Efficacy of Sishen Wan (四神丸) on dinitrobenzene sulfonic acidinduced ulcerative colitis and its effect on toll-like receptor 2/interleukin-1 receptor-associated kinase-4/nuclear factor-κB signal pathway
ZHANG Zhaohua,LIU Rong,DU Nana,ZHU Xiangdong
ZHANG Zhaohua,LIU Rong,School of Basic Medicine,Gansu university of Chinese Medicine,Lanzhou 730000,China
ZHU Xiangdong,School of Basic Medicine,Gansu university of Chinese Medicine,Lanzhou 730000,China;College of Chinese Medicine,Ningxia medical university,Yinchuan 750000,China
DU Nana,Department of Thoracic Surgery,Gansu Provincial Cancer Hospital,Lanzhou 730000,China
Abstract OBJECTIVE:To investigate the therapeutic effect of Sishen Wan (四神丸,SSW) on ulcerative colitis (UC)induced by dinitrobenzene sulfonic acid and its effect on toll-like receptor 2/interleukin-1 receptor-associated kinase-4/nuclear factor-κB (TLR2/IRAK4/NF-κB) signaling pathway in colonic tissue.METHODS:In this study,120 Sprague–Dawley rats were randomly divided into blank and model groups.The experimental UC model in rats was established by subcutaneous injection of hydrocortisone+senna gavage for 21 d+dinitrobenzene sulfonic acid (DNBS)/ethanol solution enema.The successful model rats were randomly divided into the model group;mesalazine (0.36 g/kg) group;and high-,medium-,and low-dose SSW (24,12,and 6 g/kg) groups.The model and blank groups were gavaged with equal volumes of distilled water once a day for 21 d.The general condition of the rats was observed,and the body mass,fecal properties,and occult blood were recorded for calculating the disease activity index(DAI) score.The colonic tissue of the rats was collected,and its general morphology and pathological form were noted for obtaining the colonic mucosal injury index(CMDI) score.Hematoxylin-eosin staining was used to view the pathological changes of the colon tissue in each group,apoptosis of the cells was detected using terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling staining,and quantitative real-time polymerase chain reaction was used to measure the expressions of TLR2,myeloid differentiation primary response gene 88(MyD88),IRAK4,and NF-κB p65 mRNA in the colon tissue.The expressions of TLR2,MyD88,IRAK4,and NF-κB p65 protein were detected using western blotting and immunohistochemistry assay,and the levels of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) in the colon tissue were determined using enzyme linked immunosorbent assay.RESULTS:Compared with the blank group,the general condition of the model group was relatively poor.The DAI and CMDI scores of the model group increased significantly (P <0.01),the glands and intestinal mucosa disappeared partially,and several inflammatory cells infiltrated and gathered in the mucosal layer and base layer of the rats in the model group.Furthermore,the cell apoptosis and expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 mRNA and protein in the colon tissue of rats in the model group increased significantly (P <0.01).The levels of IL-1β and TNF-α increased significantly in the colon tissue of rats in the model group (P <0.01).After treatment with SSW,compared with the model group,the general condition of the UC rats improved.Moreover,the DAI and CMDI scores of the UC rats decreased significantly (P <0.05),and the pathological changes in the colon tissue of the UC rats tended to be normal.The cell apoptosis and expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 mRNA and protein in the colon tissue of the UC rats decreased gradually (P <0.01),and the levels of IL-1β and TNF-α decreased significantly (P<0.01).CONCLUSION:SSW can improve the general condition and alleviate the intestinal mucosal injury of UC model rats.Additionally,SSW can inhibit the TLR2/IRAK4/NF-κB signaling pathway,but further studies are required to confirm it.
Keywords:colitis,ulcerative;toll-like receptor 2;interleukin-1 receptor-associated kinases;NF-kappa B;signal transduction;therapeutic uses;Sishen Wan
1.INTRODUCTION
Ulcerative colitis (UC) is a chronic nonspecific inflammatory disease characterized by inflammation of the mucosa and submucosa of the large intestine,which often occurs repeatedly and is difficult to treat.1The etiology and pathogenesis of UC are not completely understood.At present,the occurrence and development of UC are considered to be related to factors such as immune,genetic,infection,and intestinal flora.2-4Some factors,such as diet,drugs,and lifestyle,which may affect the host's microbiota or immune response to the antigen,are associated with an increased risk of UC.5The treatment regimen for UC includes anti-inflammatory agents,such as 5-aminosalicylates (sulfasalazine and mesalamine) and corticosteroids (prednisone and hydrocortisone),and immunosuppressants,such as azathioprine,mercaptopurine,cyclosporine,and infliximab,based on the etiology.6
Several studies have been conducted on the treatment of UC using Traditional Chinese Medicine.Such treatments have achieved good curative effects in clinical and basic research.7,8Arbutin isolated from the leaves of Yuejuye(Folium Vaccinii Vitis-idaeae) can alleviate the inflammatory response and maintain the intestinal mucosal barrier of UC rats induced by dextran sodium sulfate.9Xiaokui Jiedu decoction (消溃解毒汤) contains several components with pharmacological activity,which can target the hub gene and regulate the inflammation-and oxidative stress-related pathways in UC intestinal cells to achieve therapeutic effect via network pharmacology.10Ding's herbal enema (DHEP)is a traditional Chinese medicine therapy,which has been employed to treat UC in China.The therapeutic mechanism of DHEP may be related to the regulation of the gut microbiota composition and maintenance of the balance between Treg and Th17 cells.11At present,it has been found that Sishen Wan (四神丸,SSW) can regulate intestinal mucosal immunity and aid in managing UC based on literature mining,clinical practice observation,and preliminary investigations of our research group.SSW not only exerts a good therapeutic effect on colitis when used alone but also exerts a synergistic effect in the treatment of colitis when combined with other traditional Chinese medicine or western medicine in clinical research.12,13,14In basic research,Zhao et al found that SSW effectively alleviated experimental chronic colitis induced by trinitrobenzene sulfonic acid (TNBS) as a result of inhibiting the wingless-type MMTV integration site family (Wnt)/β-Catenin signaling pathway.15Wanget al16observed that SSW demonstrated a good therapeutic effect on chronic colitis,which might have been achieved by inhibiting the activation of the NF kappa B essential modulator/ NEMO-like kinase/nuclear factor-κ B (NEMO/NLK/NF-κB) signaling pathway.Owing to the multitargeting effect of SSW and the complexity of the occurrence of UC,the treatment of the disease with SSW may be related to the alterations in other molecular signaling pathways.The regulation of toll-like receptors (TLRs) and the related signal transduction can regulate immunity and reduce intestinal inflammation to treat UC.17,18Toll-like receptor 2/myeloid differentiation primary response gene 88/interleukin-1 receptor-associated kinase-4/NF-κB(TLR2/MyD88/IRAK4/NF-κB) signaling pathway is one of the key TLR-related signaling pathways and plays a pertinent role in the initiation and maintenance of inflammation.15However,whether TLRs is activated in UC has not been investigated so far.Therefore,this study aimed to explore one of the molecular mechanisms of UC based on the TLR2/MyD88/IRAK4/NF-κB signaling pathway.Furthermore,this research intended to explore whether SSW protects the immune-damaged intestinal mucosa via regulation of the TLR2/IRAK4/NF-κB signaling pathway.The findings are expected to provide a new perspective and theoretical support for the treatment of UC.
