CuAg nanoparticles on TiO2 for high-efficiency photodegradation of acetaldehyde
XU Ji-liang,WANG Liu-feng,JIANG Peng-yan,LI Shu-hui,HUANG Ji-wu ,WANG Liang-bing
(1.State Key Laboratory for Powder Metallurgy,School of Materials Science and Engineering,Central South University,Changsha 410083,China;2.Zhejiang NHU Company Ltd.,Shaoxin 312500,Zhejiang,China)
Abstract:Photodegradation of acetaldehyde serves as a novel and efficient approach for removing acetaldehyde,and TiO2 is generally used as a photocatalyst.However,TiO2 has a weak adsorption capacity for acetaldehyde,low product selectivity,and high electron-hole pair recombination rate,which significantly limit the treatment performance of acetaldehyde.In this study,we successfully constructed a highly active and stable photocatalyst for photocatalytic degradation of acetaldehyde by loading CuAg nanoparticles on TiO2 (CuAg/TiO2),which efficiently solved the inherent defects of TiO2.The degradation rate of acetaldehyde on CuAg/TiO2 was up to 42.49 % under the irradiation of natural sunlight.CuAg/TiO2 retained above 98.89% of initial activity after four successive rounds of full spectrum photodegradation.Further mechanism studies indicated that CuAg nanoparticles in CuAg/TiO2 generated hot electrons under light,followed by the transfer of hot electrons to TiO2 and oxygen adsorbed on Ag sites.The generated superoxide radicals on CuAg/TiO2 can efficiently degradate acetaldehyde,resulting in the remarkable performance in the removal process of acetaldehyde.
Key words:silver;surface plasmon resonance;copper;acetaldehyde photodegradation;plasmonic catalysis
Acetaldehyde,as a volatile organic chemical,is one of the typical air pollutants[1].Especially in the modern residential and commercial buildings,cars and other indoor spaces,the concentration of acetaldehyde is often higher than outside.Long-term inhalation of acetaldehyde pollutants could cause a range of symptoms,including headache,nausea,and even damage of liver’s function[2-3].Currently,many techniques,such as physical adsorption,biodegradation,thermal catalysis technology,and photocatalytic oxidation,have been used to remove acetaldehyde pollutant[3-4].Among them,photocatalysis has become one of the most effective techniques for acetaldehyde removalbecause of its non-toxic,low cost,convenient operation,and eco-friendly[3].Titanium dioxide (TiO2)with semiconductor properties is widely used in photocatalytic degradation of air pollutants[5-18].However,its application on degrading acetaldehyde has some limitations due to the inherent defects of TiO2[6,8,11].For instance,TiO2has low acetaldehyde adsorption capacity,low product selectivity and high electron-hole pair recombination rate[13,15,18].Thanks to the multitudinous efforts of several research groups,it has been found that the synergistic catalytic effect between plasmonic metals and semiconductors can effectively improve its photodegradation efficiency[4,19-20].For example,Jia and co-workers[4]proposed a heterogeneous catalyst consisting of plasmonic gold nanoparticles and TiO2for removing acetaldehyde.The synergistic effect enhances the photodegradation efficiency of acetaldehyde from two main aspects:1)the presence of TiO2particles leads to the enhancement of localized electric fields on the plasmonic surfaces,causing the enhanced adsorption of acetaldehyde and reaction intermediates on catalyst surfaces,and also changing its product selectivity.2) The interaction between the plasmonic particles and TiO2particles promotes the separation of photogenerated carriers and improves the life spans of catalysts[4,20].
We reported a properly designed CuAg/TiO2catalyst for high-efficiency photocatalytic degradation of acetaldehyde,which showed remarkable catalytic activity for the photodegradation of acetaldehyde and also high CO2product selectivity.During the process of photodegradation of acetaldehyde,the degradation rate of acetaldehyde on CuAg/TiO2was up to 98.97% under the irradiation of 385 nm light.Impressively,CuAg/TiO2had an acetaldehyde degradation rate of 42.49%under the irradiation of natural sunlight.Furthermore,more than 98.89% of initial activity was maintained after four catalytic cycles.Further mechanism studies indicated that oxygen adsorbed on Ag sites obtained hot electrons generated by the localized surface plasmon resonance (LSPR) effect and then formed superoxide radicals,while TiO2efficiently adsorbed acetaldehyde molecules on CuAg/TiO2,directly contributing to the performance improvement of CuAg/TiO2.
