PRKCDBP Methylation is a Potential and Promising Candidate Biomarker for Non-small Cell Lung Cancer
Jing LI, Lin QI, Mingfang ZHANG, Caiyun YAO, Jinan FENG, Zhonghua ZHENG,Chujia CHEN, Shiwei DUAN, Yuanlin QI
1School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350122, China;
2Medical Genetics Center, School of Medicine, Ningbo University, Ningbo 315211, China
Abstract
Background and objectives: The occurrence and development of lung cancer are closely linked to epigenetic modification.Abnormal DNA methylation in the CpG island region of genes has been found in many cancers. Protein kinase C delta binding protein (PRKCDBP) is a potential tumor suppressor and its epigenetic changes are found in many human malignancies. This study investigated the possibility of PRKCDBP methylation as a potential biomarker for non-small cell lung cancer (NSCLC).
Methods: We measured the methylation levels of PRKCDBP in the three groups of NSCLC tissues. Promoter activity was measured by the dual luciferase assay, with 5′-aza-deoxycytidine to examine the effect of demethylation on the expression level of PRKCDBP.
Results: The methylation levels of PRKCDBP in tumor tissues and 3 cm para-tumor were higher than those of distant (>10 cm)non-tumor tissues. Receiver operating characteristic (ROC) curve analysis between tumor tissues and distant non-tumor tissues showed that the area under the line (AUC) was 0.717. Dual luciferase experiment confirmed that the promoter region was able to promote gene expression. Meanwhile, in vitro methylation of the fragment (PRKCDBP_Me) could significantly reduce the promoter activity of the fragment. Demethylation of 5′-aza-deoxycytidine in lung cancer cell lines A549 and H1299 showed a significant up-regulation of PRKCDBP mRNA levels.
Conclusion: PRKCDBP methylation is a potential and promising candidate biomarker for non-small cell lung cancer.
Key words Lung neoplasms; PRKCDBP; DNA methylation
Introductio n
The latest data show that one in four cancer patients in the United States is lung cancer[1], and it is also the highest in cancer mortality in China[2]. Clinically, 80%-85% of lung cancer patients are non-small cell lung cancer (NSCLC)[3].The survival rate of lung cancer depends largely on the stage of diagnosis. The five-year survival rate of lung cancer diagnosed at local stage is 54%, but only 15% of patients can be diagnosed at local stage[4]. At present, clinical diagnosis of lung cancer relies on spiral computed tomography (CT)and X-ray, but it is prone to both false negative and false positive cases[5]. Therefore, finding new diagnostic markers for lung cancer is of great significance for lung cancer.
The occurrence and development of lung cancer is closely linked to epigenetic modification[6]. DNA methylation is one of the most studied epigenetics, and abnormal DNA methylation in the CpG island region of genes has been found in many cancers[7]. CpG island mainly occurs in the coding region of eukaryotic genes, and abnormal methylation of CpG island will cause down-regulation of tumor suppressor gene expression or overexpression of oncogene, thereby promoting the development of cancer[8].
The protein kinase C delta binding protein (PRKCDBP)is a potential tumor suppressor and its epigenetic changes are found in many human malignancies. ThePRKCDBPgene is located at the human chromosome 11p15.5-p15.4,which harbors frequent deletions in the tumors of breast cancer[9]and lung cancer[10], suggesting its possible role in tumorigenesis.PRKCDBPcan induce G1cell arrest,enhance the sensitivity of cells to various apoptotic stimuli, and promote apoptosis. In addition,PRKCDBPtranscription is found to be directly activated by tumor necrosis factor α (TNFα) via nuclear factor kappa-B (NF-κB) signaling pathway[11]. TNFα is an important proinflammatory cytokine that not only plays an important role in the inflammatory process, but also involves processes such as apoptosis, proliferation, differentiation,wich are often related to the induction and development of cancer[12]. As a transcriptional target of the TNFα-NF-κB signaling pathway,PRKCDBPplays a key role in TNFαinduced apoptosis[11].
To explore whetherPRKCDBPcan be used as a diagnostic biomarker for NSCLC. This experiment detected the level ofPRKCDBPmethylation in tumor tissues, 3 cm para-tumor tissues, distant non-tumor from NSCLC patients. We conducted an in-depth analysis of thePRKCDBPmethylation level and clinical pathological data,and verified the gene regulation function of the target gene fragment.