2.MATERIALS AND METHODS
2.1.Experimental materials
120 specific pathogen free (SPF) Sprague–Dawley (SD)rats weighing 180-220 g,half male and half female,were purchased from the experimental animal center of Gansu University of Chinese medicine [SYXK(Gan)2020-0009],this study was approved by Animal Experimental Ethical Inspection of Gansu University of Chinese Medicine (Ethics No.2020-260).
Dinitrobenzene sulfonic acid (DNBS) (Sigma-Aldrich,St.Louis,MO,USA);hematoxylin-eosin (HE) staining Kit (Solarbio Science &Technology Co.,Ltd.,Beijing,China);terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) apoptosis detection kit (Yeasen Biotechnology Co.,Ltd.,Shanghai,China);immunohistochemical staining kit (ZSGB-Bio Co.,Ltd.,Beijing,China);rat tumor necrosis factor-α (TNF-α)enzyme linked immunosorbent assay (ELISA) kit(Enzyme-linked Biotechnology Co.,Ltd.,Shanghai,China);rat interleukin-1β (IL-1β) ELISA kit (Enzymelinked Biotechnology Co.,Ltd.,Shanghai,China);radio immunoprecipitation assay (RIPA) lysate (Solarbio Science &Technology Co.,Ltd.,Beijing,China);nuclear protein extraction kit (Solarbio Science &Technology Co.,Ltd.,Beijing,China);total RNA extraction kit (Takara Biomedical Technology Co.,Ltd.,Beijing,China);PrimeScript™ RT reagent kit with gDNA rraser (Perfect Real Time) (Takara Biomedical Technology Co.,Ltd.,Beijing,China);TB Green®Premix Ex Taq™ (Tli RNaseH Plus) (Takara Biomedical Technology Co.,Ltd.,Beijing,China);Sodium Dodecyl Sulfate Poly Acrylamide Gel Electrophoresis (SDSPAGE) Gel kit Solarbio Science &Technology Co.,Ltd.,Beijing,China);rabbit anti-TLR2 polyclonal antibody(Huabio,Hangzhou,China),rabbit anti-IRAK4 polyclonal antibody (Bioss,Beijing,China),rabbit anti-NF-κB p65 polyclonal antibody (CST,Danforth,MA,USA),rabbit anti phospho-NF-κB p65 (Ser468) antibody(Bioss,Beijing,China),rabbit anti-MyD88 polyclonal antibody (Abcam Cambridge,UK),rabbit anti β-actin antibody (Abcam Cambridge,UK).
2.2.Drugs
SSW was composed of Buguzhi (Fructus Psoraleae),Wuzhuyu (Fructus Evodiae Rutaecarpae),Roudoukou(Semen Myristicae),Wuweizi (Fructus Schisandrae Chinensis),Shengjiang (Rhizoma Zingiberis Recens),and Dazao (Fructus Jujubae) were identified to be authentic by Professor Yang Fude of the Gansu University of Chinese Medicine.SSW were prepared into decoctions according to the indicated dose ratios(155,77.5,77.5,38.8,77.5,and 77.5 g,ratio:4∶2 ∶2 ∶1 ∶2 ∶2,respectively).Briefly,the abovementioned herbs (raw materials) were mixed according to the ratio of 4∶2 ∶2 ∶1 ∶2 ∶2 and soaked in 8 times the volume of water for 0.5 h,followed by decoction in low fire for 20 min after boiling in high fire,the liquid medicine was filtered through a gauze,and the residue was again decocted to obtain the liquid medicine as per the method.The liquid medicine obtained twice was mixed and concentrated into a liquid medicine containing 1 g/mL of crude medicine using a rotary evaporator,which was stored in the refrigerator after repackaging.A previous study19measured the main active ingredients of SSW by high performance liquid chromatographyelectrospray ion trap mass spectrometry (HPLCESIMS/MS),which included isopsoralen (1293.7 mg/g),schizandrin (258.0 mg/g),g-schizandrin (131.5 mg/g),psoralen (131.08 mg/g),and deoxyschizandrin (72.6 mg/g),among others.
Senna leaf was purchased from Lanzhou Huirentang Pharmaceutical Chain Co.,Ltd.and authenticated by Professor Yang Fude of the Gansu University of Chinese Medicine.The aqueous extract of senna leaf was obtained as follows:according to the body weight of rats,an appropriate amount of senna leaf (10 g/kg) was soaked in 8 times the volume of water for 0.5 h,followed by decoction in a low fire for 20 min after boiling in a high fire;the liquid medicine was filtered through a gauze,and the residue was decocted again to obtain the liquid medicine according to the method mentioned earlier.The liquid medicine obtained twice was mixed and concentrated into a liquid form of medicine containing 1 g/mL of the crude medicine using a rotary evaporator,followed by storage in the refrigerator after repackaging.Mesalazine Sustained Release granules (batch No.200707) was purchased from Shanghai Ethypharm Pharmaceutical Co.,Ltd.(Shanghai,China),which were dissolved in distilled water to prepare the corresponding solution according to the gavage volume (10 mL/kg) and concentration (0.36 g/kg) for standby.
2.3. Establishment of an experimental UC model in rats
According to a past study,20SPF SD rats were administered with 10 mL·kg-1·day-1of an aqueous extract of senna leafviaoral gavage and injected subcutaneously at the dose of 15 mg·kg-1·day-1of hydrocortisone once a day for 21 d.After 21 d,the rats were fasted,but allowed free access to water for 24 h.After inducing abdominal anesthesia with 2% sodium pentobarbital (0.2 mL/100 g),a 12-cm long polypropylene tube (2-mm diameter) with several holes on the side was inserted into the colon from the anus.DNBS/ethanol solution (25 mg/DNBS+0.25 mL 50%ethanol) was gently injected into the intestinal cavity of the rats up to approximately 7-8 cm depth from the anus with a rubber infusion tube and left for several minutes as such,subsequently followed by an injection of approximately 0.4 mL of air,pinching of the rat's anus,lifting the rat from the tail and keeping it upside down for 1 min to prevent the backflow of the injected liquid and ensuring that the liquid fully contacted the colon.The anesthetized rats were raised normally after waking up.The model was evaluated according to the methods introduced in the literature21to obtain rats with a successful UC model.