1 Experiments
1.1 Materials and chemicals
Silver nitrate (AgNO3) was purchased from Sinopharm Chemical Reagent Co.Ltd.Copper nitrate trihydrate (Cu(NO3)2·3H2O) was obtained from Shanghai Aladdin Biochemical Technology Co.Ltd.Hydrazine hydrate (N2H4·H2O,80%) was supplied by Hunan Huihong Chemical Reagent Co.Ltd.Commercial P25 powder was purchased from Degussa company.High purity carbon dioxide (CO2≥99.999%)was ordered from Saizhong Specail Gas Co.Ltd.All aqueous solutions were prepared using deionized (DI)water with a resistivity of 18.2 MΩ·cm-1.
1.2 Synthesis of Cu/TiO2,Ag/TiO2 and CuAg/TiO2
All catalysts were preparedviahydrazine hydrate reduction method.In general,the synthesis of Cu/TiO2catalysts is as follow:1 g of TiO2was dispersed in 5 mL of Cu(NO3)2solution (63 μmol/L),then stirred the mixture for 3 h in a 20 mL reaction flask at 80℃,and dropwisely added hydrazine hydrate into the mixture and stirred for another 1 h at 80℃;and washed the resulted precipitates with deionized (DI) water and centrifuged for several times.The obtained Cu/TiO2was dispersed in DI water for further use.The preparation methods of Ag/TiO2and CuAg/TiO2were similar to that of Cu/TiO2,except that 1 g of TiO2was dispersed in 5 mL of AgNO3(37 μmol/L) solution or a solution of AgNO3(23.3 μmol/L) and Cu(NO3)2(23.3 μmol/L).
1.3 Photocatalytic tests
The photocatalytic degradation of acetaldehyde was conducted in a 352 mL quartz topirradiation-type reaction vessel.First,100 mg of catalysts was evenly spread on the bottom of reaction vessel by gradually spraying the suspended catalyst mixture into the reactor,then injected acetaldehyde gas (~400×10-6) into the reactor.In order to achieve adsorption-desorption equilibrium between acetaldehyde and the photocatalyst,the reactor was kept under dark for 2 hours.After illumination,the concentration of acetaldehyde in the reactor was monitored at set intervals by a gas chromatography (GC) (9790Ⅱ,FULI) equiped with a flame ionization detector (FID).The acetaldehyde degradation (X,%) was calculated by the formula:
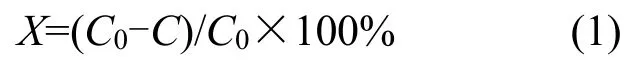
whereC0andCrepresent the initial concentration and real-time concentration of acetaldehyde,respectively.
1.4 Characterization
The XRD patterns were obtained by a Rigaku Xray diffractometer (Rigaku,MiniFlex 600) with Cu-Kα radiation (λ=1.54178 Å).TEM and HAADF-STEM images were obtained on a field-emission transmission electron microscope (JEOL,ARM-200F) with an acceleration voltage of 200 kV.X-ray photoemission spectroscopy (XPS) experiments were implemented at BL10B beamline in the National Synchrotron Radiation Laboratory (NSRL) in Hefei,China.The UVVis absorption experiments were carried out on a double beam UV-Vis spectrophotometer (Persee,TU-1901) at room temperature.The inductively coupled plasma optical emission spectrometry (ICP-OES)experiments were implemented on a plasma spectrometer (Spectro,Spectro Blue SOP).The CO2concentration during the photodegradation of acetaldehyde was investigated using a gas chromatography (GC) (9790Ⅱ,FULI) equipped with a thermal conductivity detector (TCD).
2 Results and discussions
2.1 Structure characterizations of photocatalysts
The samples were characterized by TEM,HAADF-STEM,and EDX elemental mapping,as shown in Fig.1.

