Efficacy of phospholipid complex of flavonoids from persimmon leaves on atherosclerosis,and possible mechanism
CHEN Jinpeng,ZHANG Kexia,LIU Yi,JIN Song,GAI Xiaohong,REN Tao,TIAN Chengwang
CHEN Jinpeng,LIU Yi,GAI Xiaohong,REN Tao,TIAN Chengwang,State Key Laboratory of Drug Delivery and Pharmacokinetics,Tianjin 300301,China;Tianjin Key Laboratory of TCM quality markers,Tianjin 300301,China;Tianjin Institute of Pharmaceutical Research,Tianjin 300301,China
ZHANG Kexia,Tianjin Pharmaceutial Research Institute Co.Ltd.,Tianjin 300462,China;
JIN Song,Sinopharm Group Tianjin Co.Ltd.,Tianjin 300040,China Supported by National Science and Technology Major Project of China(Research on Evaluation Technology of New Traditional Chinese Medicine Based on Big Data of Classical Prescription,No.2019ZX09201005) and International Cooperation Project of Traditional Chinese Medicine (China-Germany International Cooperation in Innovative Research and Development of Traditional Chinese Medicine and Botanical Medicine,No.0610-2140NF 020630)
Abstract OBJECTIVE:To investigate the efficacy of phospholipid complex of flavonoids from persimmon leaves (PLF-PC)on atherosclerosis,and to study its mechanism behind the action.METHODS:To clarify the constituents of the flavonoids from persimmon leaves (PLF),an ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry method was established.To enhance the anti-atherosclerotic effect of PLF,a newly emerging approach based on the combination of phospholipid complexation technique was employed.PLF-PC was prepared by the solvent-evaporation method then characterized using Fourier transform infrared spectroscopy,Powder X-Ray Diffractometry and Scanning electron microscopy.A model of oxidized lowdensity lipoprotein-induced injury on human umbilical vein endothelial cells was established to investigate the anti-atherosclerotic effect of PLF-PC versus PLF.The levels of nitric oxide,endothelial nitric oxide synthase,intracellular adhesion molecule-1,reactive oxygen species,superoxide dismutase,tumor necrosis factorαand nuclear factor-κB were observed via assay kits.RESULTS:A total of 31 compounds were identified in PLF.PLF-PC showed better anti-atherosclerotic power compared with PLF,moreover,enzyme linked immuneosorbent assay analysis showed that the PLF-PC may effect on endothelial dysfunction and atherosclerosis via antioxidant-related mechanisms.CONCLUSIONS:Our findings elucidated that PLF-PC significantly enhanced the PLF’s efficacy on atherosclerosis.
Keywords:flavonoids;chromatography,high pressure liquid;mass spectrometry;phospholipids;lipoproteins,LDL;endothelial cells
1.INTRODUCTION
Atherosclerosis (AS) is one of the primary causes of death for its morbidity and mortality in many countries.AS is a chronic inflammatory disease of the arterial wall and that results in the formation of plaques in large and mid-sized arteries.1Endothelial cells play a critical role in maintaining cardiovascular homeostasis through modulating blood vessel tone,regulating local cellular growth and extracellular matrix deposition,and controlling homeostatic as well as inflammatory responses.2Previous evidence suggests that endothelial dysfunction is considered an early marker for AS,importantly,endothelial dysfunction not only precedes AS but greatly contributes to atherogenesis in all disease stages.3,4There are many atherosclerotic risk factors,e.g.,oxidized low-density lipoprotein (ox-LDL),smoking,diabetes mellitus,hypertension,sedentary lifestyle,diet and several other conditions.5Among them,ox-LDLinduced oxidative injury has been recognized as a key mechanism for endothelial cell damage and atherosclerotic plaque formation.6,7Ox-LDL could enhance the production of reactive oxygen species (ROS),decrease the release of nitric oxide (NO),and attenuate the antioxidant system.8Moreover,ox-LDL can induce foam cell formation and exacerbate vascular inflammation.9Therefore,protection of endothelial cells against ox-LDL-induced injury is a medical key issue for AS.