2.4.Group administration
According to a previous study,22the high-,medium-,and low-dose SSW groups were administered with SSWviaoral gavage at the doses of 24,12,and 6 g/kg,respectively.The mesalazine group was administered with 0.36 g/kg mesalazineviaoral gavage.The blank and model groups were administered with an equal volume of distilled water via oral gavage once a day for 21 d.All groups were administered with the oral gavage on the second day after modeling once a day for 21 d.
2.5.Sample collection
All animals were anesthetized with 3% sodium pentobarbital (0.2 mL/100 g) via an abdominal injectionafter the intervention,followed by execution by cervical dislocation.The segment of the colon tissues with the most serious lesion in the rats was intercepted,washed with normal saline,and then divided into two portions,and one part was fixed in the 4% paraformaldehyde solution.The other part was directly placed in a centrifuge tube and frozen in liquid nitrogen for Western blotting and the quantitative real-time polymerase chain reaction (qPCR) assay.
2.6.Observation of the general conditions
The eating,drinking,activity,hair color,stool characteristics,and blood in the stool of rats were observed every day,and the body mass of rats was monitored regularly,with the disease activity index (DAI)scored as suggested elsewhere.23
The most severely injured parts of the colon and ileum were dissected,and the colonic mucosal injury index(CMDI) was calculated as suggested elsewhere.24
2.7.HE staining
After fixing the cells with 4% polyformaldehyde solution,dehydrated in the alcohol solutions at different concentrations and embedded in paraffin,the colon tissues were sectioned into 5-μm thick slices.The sections were then stained with hematoxylin staining solution for 5 min,rinsed with tap water for 5 min,differentiated with 1% hydrochloric acid ethanol for 30 s,rinsed with tap water for 5 s,dyed in 0.5% eosin staining solution for 3 min,rinsed with distilled water for 30 s,and dehydrated in gradient alcohol solutions.The pathological features of the colon were observed under a bright-field microscope.
2.8.TUNEL staining
After dewaxing with the alcohol solutions and dimethylbenzene solutions,the 5-μm-thick paraffin sections of the colon tissues were incubated in 20 μg/mL protease K solution at room temperature for 30 min,washed with PBS,incubated in a 100-μL equilibration buffer at room temperature for 15 min,treated with 50-μL TdT buffer,covered with a sealing film,and incubated at 37 ℃ for 60 min in the dark,followed by washing with PBS,treating with 2 μg/mL PI for incubation at room temperature for approximately 5 min in the dark,and washing with PBS.The apoptotic cells of the colon tissues were observed by fluorescence microscopy.
2.9.ELISA assay
The colon tissues were used to detect the level of TNF-α and IL-1β by ELSIA according to the kit instruction.The absorbance (A) was measured by a microplate reader at 450 nm,and the concentration of TNF-α and IL-1β in each sample was calculated according to the formula fitted by the standard curve.
2.10.Western blotting
The colon tissues were crushed and grounded into a powder in liquid nitrogen and then treated with RIPA lysate containing 100-μM PMSF,followed by incubation for 30 min at 4 ℃ to extract the total protein.BCA assay was performed to estimate the protein concentration.Then,5 × loading buffer was added to the total protein extract.Equal amounts of total protein (60-80 μg) were separated by 10% SDS-PAGE and transferred onto 0.45-μm PVDF membranes.After blocking with 5% fat-free milk in Tween-20 buffered TBS,the membranes were incubated in primary antibodies against TLR2,MyD88,IRAK4,and NF-κB p65 proteins at 4 ℃ overnight.Subsequently,the membranes were washed with TBST buffer and incubated in horseradish peroxidase–conjugated secondary antibodies at room temperature for 2 h.An enhanced luminescence reagent was used to detect the labeled protein bands by chemiluminescence and then photographed by the HP Scanjet 5500 (Hewlett-Packard,Palo Alto,CA,USA),while Image J (National Institutes of Health,Bethesda,MD,USA) was used to analyze the gray value of the band in order to measure the relative protein concentration.
2.11.qPCR assay
The total RNA in the colon tissues was extracted with Trizol,quantified with a micro spectrophotometer,and subsequently reverse-transcribed into cDNA,which were subjected to fluorescence quantitative detection by RT-PCR with the S6 Universal SYBR qPCR Mix.A two-step process was conducted as follows for RT-PCR:Step1∶ 1 cycle of 95 ℃ for 30 s;Step2:40 cycles of 95℃for 10 s,60 ℃ 30 s.According to the PCR standard amplification curve,the original Ct values of the target genes were obtained,and the 2−△△Ctmethod was applied for semiquantitative analysis.Table 1 shows the detailed primer sequence of each gene.

Table 1 primer sequence
2.12.Immunohistochemistry
After dewaxing with the alcohol and dimethylbenzene solutions,the 5-μm-thick paraffin sections of the colon tissues were placed in 0.01-M citric acid sodium buffer(pH 6.0) and boiled in the microwave oven to the boil microwave acid antigen repair,incubated with 3%hydrogen peroxide at room temperature for 10 min,and blocked with goat serum at room temperature for 15 min.The sections were incubated in an appropriate amount of diluted primary antibodies overnight at 4 ℃,washed with PBS,and then incubated in an appropriate amount of biotin-labeled secondary antibodies at 37 ℃ for 30 min.The horseradish peroxidase-labeled Streptomyces ovalbumin working solution was then added to the sections and incubated at 37 ℃ for 10 min.A color reaction was developed by incubation with the DAB solution for 15 min at room temperature.After washing,the sections were counterstained with hematoxylin and differentiated with 0.5% hydrochloric acid and alcohol.Subsequently,the sections were dehydrated in alcohol solutions at different concentrations.The expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 proteins were observed by a microscope under the bright field.Image J software (National Institutes of Health,Bethesda,MD,USA) was applied to measure the integrated optical density (IOD) of the colored portion in the photo.The actual IOD value of each group was divided by the mean IOD of the control group,and the obtained ratio was statistically analyzed to indicate the relative expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 proteins.
2.13.Statistical methods
SPSS 22.0 statistical software (IBM Corp.,Armonk,NY,USA) was used to analyze the raw data,and the relevant normal measurement data were expressed as mean ±standard deviation (χ ¯).One-way analysis of variance was used to analyze the raw data which obeyed normal distribution for multiple comparisons followed by the least significant difference test for Post Hoc Test.P<0.05 indicated that the difference was significant.