Materials and methods
Collection of tissue samples
We have collected tissues samples of NSCLC patients under the guidance of pathologists since February 2018.The tissue samples comprised tumor tissues, para-tumor tissues (3 cm away from tumor tissue) and distant nontumor tissues (more than 10 cm from tumor tissues). We collected a total of 119 samples, including 44 pairs of tumor tissues and distant non-tumor tissues, and 31 para-tumor tissues. We collected clinical pathology information for NSCLC patients, including age, gender, family history, and pathological type. The study protocol was approved by the Ethics Commiはee of the First Affiliated Hospital of Fujian Medical University, and each patient signed an informed consent form.
Bisulfite conversion
DNA of tissue samples were extracted using EZNA Tissue DNA Extraction Kit (Omega Bio-Tek, Norcross, GA, USA)and quantified using a NanoDrop 1000 spectrophotometer(Thermal Scientific Co. Ltd., Wilmington, MA, USA).The bisulfite modification was performed according to the instruction of EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA, USA). The converted DNA was stored at -20oC for methylation detection.
Quantitative methylation specific PCR (qMSP)
The qMSP primers were designed to amplify the CpG rich region ofPRKCDBPgene, and the qMSP product was 96 bp in length. The forward primer was 5’-ATA GGTCGGTAAAGGTTT-3’, and the reverse primer was 5’-CCACGAACTACTAATAACG-3’. We used a SYBR-green-based qMSP to assess the level of methylation ofPRKCDBPin three groups of NSCLC tissue samples. In order to avoid errors in the amount of sample loading,each sample used ACTB as an internal control. The primer sequences of ACTB were as follows: Forward,5′-TGGTGATGGAGGAGGTTTAGTAAGT-3′ and reverse,5′-AACCAATAAAACCTACTCCTCCCヰAA-3′. At the same time, M-SssI-treated human sperm DNA and nuclease-free water were used as positive and negative controls, respectively,to ensure the accuracy of the experiment. The qMSP product was randomly selected for capillary electrophoresis(Bioptic, Taiwan, China) and Sanger sequencing to verify the homogeneity of product and the successful conversion of sodium bisulfite. We used the formula to calculate the methylation reference percentage (PMR) value. The relevant formula was as follows: [PMR=2-ΔΔCt×100%, ΔΔCt=sample DNA (CtPRKCDBP-CtACTB) - fully methylated DNA (CtPRKCDBPCtACTB)].
Dual luciferase and real-time PCR for gene expression detection
The PKCDBP CpG-rich fragment (+181 bp to +581 bp) was selected to construct the pGL3-PRKCDBPplasmid of the target gene. To avoid the difference in the number of cells,the pGL3-basic plasmid and the pRL-SV40 plasmid were cotransformed as a negative control, and the pGL3-promoter plasmid and the pRL-SV40 plasmid were co-transformed as a positive control. Human embryonic kidney 293T cell line was used for the transformation. After 24 h of transfection,luciferase assays were performed using SpectraMax 190(Molecular Devices, Sunnyv, USA) following the Promega Dual Luciferase Reporting System kit instructions. Luciferase activity was finally calculated using the Dual Luciferase Reporter Assay Systems (Promega, Madison City, WI, USA).
Lung cancer cell lines A549 and H1299 were purchased from the cell bank of Shanghai Academy of Sciences, and cells were treated with 5’-aza-deoxycytidine. RNA was extracted by TRIzol one-step method before and after treatment, and product cDNA was obtained using Takara reverse transcription kit. The forward RT-PCR primer forPRKCDBPwas 5’-GCGGGAAGCTCCACGヰC-3’, and the reverse primer was 5’-GCTCTGTACCTTCTGCAATCCG-3’. In order to avoid the error in the amount of sample loading, each sample had ACTB as the internal reference. The forward RT-PCR primer for ACTB was 5’-AGCACAGAGCCTCGCCTTT-3’, and the reverse primer was 5’-AGGGTGAGGATGCCTCTCTT-3’. A negative reference was also made using nuclease-free water.The real-time PCR system was configured according to the Takara TB Green kit instructions, and the mRNA expression ofPRKCDBPwas detected using an StepOnePlusTM(96) realtime PCR instrument (Applied Biosystems, Foster City, CA,USA).