Fig.1 TEM images of (a) TiO2,(b) CuAg/TiO2 and (c) HAADF-STEM and the corresponding EDX elemental mapping images of CuAg/TiO2图1 TiO2(a)和CuAg/TiO2(b)的透射电子显微镜图像及CuAg/TiO2 的HAADF-STEM 及相应的EDX 元素映射图(c)
Fig.1(a) showed the TEM image of TiO2,where the average particle size of TiO2was~50 nm.Fig.1(b)illustrated the TEM image of CuAg/TiO2preparedviahydrazine hydrate reduction method.Isolated metal nanoparticles with extremely small sizes were marked with white circles,and metal nanoparticles uniformly distributedn on the surface of TiO2nanoparticles.HAADF-STEM image and the corresponding energy dispersive X-ray (EDX) elemental mapping of CuAg/TiO2showed thatCu,Ag,Ti,and O elements evenly distributed in the nanostructure (Fig.1(c)).
The crystalline structures and compositions of the as-synthesized samples were further analyzed by X-ray diffraction (XRD).As shown in Fig.2,the main lattice type of TiO2nanoparticles was anatase.As for Cu/TiO2,Ag/TiO2,and CuAg/TiO2,the characteristic peaks of Cu and Ag were weak,which might be due to the amorphous Cu,Ag,and CuAg nanoparticles.The XRD results agreed with the analysis of TEM images.
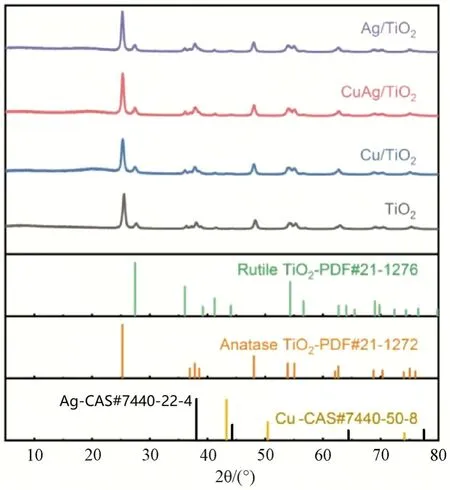
Fig.2 XRD patterns of samples图2 样品的X 射线衍射图谱
2.2 Catalytic performances of photocatalysts in the degradation of acetaldehyde
The catalytic performances of as-obtained Cu/TiO2,Ag/TiO2,CuAg/TiO2,and TiO2in photocatalytic degradation of acetaldehyde were evaluated,and the experimental methods were described in the Experiments section.A blank test was conducted without any catalyst,and no product was observed.
Fig.3(a) illustrated the reaction profiles of TiO2,Ag/TiO2,Cu/TiO2and CuAg/TiO2versus time during photocatalytic degradation of acetaldehyde.CuAg/TiO2attained the highest acetaldehyde conversion rate of 98.84% within 20 minutes.In comparison,the degradation rates of acetaldehyde on Cu/TiO2,Ag/TiO2,and TiO2reduced to 95.76%,94.11%,and 98.08% under the same conditions,respectively.The photocatalytic activities of as-obtained catalysts under visible light (λ > 400 nm) irradiation were evaluated.As shown in Fig.3(b),the acetaldehyde concentration on CuAg/TiO2was quickly decreased,reaching a turning point at about 15 min,and a acetaldehyde conversion rate of 98.36% was obtained within 45 min.The catalytic performance of Cu/TiO2was similar to that of CuAg/TiO2,and its acetaldehyde conversion rate reached to 98.24%.The catalytic performances of Ag/TiO2and TiO2were far inferior to Cu/TiO2and CuAg/TiO2,only 12.52% and nearly 0 conversion rate under visible light irradiation were obtained,respectively.To explore reaction path of acetaldehyde photodegradation,we collected products during reaction.Fig.3(c) showed the time function of generating CO2from the photodegradation of acetaldehyde under visible light irradiation.There was no CO2released on TiO2catalyst,and the amount of produced CO2also followed the order of CuAg/TiO2>Cu/TiO2>Ag/TiO2.When the reaction time was 60 min,the concentration of CO2on CuAg/TiO2catalyst reached to 651.42×10-6,which was much higher than that of other catalysts.Fig.3(d) revealed that the conversion rate of acetaldehyde increased with catalyst mass.The effects of different wavelengths of light irradiation on acetaldehyde degradation were investigated.As shown in Fig.3(e),CuAg/TiO2had an optimal degradation rate of 98.97% under the light wavelength of 385 nm,which was much higher than that of other wavelengths.Impressively,the degradation rate of acetaldehyde on CuAg/TiO2was up to 42.49% under the irradiation of natural sunlight,which was of great significance for practical application.