3.RESULTS
3.1.Evaluation of the UC model
The rats in the blank group always showed quick response,normal dietary habit,normal activity,thick and soft hair with luster,and brown particles in feces.The model group of UC rats demonstrated symptoms of UC,including emaciation (the weight of the model group was 20-30 g less than that of the blank group on the 11th and 21th day of modeling),loose stool,pollution around the anus,inactivity and reduced feeding,fear of cold,curling or arched back in groups,and withered and scattered hair.Furthermore,the DAI score of the model group was significantly higher than that of the normal group (P<0.05).The above results indicated that the modeling was successful.
3.2.General conditions
The body mass of the rats in the blank group,which were energetic and responsive,increased significantly.The rats displayed normal dietary habits,had glossy hair,and there was no loose stool or pus,blood,and mucus in the stool.After modeling,the rats exhibited changes such as reduced food intake;weight loss;less movement;poor spirit;and irregular,purulent,and bloody stool.After intervention with SSW and mesalazine,the activity,appetite,and body mass of the rats increased.The general condition of the rats in the high-and medium-dose groups and mesalazine group improved significantly.
3.3.Effects of SSW on body weight and DAI and CMDI scores of UC model rats
Compared with the blank group,the body weight of the rats in the model group decreased significantly (P<0.05)and the DAI and CMDI scores of the rats in the model group increased significantly (P<0.05).Compared with the model group,the body weight of rats in the mediumand high-dose SSW groups and mesalazine group increased significantly (Model groupvsmesalazine group,P<0.05) and the DAI and CMDI scores of the rats in the medium-and high-dose SSW groups and mesalazine group decreased significantly (P<0.05)(Figure 1A,B,C).