Bioinformatics analysis
PRKCDBPmethylation levels of tumor tissuses and paratumor tissues from 51 lung cancer patients were downloaded from TCGA database (https://genome-cancer.ucsc.edu/).In addition, we obtained data of 230 samples from the cBioPortal database (hはp://www.cbioportal.org) to analyze the relationship betweenPRKCDBPmethylation level and mRNA expression.
Statistical analysis
We performed statistical analysis using SPSS version 20.0(SPSS Inc. Chicago, IL, USA). Nonparametric Friedman test was used to determine methylation differences between two groups among tumor tissues, 3-cm para-tumor tissues,and distant non-tumor tissues. AdjustedPwas obtained byBonferroni’scorrection. CpG site methylation of tumor tissues and para-tumor tissues from TCGA were compared using aWilcoxon signed-ranktest.Kaplan-Meiersurvival analysis was used to assess the prognostic value of the level ofPRKCDBPmethylation. ROC curve was used to evaluate the diagnostic value of distalPRKCDBPmethylation for NSCLC.P<0.05 was considered statistically significant.
Results
Descriptions of tissue samples and promoter region of PRKCDBP
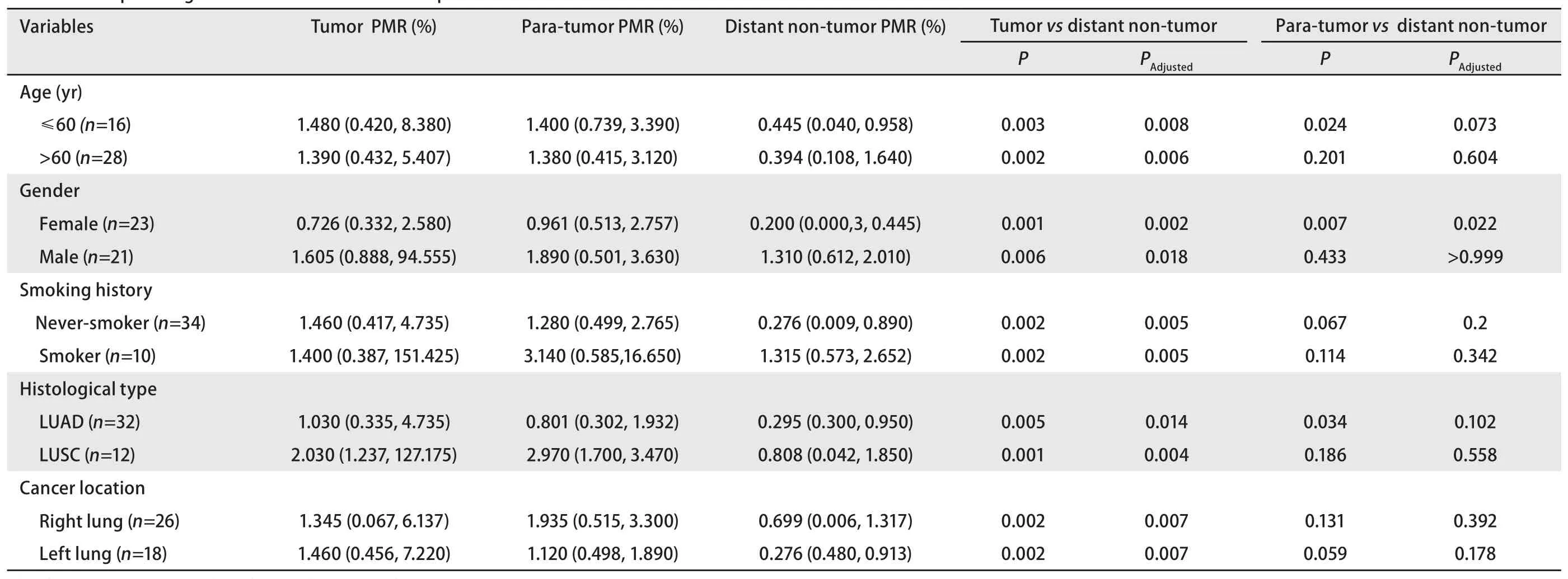
We collected paired tissue samples from 44 patients with NSCLC and classified their clinical pathology information(Tab 1). The average age of the patients was 62.91±9.05 years old, including 23 females and 21 males. There were 32 cases of adenocarcinoma and 12 cases of squamous cell carcinoma.