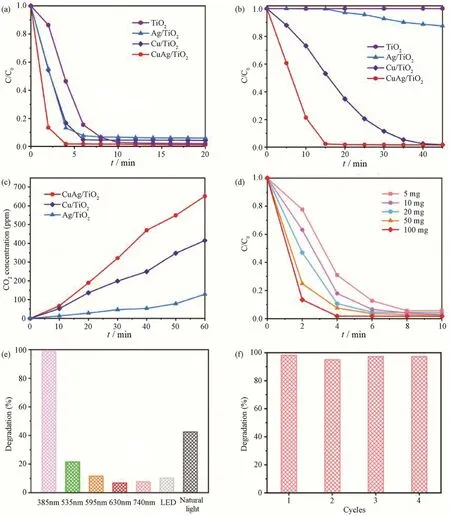
Fig.3 Time course under irradiation图3 光照下反应时间历程
The cyclic catalytic performance of CuAg/TiO2photocatalyst was tested.As shown in Fig.3(f),CuAg/TiO2retained more than 98.89% of the initial activity after four successive rounds,indicating its excellent stability.In brief,CuAg/TiO2showed a good applicability in the photocatalytic degradation of acetaldehyde.
Besides the degradation of acetaldehyde,we also evaluated the catalytic performances of CuAg/TiO2and TiO2for the photodegradation of benzene.As shown in Fig.4(a),both CuAg/TiO2and TiO2exhibited catalytic activities under full spectrum irradiation of xenon lamp.The degradation rate of benzene on CuAg/TiO2was higher than that of TiO2,which reached to 98.63%within 30 minutes.Under the irradiation of visible light,there was no activity on TiO2catalyst,while the optimal conversion rate on CuAg/TiO2was 96.15% (Fig.4(b)).Thus,CuAg/TiO2catalyst can be extended to photodegradation of benzene in the air.
Fig.4 Time course of photocatalytic degradation of benzene on CuAg/TiO2,and TiO2 under irradiation图4 CuAg/TiO2 和TiO2 光催化降解苯的时间历程
2.3 Mechanism studies of excellent catalytic activity for CuAg/TiO2 in photocatalytic degradation of acetaldehyde
In order to elucidate the mechanisms of the remarkable catalytic activity of CuAg/TiO2in photodegradation of acetaldehyde,we investigated its optical and electronic structure.
Fig.5(a) showed the diffuse reflectance ultravioletvisible (UV-Vis) spectra,where CuAg/TiO2and Ag/TiO2exhibited similar optical properties with strong absorption in the full spectrum of 300~750 nm.A broad characteristic plasmonic absorption band of CuAg/TiO2at~510 nm was clearly observed,while that of Ag/TiO2was showed at~490 nm.In contrast,Cu/TiO2had no such characteristic absorption band in the visible light region while the absorbance of light was constantly enhanced.It can be inferred that CuAg/TiO2had the effects of both Ag/TiO2and Cu/TiO2,resulting in the high absorption at about 510 nm.The absorbance of CuAg/TiO2in the visible light region was much higher than that of Ag/TiO2,resulting in the outstanding degradation rate of acetaldehyde on CuAg/TiO2catalyst under visible light irradiation.

Fig.5 (a).UV-Vis absorption spectroscopy;(b) Schematic diagram of the indirect charge-transfer mechanism on CuAg/TiO2;(c) Transient photocurrent responses of CuAg/TiO2 under irradiation of different wavelengths of light图5 (a).紫外-可见吸收光谱;(b).CuAg/TiO2间接电荷转移机理示意图;(c).CuAg/TiO2在不同波长光照射下的瞬态光电流响应
In addition,a Schottky barrier was created at the interface between CuAg nanoparticles and TiO2(Fig.5(b)).The hot electrons generated by the LSPR effect can transfer to the conduction band of TiO2phase,and enter into lowest unoccupied molecular orbital (LUMO) of the adsorbed acetaldehyde through an indirect charge-transfer mechanism[21].The absorbed acetaldehyde molecules were activated,leading to the next level chemical transformation.Fig.5(c) revealed the changes of photocurrent on CuAg/TiO2catalyst under the irradiation of monochromatic light across 385~740 nm.The highest photocurrent signal located at 535 nm,which agreed with the results of UV-Vis absorption spectra.Even under the irradiation of monochromatic light of 740 nm,the signal of photocurrent was also observed.