Figure 1 Effects of SSW on body weight,DAI score and CMDI score of UC model rats
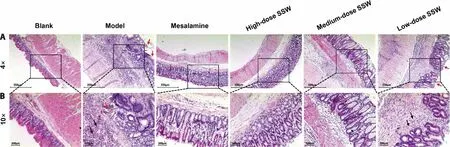
Figure 2 SSW improved the pathological lesions of colon tissue in UC model rats (hematoxylin-eosin staining)
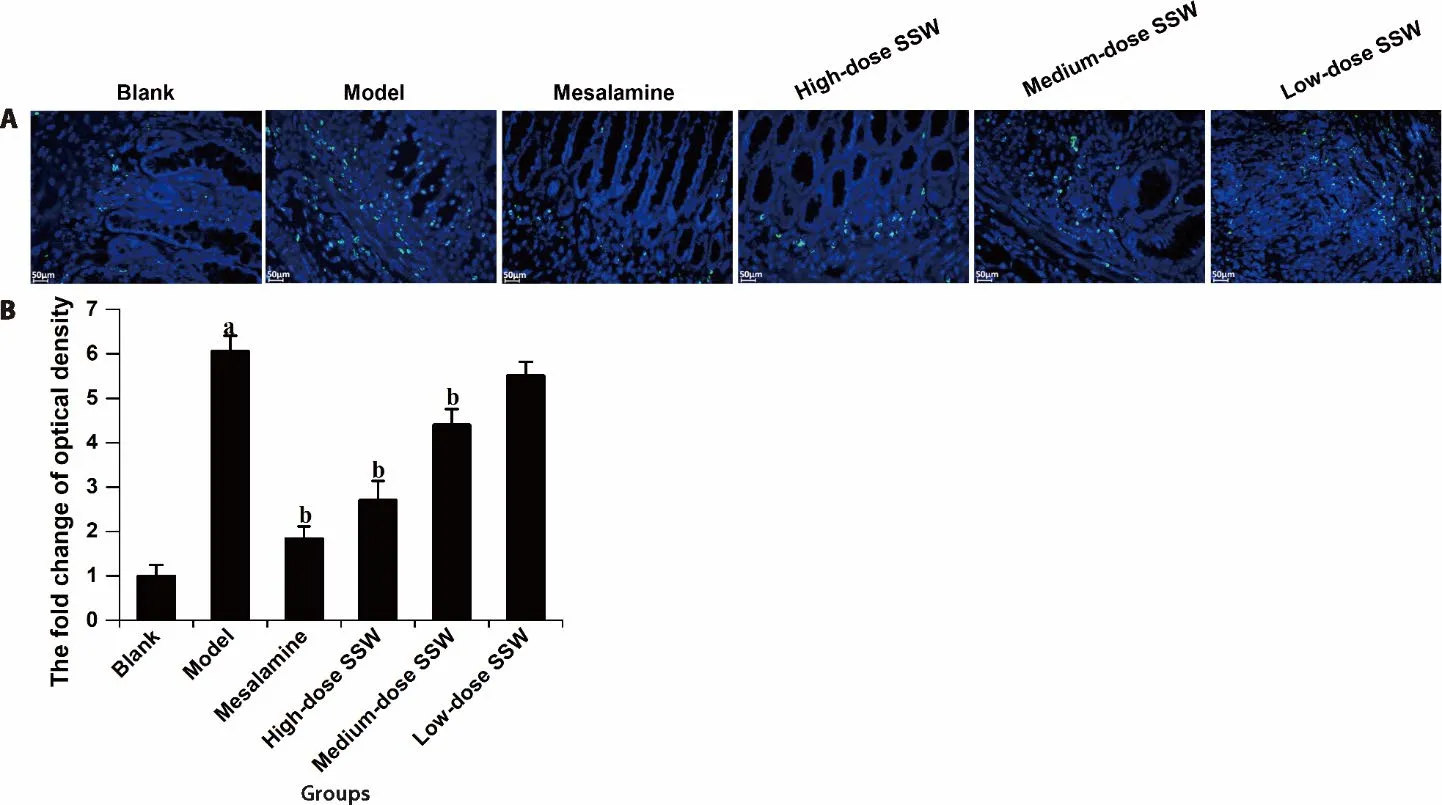
Figure 3 SSW alleviated the apoptosis of colonic cells in UC model rats

Figure 4 SSW reduced the level of IL-1 and TNF-α in colon tissues of UC model rats (ELISA assay)
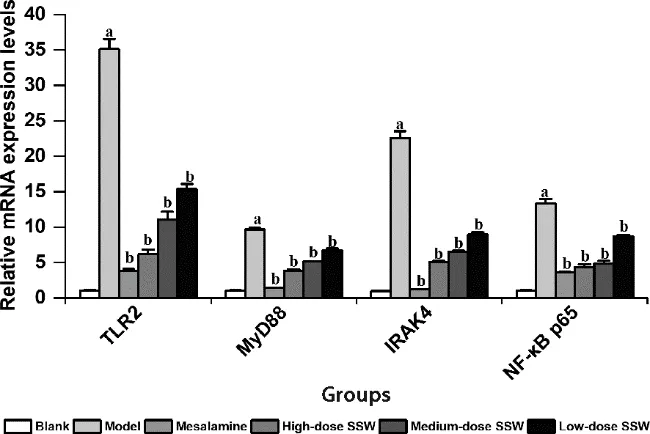
Figure 5 SSW reduced the level of TLR2,MyD88, IRAK4 and NFκB p65 mRNAs in colon tissue of UC model rats,which were detected by qPCR
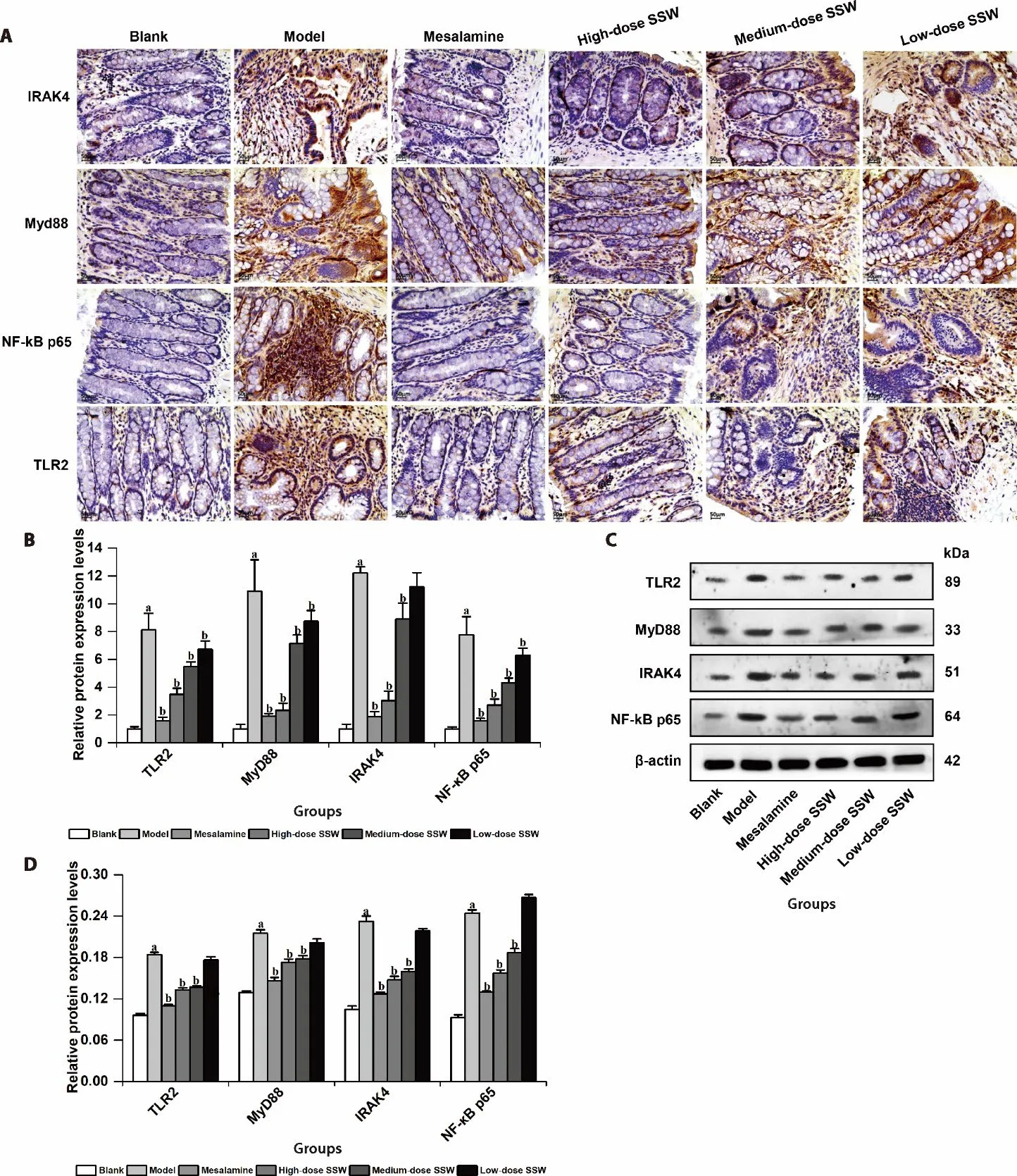
Figure 6 SSW reduced the level of TLR2,MyD88,IRAK4 and NF-κB p65 proteins in colon tissue of UC model rats
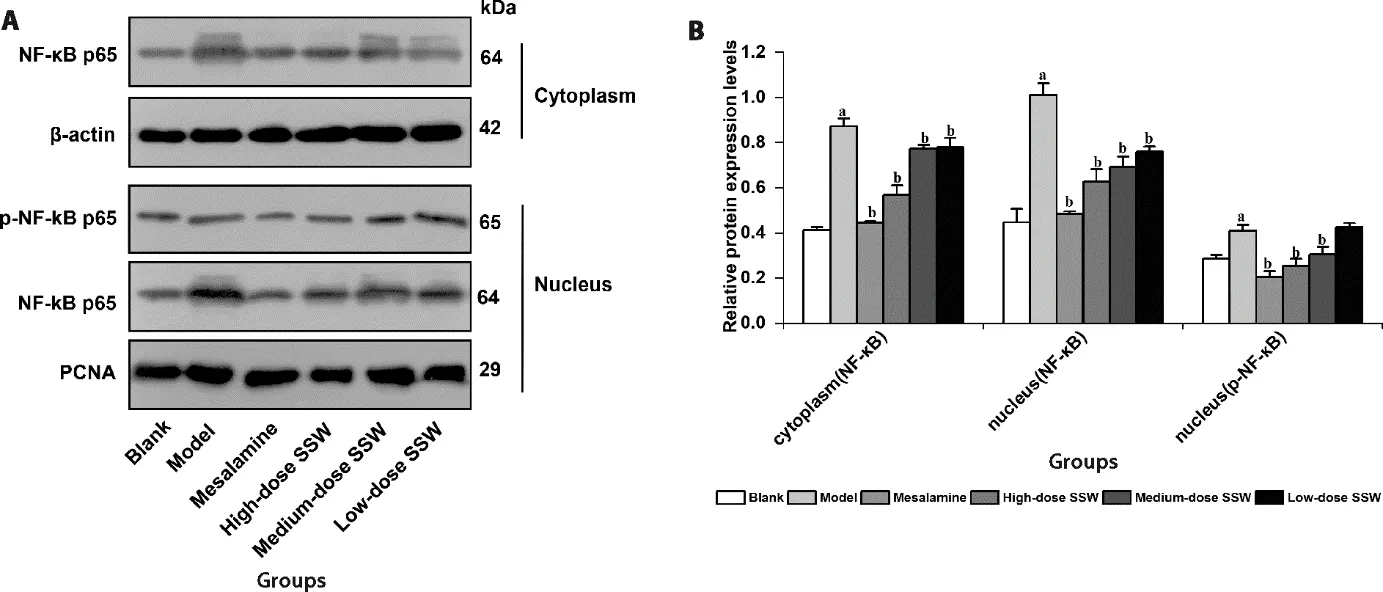
Figure 7 SSW reduced the level of NF-κB p65 protein in the nucleus of colon tissue of UC model rats
3.4.SSW alleviated the pathological lesions in the colon tissue of UC model rats
In the rats belonging to the blank group,the structure of the colon tissue was normal,the structure of the intestinal mucosa was complete,and the glands were evenly distributed.Compared with the blank group,the intestinal mucosa disappeared partially,the glands disappeared,and several inflammatory cells infiltrated and gathered in the mucosal layer and grass-roots level in the rats of the model group.Compared with the model group,the inflammatory cells decreased and the mucosal structure recovered to normal in varying degrees in the SSW and mesalazine groups.The treatment effect of mesalazine and high-dose SSW is considered to be optimum when the mucosal structure is close to that of the blank group.