Since the silencing of many tumor suppressor factors is due to the methylation of CpG islands, we selected a fragment in the CpG island region in the promoter region ofPRKCDBP(GRCh37/hg19, chr6341276-chr66341371; Fig 1A). There are two CG sites (cg18959478 and cg18392783;see also Fig 4C) in the selected fragment (Fig 1A). Capillary electrophoresis showed the fragment size of qMSP product was the same as we expected (Fig 1C). The qMSP products were also subjected to Sanger sequencing and the results showed that the amplified sequences were correct, suggesting that the bisulfite conversion was complete (Fig 1B).
Hypermethylation of PRKCDBP promoter in tumor tissues
The comparison between groups in the three groups of samples was performed using the nonparametric Friedman test.Padjustedis the Bonferroni correctedPvalue, which adjusts the type I error in multiple comparisons, and thus it is more accurate than the originalPvalue. Paired comparison ofPRKCDBPmethylation levels showed that the methylation level of tumor tissues and 3 cm para-tumor tissues was higher than that of distant non-tumor tissues (tumor tissuesvsdistant non-tumor tissues:Padjusted<0.001; 3 cm para-tumor tissuesvsdistant non-tumor tissues:Padjusted=0.05; Fig 2A).
Through the TCGA database, we analyzed the methylation levels of four CpG sites located in the promoter region ofPRKCDBP. The methylation levels of 3 CpG sites in tumor tissues was higher than those in matched paratumor tissues (cg18959478,P=0.011; cg18392783,P=0.009;cg05628549,P=0.001, Fig 4C).
Potential diagnosis and prognosis values of PRKCDBP methylation
Our ROC curve analysis showed thatPRKCDBPmethylation was of diagnostic value for NSCLC (AUC=0.717,P=0.000,5,95%CI: 0.609-0.824, sensitivity=59.09%, specificity=77.27%,Fig 2B). In addition, the ROC curve results of tumor tissues and para-tumor tissues in the TCGA database also showed thatPRKCDBPmethylation was a potential diagnostic biomarker for lung cancer (AUC=0.686,P=3E-6, 95%CI:0.612-0.760, sensitivity=57.94%, Specificity=81.30%, Fig 4E).Kaplan-Meiersurvival analysis of TCGA data also indicated that overall survival was significantly lower in lung cancer patients with hypermethylation ofPRKCDBPthan in patients with hypomethylation ofPRKCDBP(P=0.012, Fig 4D).
Subgroup analysis of PRKCDBP methylation
Subgroup comparisons by age, gender, smoking history,lesion location and pathological type showed that thePRKCDBPmethylation level in tumor tissues was higher than that in distant non-tumor tissues (allPadjusted<0.05, Tab 1). There was no significant difference in the methylation level between the tumor tissues and the 3 cm para-tumor tissues in the subgroup comparisons (allPadjusted>0.05). This may be due to the close proximity of the para-tumor tissues and tumor tissues.
Tab 1 Clinical pathological information
PRKCDBP mRNA expression is regulated by promoter methylation
We performed a dual luciferase assay to verify that the selected gene fragment had promoter activity. Our results showed that the transcriptional activity of the target gene plasmid pGL3-PRKCDBPwas significantly higher than that of the pGL3-basic control plasmid, which confirmed that the fragment we analyzed could initiate gene expression [fold change (FC)=9.54,P=0.002]. And our results also suggest that thein vitromethylation of the fragment (PRKCDBP_Me)can significantly reduce the promoter activity of the fragment(FC=0.273;P=0.006, Fig 3). TCGA data analysis showed an inverse correlation betweenPRKCDBPmethylation and mRNA expression (r=-0.437,P=4.88E-10; Fig 4A). In addition, we used 5’-aza-deoxycytidine demethylation on lung cancer cell lines A549 and H1299, and found that the two demethylated cell lines showed significant up-regulation of mRNA levels (A549:P=0.003, FC=2.193; H1299:P=5.713E-7, FC=32.081; Fig 4B).
Discussion
In this study, the promoter methylation level ofPRKCDBPwas used as a potential marker for the possibly early detection of NSCLC. We found that the level ofPRKCDBPpromoter methylation was higher in tumor tissue samples,and its mRNA expression level was inversely correlated with methylation level. In the further analysis of clinical pathological data, it was found thatPRKCDBPmethylation levels of tumor tissues in all clinical pathological subtypes were higher than that in the distant non-tumor tissues. In addition, thePRKCDBPmethylation levels between tumor tissues and para-tumor tissues were statically significant in female patients, suggestingPRKCDBPmethylation should be a more sensitive biomarker for female NSCLC patients.These results demonstrated thatPRKCDBPmethylation should become a potential biomarker in NSCLC diagnosis.
