Ebbetween Rh and C9N4is−3.90 eV,and the bulk cohesive energy of Rh is−5.8 eV.[33]The ratio of Eb/Ecohequals 0.67,which claims Rh atoms prefer to be anchored separately.Moreover,the diffusion energy barrier of Rh atom to the adjacent stable points is 2.1 eV,implying Rh atom bound stably at the initial site.The large binding energy and the high diffusion barrier prohibit the aggregation of metal atoms.
Strain has also been adopted to adjust the electronic interaction between metal and substrate.[34]We apply a small tensile strain to Rh@C9N4,and compare theΔGH∗,the d-band center and the binding energy with the unstrained case.The data are listed in Table 3,the absolute values ofΔGH∗are less than 0.09 eV,implying that the catalyst is superior to Pt on the HER.Moreover,the d-band center,εd,of Rh is closer to the Fermi level with the increase of tensile strain,indicating the enhanced activity of the catalyst.[35]The binding energies of the strained cases keep large,which prevents the diffusion of Rh.As shown in Fig.4,the conductivity of the strained systems remain as high as the unstrained case.The above results show that the Rh@C9N4sheet has good ductility,under a tiny strain,its structural and electronic properties are robust,its catalytic activity is enhanced.

Table 3.ΔGH∗,Eb,and d-band centerεd between Rh and C9N4 nanosheets at the absence and presence of strain.

Fig.4.PDOS of the p orbital of N with the d orbital of Rh at the absence and presence of strain,respectively.The Fermi level that is set at the zero and the d band center is marked by the vertical short black line,respectively.
In experiments,a big issue is how to accurately achieve such a small strain.The reported lattice constants of Ge3N,InN,and SnC are 7.11˚A,3.63˚A,and 3.60˚A,[29,36–38]respectively.It is possible to respectively combine the 2D sheet Ge3N,InN,and SnC with Rh@C9N4,and their lattices match well.We explore to build a heterojunction using these 2D materials and Rh@C9N4.The lattice constants of Ge3N,InN,and SnC are optimized to be 7.17˚A,3.63˚A,and 3.60˚A,respectively,which are in good agreement with the literatures.The lattice mismatch between each of them and Rh@C9N4is 1%,1.3%,and 1.7%,respectively.TheΔG and the binding energy of the junctions are listed in Table 2.Amazingly,the Gibbs free energy change is basically consistent with the applied strain.The small negative value of Ebindicates that the interaction between the two components is weak van de Waals effect.We propose,to apply tiny tensile strain on Rh@C9N4in the experiment,an effective and feasible method is to construct a junction with the Ge3N,InN or SnC sheets.
The last but not least,we consider the Rh atom as the active site for oxygen evolution reaction.In order to get the rate-determining step of OER on Rh@C9N4,we compute the change of free energy for each basic step.From Fig.5,it can be obviously seen that the last step is the ratedetermining step,and overpotential of OER(ηOER)is calculated to be 0.58 V.For the better evaluation of OER activity,we have compared more systems.For instance,the overpotential(η)of IrO2as OER catalyst is calculated to be 0.56 V.As the HER/OER bifunctional electrocatalyst,ηis 0.52 V and 0.67 V for Ni@Mo2B2and Mn@C2N,respectively,and most of the M@C materials.[7,39–42]Based on the similar methodology,Rh@C9N4,as bifunctional electrocatalyst,exhibits good catalytic activity,and the value of HER/OER is−0.02 eV/0.58 V,respectively,indicating better HER/OER catalytic performance than Mn@C2N(−0.15 eV/0.67 V).
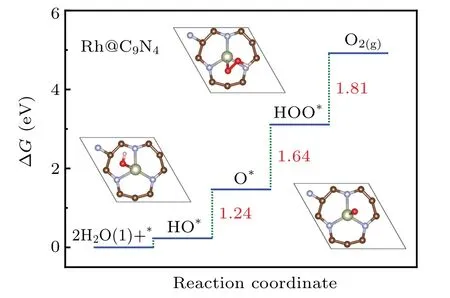
Fig.5.Free energy diagram for OER on Rh@C9N4.
4.Conclusion
Using first principles calculations,we investigated the dual-performance of C9N4nanosheet for water splitting.C9N4nanosheet exhibits a metallic characteristic and shows excellent catalytic activity of HER with hydrogen atoms adsorbed on the 12-membered ring and the 9-membered ring,respectively.With the increasing tensile strain applied to C9N4,the Gibbs free energy change reduces gradually.Practically,constructing heterojunction with BiN or GaAs,and anchoring Rh atom on C9N4,could realize the tensile strain,the hybrid do promote the process of HER.For Rh@C9N4,the moderate tensile strain(~2%)may help to achieve the excellent effect of the HER as well.We demonstrate that it is a feasible way to introduce the tensile strain by building the heterojunctions between 2D sheets Ge3N,InN or SnC and Rh@C9N4.ΔGH∗of HER can be as low as−0.03 eV and the overpotentialηOERis 0.58 V,therefore,Rh@C9N4emerges an excellent bifunction catalyst for both HER and OER.The C9N4nanosheet is expected to open up a new way of high efficiency and promising dual-functional electrocatalysts for water splitting.
- Chinese Physics B的其它文章
- Multiple solutions and hysteresis in the flows driven by surface with antisymmetric velocity profile∗
- Magnetization relaxation of uniaxial anisotropic ferromagnetic particles with linear reaction dynamics driven by DC/AC magnetic field∗
- Influences of spin–orbit interaction on quantum speed limit and entanglement of spin qubits in coupled quantum dots
- Quantum multicast schemes of different quantum states via non-maximally entangled channels with multiparty involvement∗
- Magnetic and electronic properties of two-dimensional metal-organic frameworks TM3(C2NH)12*
- Preparation of a two-state mixture of ultracold fermionic atoms with balanced population subject to the unstable magnetic field∗