3.5.SSW alleviated apoptosis of the colonic cells of UC model rats
Compared with the blank group,the proportion of apoptotic cells in the colon tissue of the model group increased significantly (P<0.05).Compared with the model group,the proportion of colonic cell apoptosis in the medium-and high-dose SSW groups and mesalazine group decreased significantly (P<0.05).However,there was no significant difference in the proportion of apoptotic cells between the high-dose SSW group and mesalazine group.
3.6.SSW reduced the levels of IL-1 and TNF-α in the colon tissue of UC model rats
Compared with the blank group,the levels of IL-1 and TNF-α in the colon tissue of model group increased significantly (P<0.05).Compared with the model group,the levels of IL-1β and TNF-α in the colon tissue of the medium-and high-dose SSW groups and mesalazine group decreased significantly (P<0.05).
3.7.SSW reduced TLR2,MyD88,IRAK4,and NF-κB p65 mRNA levels in the colon tissue of UC model rats
Compared with the blank group,the expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 mRNAs in the colon tissue of the model group increased significantly(P<0.05).Compared with the model group,the expression levels of TLR2,MyD88,IRAK4,and NF-κB p65 mRNAs in the colon tissue of the medium-and highdose SSW groups and mesalazine group decreased significantly.
3.8.SSW reduced TLR2,MyD88,IRAK4,and NF-κB p65 protein levels in the colon tissue of UC model rats
Compared with the blank group,the levels of TLR2,MyD88,IRAK4,and NF-κB p65 proteins in the colon tissue of the model group increased significantly (P<0.05).Compared with the model group,the levels of TLR2,MyD88,IRAK4,and NF-κB p65 proteins in the colon tissue of the medium-and high-dose SSW groups and mesalazine group decreased significantly (P<0.05).
3.9.SSW reduced the level of NF-κB p65 protein and inhibited the activation of NF-κB p65 in the nucleus of the colon tissue of UC model rats
Compared with the blank group,the levels of NF-κB p65 and p-NF-κB p65 protein in the nucleus of the colon tissue in the model group increased significantly(P<0.05).Compared with the model group,the level of NF-κB p65 and p-NF-κB p65 protein in the nucleus of the colon tissue in the medium-and high-dose SSW groups and mesalazine group decreased significantly (P<0.05).
4.DISCUSSION
This study established that SSW can significantly alleviate the pathological changes in the colon tissue of UC model rats,reduce the apoptosis of the cells in the colon tissue,and exert a therapeutic effect on UC.Further investigations revealed that SSW could reduce the mRNA and protein levels of TLR2,MyD88,IRAK4,and NF-κB p65 as well as the levels of IL-1β and TNF-α in the colon tissues and decrease the NF-κB p65 protein level in the colonic nucleus of UC model rats.As the TLR2/IRAK4/NF-κB signaling pathway plays a key role in the occurrence,development,and maintenance of UC,the results suggest that SSW can inhibit the activation of this pathway and reduce the inflammatory response in the colon tissue of UC model rates,thus exerting a therapeutic effect on UC.