In order to verify the electrons transferring process and the reaction intermediates of photodegrading acetaldehyde,we analyzed thein situX-ray photoelectron spectroscopy (XPS) spectra of CuAg/TiO2during reaction process (in Fig.6).
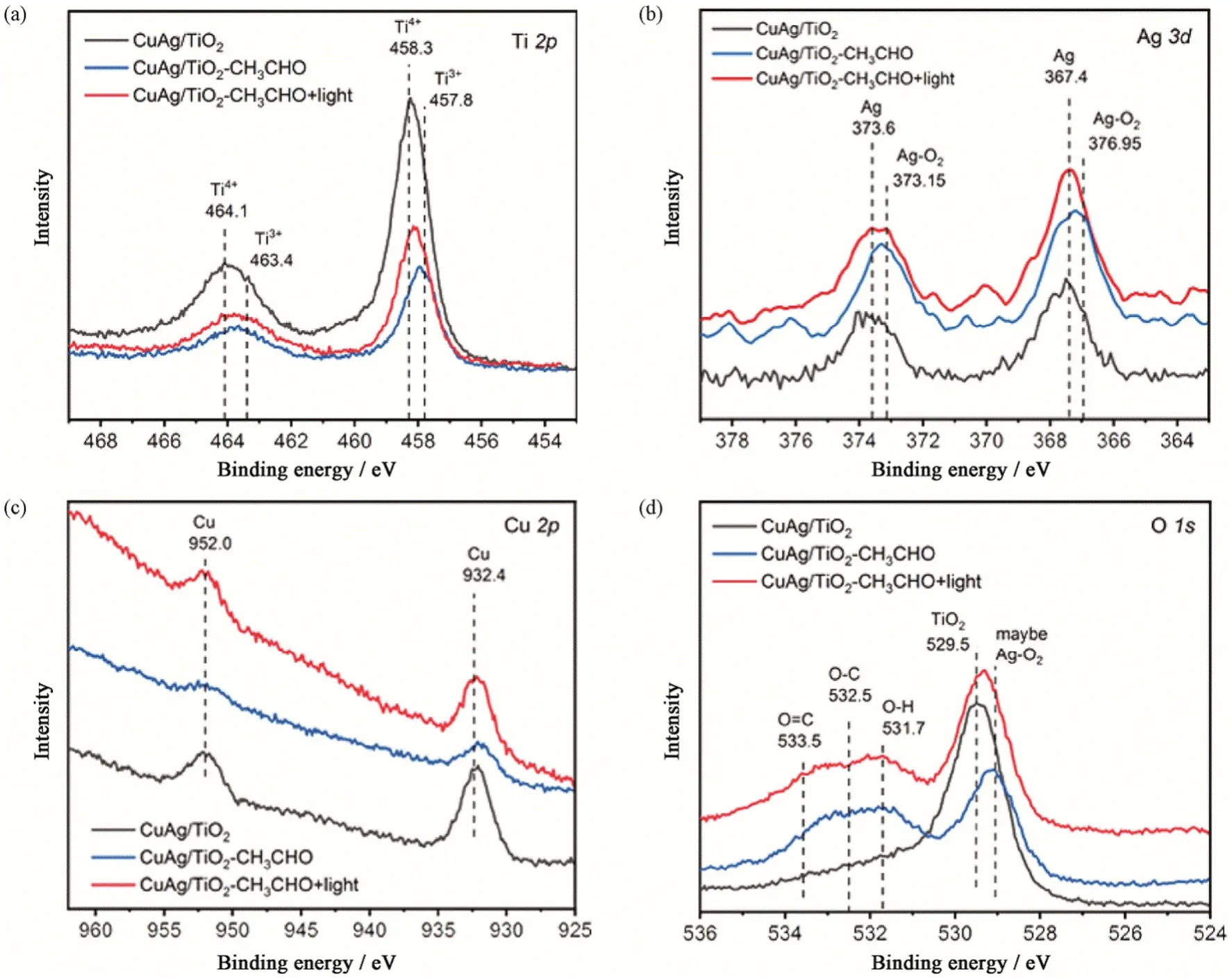
Fig.6 In situ XPS spectra of CuAg/TiO2 with/without light irradiation or CH3CHO图6 有光照及无光照、有甲醛及无甲醛时CuAg/TiO2 的原位X 射线光电子能谱
As displayed in the Ti 2p XPS spectra of CuAg/TiO2(Fig.6(a)),a peak of Ti4+appeared in the CuAg/TiO2catalyst,which was corresponding to TiO2[22].After adding acetaldehyde,a peak of Ti3+appeared in the sample without light irradiation,which was presumed to be the peak of acetaldehyde adsorbed on the Ti sites (Ti-C).The peak of Ti3+disappeared under illumination,indicating the change of Ti-C into TiO2owing to the conversion of acetaldehyde.The Ag 3d XPS spectra (Fig.6(b)) showed the peak binding energy of the oxygen adsorbed on the Ag sites was 376.95 eV without illumination.The peak of Ag-O2changed back to the peak of Ag,indicating that O2on Ag sites was reacted under light irradiation.In the absence of light,acetaldehyde molecules and oxygen were attached to the surface of the catalyst,so the oxygen got hot electrons from Ag sites to form superoxide radicals that reacted quickly with acetaldehyde molecules to produce carbon dioxide[20].As shown in the Cu 2p XPS spectra (Fig.6(c)),the valence state of Cu did not change significantly before and after lighting,indicating that Cu was indeed light absorption units that generated a lot of hot electrons by the LSPR effect under light irradiation rather than active sites.Hot electrons generated by the LSPR effect could activate acetaldehyde molecules and produce superoxide radicals.The O 1s XPS spectra of CuAg/TiO2(Fig.6(d)) exhibited that there were three peaks whose binding energy was respectively corresponding to C=O,C-O and O-H under illumination,manifesting that the products of the photodegradation of acetaldehyde at least had CO2,while other products need to be further confirmed[23-27].