PRKCDBPpromoter hypermethylation have been found in colorectal[11], ovarian[13],gastric[14], breast[15]and lung cancer[16]. Our qMSP study provided a quantitative method which is more accurate than MSP applied in the previous studies[17]in the detection of thePRKCDBPmethylation level.PRKCDBPmethylation was inversely correlated with mRNA expression in NSCLC patients in TCGA dataset. We also treated lung cancer cell lines (A549 and H1299) with DNA demethylation reagent 5’-aza-deoxycytidine and found the mRNA expression ofPRKCDBPincreased after treatment. These results indicated the expression of genePRKCDBPwas regulated by promoter methylation.We then performed double luciferase assays to determine the promoter activity of the fragment analyzed in this study and confirmed this fragment had transcriptional promoting function.
Cavin-3 (PRKCDBPencoding protein) is a member of the Cavin family, which is involved in the formation and functions of caveolae,including signal transduction, lipid regulation,endocytosis, and tumorigenesis[18]. The Cavin family gene expression is down-regulated in breast cancer. In studies of neuroblastoma[19]and breast cancer[15], it was found thatPRKCDBPmethylation can determine its prognosis. Our study found that the promoter ofPRKCDBPwas hypermethylated in lung cancer tissue as well as in para-tumor tissues,while was hypomethylated in the distant non-tumor tissues. It suggested that the methylation level ofPRKCDBPmight affect the development of NSCLC and the methylation changes might be detected at the early stage of its onset.
The incidence of lung cancer in China is higher in male patients than in female patients. In recent years, due to changes in smoking habits, the incidence of lung cancer in women has also shown a growing trend[20]. It was reported that PRKCDBP could interact with BRCA1 protein and be involved in DNA damage response and participate in the BRCA1-mediated tumor suppressor pathway[21].Due to the limited sample size, we did not verify the relationship between PRKCDBP and BRCA1. However,the methylation levels of PRKCDBP promoterin female patients is higher in both tumor tissues and para-tumor tissues than in distant non-tumor tissues. It seems that PRKCDBP hypermethylation should be more sensitive in female NSCLC patients than males. This phenomenon might be related to the biologic property of PRKCDBP protein interacting with BRCA1[10].
PRKCDBPwas a downstream target of TNF α-induced tumor cell apoptosis signaling, and was activated by NF-κB[12]. It was also found thatPRKCDBPcould enhance the protein stability of P53, and promote apoptosis by enhancing P53 function. The hypermethylation ofPRKCDBPcauses down-regulation of P53, which promotes the progression of malignant tumors[14]. This also suggests a multifaceted function ofPRKCDBPin NSCLC.
In summary, this study showed that the level ofPRKCDBPmethylation is indeed associated with NSCLC.PRKCDBPmethylation is a potential and promising candidate biomarker for non-small cell lung cancer.The mechanism ofPRKCDBPon NSCLC remains unclear and further experimental verification is needed in the future.
Acknowledgement
The research was supported by the grants from National Natural Science Foundation of China (to Yuanlin QI,No.81773055), Natural Science Foundation of Fujian Province (to Yuanlin QI, No.2018J01829 and to Mingfang ZHANG, No.2019J01293), Key Talents Training Program of Fujian Provincial Health Commission (to Yuanlin QI,No.2017-ZQN-59 and to Mingfang ZHANG, No.2017-ZQN-60), Joint Funds for the Innovation of Science and Technology of Fujian Province (to Yuanlin QI,No.2017Y9113), and K.C.Wong Magna Fund in Ningbo University (to Shiwei DUAN).
Conflict of interest
All authors have read the journal’s policy on the disclosure of potential conflicts of interest and have none to declare.
Authors’ contributions
Li J and Qi L collected tissue samples and patient data,analyzed the data and wrote the manuscript. Zhang MF and Duan SW carried out literature research and data interpretation. Yao CY, Feng JN, Zheng ZH and Chen CJ performed the experiments. Qi YL designed the study and critically revised the manuscript for intellectually important content. The authors declare that all data were generated inhouse and that no paper mill was used.
Ethics approval
The present study was approved by The Ethics Committee of the First Affiliated Hospital of Fujian Medical University(Fuzhou, China; approval number: 2016KY009).