The main clinical manifestations of UC are diarrhea with purulent and bloody stool,which are accompanied by abdominal distension,loss of appetite,nausea,vomiting,etc.This study found that SSW can significantly improve the clinical manifestations,alleviate the diarrhea and bloody stool,and enhance the mental status of UC rats.Furthermore,the improvement of colonic histopathology in UC rats confirmed the therapeutic effect of SSW.After treatment with SSW,the number of inflammatory cells infiltrating the colonic tissue of UC rats reduced significantly;moreover,the edema and the number of microvessels reduced significantly,and the damaged colonic epithelial structure healed gradually.These findings are consistent with the results of previous studies,which have confirmed the therapeutic effect of SSW on UC.15,16Apoptosis occurs in the colonic epithelial cells under prolonged inflammatory conditions,which destroy the colonic epithelial tissue.In turn,the apoptotic colonic epithelial cells induce and aggravate the inflammatory response,which leads to a vicious cycle in the occurrence and development of UC.These events result in the recurrence of UC and amplify the clinical manifestations of the disease.Inhibiting the inflammation or apoptosis of the colonic cells is one of the main strategies and targets in the management of UC.25This study observed that SSW could inhibit apoptosis in the colonic cells of UC model rats;furthermore,the levels of IL-1 and TNF-α decreased significantly in the colon tissues after the intervention with SSW.IL-1 and TNF-α are among the main factors that induce and maintain the inflammatory response of UC.The inhibitory effect of SSW on IL-1 and TNF-α further confirms the therapeutic effect of SSW on UC.The molecular pathway of SSW in the treatment of UC has been shown to be involved in the inhibition and activation of multiple signaling pathways.Wanget al16documented that the therapeutic effect of SSW on chronic colitis was achieved by inhibiting the NEMO/NLK signaling pathway for the activation of NFκB.Zhaoet al15identified that SSW can also effectively alleviate the experimental chronic colitis induced by TNBSviainhibition of the Wnt/β-Catenin signaling pathway.In addition,our research group found that SSW can improve the general condition and alleviate the intestinal mucosal injury of UC model rats,whose mechanism may be related to the inhibition of the PI3K/Akt/mTOR signaling pathway.26,27SSW includes several Chinese herbal medicines with complex components.UC also involves many pathways related to inflammation,apoptosis,and proliferation that promote the occurrence and development of the disease at the molecular,cellular and organ levels.Hence,SSW showed multitargeted effect in the treatment of UC.SSW may achieve the effect of systematic treatment by inhibiting or activating multiple signaling pathways to treat UC.NF-κB is the main mediator of inflammation,28which is activated in many chronic diseases and autoimmune diseases.29,30NF-κB is also activated in UC,thereby promoting inflammation and creating a positive and negative feedback loop by regulating the expression of inflammatory factors,such as TNF-α,IL-1,and IL-17.Thus,the inflammatory response is aggravated,which results in chronic diseases.31This study observed that the expression of NF-κB increased significantly and that the levels of NF-κB entering the nucleus and undergoing phosphorylation increased significantly in UC rats.Intervention with SSW can significantly reduce the expression of NF-κB as well as the amount of NF-κB entering the nucleus and undergoing phosphorylation in UC rats.The above results suggest that SSW can inhibit the activation of NF-κB to block the inflammatory response in the colon of UC rats to achieve the therapeutic effect.As the main mediator of inflammatory activation and maintenance,multiple pathways can activate NF-κB,including PI3K/Akt,NEMO/NLK,TLR4/MyD88,and MAPK pathways.31-34Previous studies have shown that TLR2 gene is highly expressed in the colonic mucosa of UC rats.35,36TLR2 is an important member of the pattern recognition receptor family,which can recognize several exogenous and endogenous pattern molecules.Subsequent to the activation of TLR2,the downstream signaling pathways are activated,which can lead to the secretion of class I interferons and inflammatory cytokines,thereby initiating the immune response.IL-1 receptor associated kinase 4 (IRAK4) is one of the downstream kinases of the TLR2-mediated signaling pathway.37Upon the activation of IRAK4,the signal is amplified via a complex phosphorylation cascade.On the one hand,the activated IRAK4 maintains the stability of IL-1R and other factors so that the pathway can be continuously activated by IL-1.On the other hand,IRAK4 activates a series of kinases,such as IRAK1,viaphosphorylation.This step is followed by the activation of NF-κB,which leads to the release of inflammatory factors,including TNF-α,IL-1β,and many other cytokines.38It has been shown that the TLR2/IRAK4/NF-κB pathway is closely related to inflammatory bowel disease,rheumatoid arthritis,psoriasis,gout,asthma,and cancer.39This study found that the expression levels of TLR2 and IRAK4 in the colon tissue of UC rats are significantly increased.However,SSW significantly reduced the expression levels of TLR2 and IRAK4 in UC rats,which suggests that the TLR2/IRAK4/NF-κB pathway is activated and that SSW can inhibit the pathway and assuage the inflammatory response in the colon tissue of UC.In conclusion,SSW has a good therapeutic effect on UC and can significantly reduce the pathological changes in the colon tissue of UC rats.Moreover,SSW can inhibit apoptosis,alleviate the inflammatory response,and inhibit the activation of the TLR2/IRAK4/NF-κB pathway closely related to inflammation at the molecular level.This pathway may be one of the important molecular pathways of SSW in the treatment of UC.Owing to the multitargeting effect of SSW and the complexity of UC pathogenesis,SSW may cause systematic changes in the cellular signaling pathway in the treatment of UC;however,further research is needed in the future to confirm the findings.
5.REFERENCES:
1.Eisenstein M.Ulcerative colitis:towards remission.Nature 2018;563:S33.
2.Kobayashi T,Siegmund B,Le Berre C,et al.Ulcerative colitis.Nat Rev Dis Primers 2020;6:73.
3.Ungaro R,Mehandru S,Allen PB,et al.Ulcerative colitis.Lancet 2017;389:1756-70.
4.Ramos GP,Papadakis KA.Mechanisms of disease:inflammatory bowel diseases.Mayo Clin Proc 2019;94:155-65.
5.Du L,Ha C.Epidemiology and pathogenesis of ulcerative colitis.Gastroenterol Clin North Am 2020;49:643-54.
6.Wehkamp J,Stange EF.Recent advances and emerging therapies in the non-surgical management of ulcerative colitis.F1000Res 2018;7:F1000 Faculty Rev-1207.
7.Zheng L,Wen XL,Dai YC.Mechanism of Jianpi Qingchang Huashi Recipe in treating ulcerative colitis:A study based on network pharmacology and molecular docking.World J Clin Cases 2021;9:7653-70.
8.Chen W,He L,Zhong L,et al.Identification of active compounds and mechanism of Huangtu decoction for the treatment of ulcerative colitis by network pharmacology combined with experimental verification.Drug Des Devel Ther 2021;15:412540.
9.Zhang C,Zhu H,Jie H,et al.Arbutin ameliorated ulcerative colitis of mice induced by dextran sodium sulfate (DSS).Bioengineered 2021;12:11707-15.
10.Wang B,Liu Y,Sun J,et al.Exploring the potential mechanism of Xiaokui Jiedu Decoction for ulcerative colitis based on network pharmacology and molecular docking.J Healthc Eng 2021;2021:1536337.
11.Tan YY,Ding Y,Zheng X,et al.Ding's herbal enema treats dextran sulfate sodium-induced colitis in mice by regulating the gut microbiota and maintaining the Treg/Th17 cell balance.Exp Ther Med 2021,22:1368-79.
12.Ma JZ.Effect of Sishen Wan combined with Shenling Baizhu San on ulcerative colitis of spleen kidneyYangdeficiency type.Hefei:Anhui University of Chinese Medicine,2021:12-56.
13.Cheng XH,Zhang QL,Gong XL,et al.Clinical study on Sishen Pills combined with mesalazine in treatment of ulcerative colitis.Xian Dai Yao Wu Yu Lin Chuang 2018;33:1085-8.
14.Xie H,Ming H.Sishen Pill combined with vesiculation cupping in the treatment ofYangdeficiency of spleen and kidney diarrhoea for 30 Cases.Zhong Guo Zhong Yi Yao Xian Dai Yuan Cheng Jiao Yu 2019;17:73-5.
15.Zhao HM,Liu Y,Huang XY,et al.Pharmacological mechanism of Sishen Wan(®) attenuated experimental chronic colitis by inhibiting wnt/β-catenin pathway.J Ethnopharmacol 2019;240:111936.