The reaction process and product compositions of photodegrading acetaldehyde were further verified byin situdiffuse reflectance infrared Fourier transform(DRIFT) measurement.As shown in the DRIFT spectra(Fig.7),the inverted peaks indicated that CH3CHO was consumed during the reaction process.The absorption peaks which were corresponding to OH,CO2,and C=C species appeared at the same time[28].After 10 minutes reaction under light irradiation,the amount of OH species in the product significantly increased comparedwith that of under unilluminated condition,suggesting that acetaldehyde degradation occurred rapidly under light irradiation.Cu not only promoted the light absorption,but also solved the intrinsic problems of a wide band gap and short lifetime of charge carriers in TiO2through the indirect charge-transfer mechanism.In addition,TiO2efficiently adsorbed acetaldehyde molecules without light irradiation,while oxygen was absorbed on Ag sites in CuAg/TiO2.Upon the irradiation of light,oxygen absorbed on Ag sites obtained hot electrons and transformed into superoxide radicals,which reacted with acetaldehyde quickly.
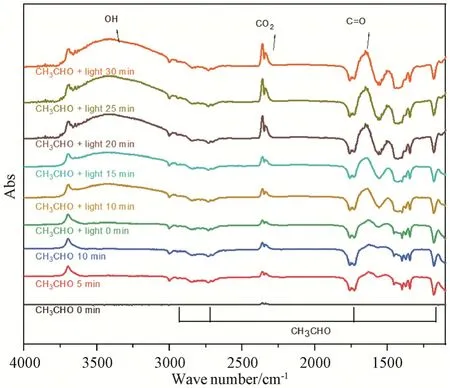
Fig.7 In situ DRIFT spectra for CuAg/TiO2图7 CuAg/TiO2 的原位漫反射红外傅里叶变换光谱
3 Conclusions
In conclusion,we reported a well-designed CuAg/TiO2catalyst with remarkable catalytic performance towards the acetaldehyde photodegradation.CuAg/TiO2exhibited a splendid catalytic performance under the full spectrum and visible light irradiation.After four cycles of catalytic experiments,CuAg/TiO2still retained 98.89% of the initial activity,proving that CuAg/TiO2had an excellent stability and was of great significance on practical application.This work not only exploits a hybrid catalyst with plasmonic metals and semiconductors to achieve an efficient performance toward photodegradation of acetaldehyde,but also opens up new possibilities for designing a series of highly efficient catalysts for removing volatile organic chemicals in the air.