16.Wang HY,Zhao HM,Wang Y,et al.Sishen Wan(®) ameliorated trinitrobenzene-sulfonic-acid-induced chronic colitisviaNEMO/NLK signaling pathway.Front Pharmacol 2019;10:170-80.
17.Wang G,Xu B,Shi F,et al.Protective effect of methane-rich saline on acetic acid-induced ulcerative colitisviablocking the TLR4/NF-κB/MAPK pathway and promoting IL-10/JAK1/STAT3-mediated anti-inflammatory response.Oxid Med Cell Longev 2019;2019:7850324.
18.Yang C,Guo X,Wang J,et al.Relationship between small intestinal bacterial overgrowth and peripheral blood ET,TLR2 and TLR4 in ulcerative colitis.J Coll Physicians Surg Pak 2020;30:245-9.
19.Zhang XX,Li XN,Hu S,et al.Simultaneous determination of nine bioactive components in Sishen Pills by HPLC-ESI-MS/MS.Zhong Cao Yao 2018;49:2070-5.
20.Zheng J,Cao ZB,Zhu Y.Discussion on establishment and evaluation of ulcerative colitis animal models with spleen-kidney yang deficiency pattern based on the theory of "combination of disease with syndrome".Zhong Guo Zhong Yi Yao Xin Xi Za Zhi 2016;23:16-8.
21.Wu YH,Xu YQ,Li HL,et al.Preparation of a rat model of ulcerative colitis with spleen and kidney Yang deficiency.Zhong Guo Shi Yan Dong Wu Xue Bao 2016;24:116-9.
22.Cheng XL,Zhu XD,Wang Y,et al.Effects of Sishen Wan on the contents of serum IL-23 and IL-27 and the expression of Foxp3 mRNA in colonic mucosa of rats with ulcerative colitis.Zhong Yi Yan Jiu 2016;29:66-70.
23 Totsuka T,Kanai T,Iiyama R,et al.Ameliorating effect of antiinducible costimulator monoclonal antibody in a murine model of chronic colitis.Gastroenterol 2003;124:410-21.
24.Ke J,Bian X,Liu H,et al.Edaravone reduces oxidative stress and intestinal cell apoptosis after burn through up-regulating miR-320 expression.Mol Med 2019;25:54.
25.Liu D,Huang X,Cheng S,et al.Regulation of sishen wan on Bax/Bcl-2 mRNA,Fas/FasL in colonic tissue from rats with colitis.Zhong Guo Zhong Yao Za Zhi 2011;36:3484-8.
26.Wang Y,Liu R,Zhu XD.Immunohistochemical effect of sishen pill on PI3K/Akt/mTOR signal pathway in colonic tissue of rats with ulcerative colitis model of spleen kidneyYangdeficiency.Zhong Guo Shi Yan Dong Wu Xue Bao 2021;29:42-8.
27.Liu R,Wang Y,Zhu XD,et al.Effect of Sishenwan on PI3K/Akt/mTOR signal pathway in colonic tissue of rats with ulcerative colitis model of spleen kidney Yang deficiency.Zhong Guo Shi Yan Fang Ji Xue Za Zhi 2021;27:16-23.
28.Lawrence T.The nuclear factor NF-kappaB pathway in inflammation.Cold Spring Harb Perspect Biol 2009;1:a001651.
29.Dolcet X,Llobet D,Pallares J,et al.NF-kB in development and progression of human cancer.Virchows Arch 2005;446:475-82.
30.Kunnumakkara AB,Shabnam B,Girisa S,et al.Inflammation,NF-κB,and chronic diseases:how are they linked? Crit Rev Immunol 2020;40:1-39.
31.Chen Y,Chen Y,Cao P,et al.Fusobacterium nucleatum facilitates ulcerative colitis through activating IL-17F signaling to NF-κBviathe upregulation of CARD3 expression.J Pathol 2020;250:170-82.
32.Gray CM,Remouchamps C,Mccorkell KA,et al.Noncanonical NF-κB signaling is limited by classical NF-κB activity.Sci Signal 2014;7:ra13.
33.Liu AH,Wu YT,Wang YP.MicroRNA-129-5p inhibits the development of autoimmune encephalomyelitis-related epilepsy by targeting HMGB1 through the TLR4/NF-kB signaling pathway.Brain Res Bull 2017;132:139-49.
34.Torrealba N,Vera R,Fraile B,et al. TGFβ/PI3K/AKT/mTOR/NF-kB pathway. Clinicopathological features in prostate cancer.Aging Male 2020;23:801-11.
35.Candia E,Díaz-Jiménez D,Langjahr P,et al.Increased production of soluble TLR2 by lamina propria mononuclear cells from ulcerative colitis patients.Immunobiology 2012;217:634-42.
36.Tan Y,Zou KF,Qian W,et al.Expression and implication of tolllike receptors TLR2,TLR4 and TLR9 in colonic mucosa of patients with ulcerative colitis.Hua Zhong Ke Ji Da Xue Xue Bao Yi Xue Ban 2014;34:785-90.
37.Pennini ME,Perkins DJ,Salazar AM,et al.Complete dependence on IRAK4 kinase activity in TLR2,but not TLR4,signaling pathways underlies decreased cytokine production and increased susceptibility to Streptococcus pneumoniae infection in IRAK4 kinase-inactive mice.J Immunol 2013;190:307-16.
38.Padwal MK,Sarma U,Saha B.Comprehensive logic based analyses of toll-like receptor 4 signal transduction pathway.PLoS One 2014;9:e92481.
39.Bahia MS,Kaur M,Silakari P,et al.Interleukin-1 receptor associated kinase inhibitors:potential therapeutic agents for inflammatory-and immune-related disorders.Cell Signal 2015;27:1039-55.
 Journal of Traditional Chinese Medicine2022年4期
Journal of Traditional Chinese Medicine2022年4期
- Journal of Traditional Chinese Medicine的其它文章
- Effectiveness of redcore lotion in patients with vulvovaginal candidiasis:a systematic review and Meta-analysis
- Efficacy and safety of external application of Chinese herbal medicine for psoriasis vulgaris:a systematic review of randomized controlled trials
- Effectiveness and safety of electroacupuncture for the treatment of pain after laparoscopic surgery:a systematic review
- Effect of astragaloside IV on the immunoregulatory function of adipose-derived mesenchymal stem cells from patients with psoriasis vulgaris
- Shenqihuatan formula (参七化痰方) reduces inflammation by inhibiting transforming growth factor-beta-stimulated signaling pathway in airway smooth muscle cells
- Drug response biomarkers of Pien Tze Huang (片仔癀) treatment for hepatic fibrosis induced by carbon tetrachloride