The flavonoids from persimmon leaves (PLF),isolated from the leaves ofDiospyros kakiL.DispryoslandEbenaceae,has been used for years for the treatment of stroke and syndrome of apoplexy in China to improve the outcome of ischemia stroke.10Studies have shown that PLF presents anti-oxidative and anti-inflammatory properties,and have beneficial effects on haemostasis,hypertension,apoplexy and atherosclerosis.11,12However,the oral bioavailabilities of flavonoids are relatively low due to their low liposolubility and poor solubility and severely limiting their ability to pass across the lipid-rich biological membranes13Our previous studies showed that phospholipid complex of flavonoids from persimmon.leaves (PLF-PC) hold a promising potential for increasing the oral bioavailability of PLF,furthermore,PLF-PC exerted better therapeutic potential in rat atherosclerosis than PLF.14Additionally,the successful cases of phospholipid complex of flavonoids fromGinkgo bilobain the treatment of anti-atherosclerotic also give us inspiration.13
Until this point,whether PLF-PC is associated with ox-LDL-induced oxidative injury and its effect on endothelial cells is still unknown.There is still less research on the chemical compositions of PLF.Therefore,the purpose of this study was to investigate the efficacy of PLF-PC on atherosclerosis,and to study its mechanism behind the action.To clarify the constituents of the PLF,an ultra-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry (UPLC-QTOF/MS) method was established.
2.MATERIALS AND METHODS
2.1.Materials and reagents
The raw materials of persimmon leaves were collected from Lianyungang,Jiangsu province,China.Soya phosphatidyl choline (Lipoid S 100) was purchased from Lipoid GmbH (Ludwigshafen,Germany).Ox-LDL,human (AngYu Bio,Shanghai,China) was stored at 4°C.RPMI1640 medium,fetal bovine serum (FBS) and penicillin/streptomycin were purchased from GIBCO Co.(Grand Island,NY,USA).Dimethylsulfoxide (DMSO)and 3-(4,5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT) were obtained from Sigma-Aldrich(St.Louis,MO,USA).The nitric oxide (NO),endothelial nitric oxide synthase (eNOS),intracellular adhesion molecule-1 (ICAM-1),reactive oxygen species (ROS),tumor necrosis factor-α (TNF-α) and nuclear factor-κB(NF-κB) and superoxide dismutases (SOD) assay kits were produced by Shanghai Enzyme-linked Biotechnology Co.Ltd.(Shanghai,China).All other chemical reagents employed in the experiments were analytical grade.
2.2.Preparation of PLF
The persimmon leaves were confirmed as the leaves ofDiospyros kakiL.DispryoslandEbenaceaeby Researcher Chengwang Tian,Tianjin Institute of Pharmaceutical Research,Tianjin,China.The dried persimmon leaves powder was extracted with 50% EtOH(powder material:solvent,1∶10,w/v) under reflux for 3 h.The extract was concentratedin vacuoat 50℃ to remove EtOH,and then the obtained water layer was extracted with three times of ethyl acetate.The ethyl acetate layer was followed by transferred and evaporated to dryness to get the PLF.
2.3.UPLC-Q-TOF/MS analysis of PLF
2.3.1.Sample preparation
About 0.5 mg of PLF was dissolved in 1 mL methanol and centrifuged at 13 000 rpm for 10 min and the supernatant was injected into UPLC-Q-TOF-MS system directly.
2.3.2.Instrumentation and analytical conditions
Chromatography was performed on a Waters ACQUITY UPLCTMsystem equipped with a binary solvent system and an auto-sampler (Waters Corp.,Milford,MA,USA).Chromatographic separation was performed on an ACQUITY UPLC® HSS C18 column (100 mm × 2.1 mm i.d.,1.8 µm) and the temperature of column and auto-sampler were maintained at 35 ℃ and 4 ℃,respectively.The mobile phase consisted of 0.1% formic acid water (A) and acetonitrile (B).The gradient program was optimized as follows:0-5 min,12% B;5-7 min,12-26% B;7-13 min 26% B;13-16 min,26-65% B;16-21 min,65-70% B;21-24 min,70-80% B;24-26 min,70-50% B;26-28 min 50-12% B;28-30 min 12% B.The flow rate was set at 0.2 mL/min and the injection volume was 2 μL.
Mass spectrometric detection was performed on a XevoTMG2 QTof (Waters MS Technologies,Manchester,UK),equipped with an electrospray ionization (ESI)source operating in both positive and negative ion modes.The mass acquisition range was from m/z 100 to 1000.The parameters of the mass spectrometer under the ESI mode were as follows:the nebulizer gas was set to 800 L/h at a temperature of 450 ℃.The cone gas was set to a flow rate of 50 L/h,the source temperature was set to 130 ℃.The capillary voltage was 3.5 kV and 3 kV in the positive and negative mode,respectively.Cone voltage was set to 30 V.Leucine-enkephalin was used as the lock mass to generate a reference ion at m/z556.2771 for positive ion mode and at m/z554.2615 for negative ion mode.In MSEmode,the parent and fragment mass information of the compounds were obtained as follows:function 1:m/z 100-1000,0.5 s scan time,0.02 s interscan delay,6 eV collision energy;function 2:m/z 100-1000,0.5 s scan time,0.02 s inter-scan delay,collision energy ramp of 25-40 eV.All data collected in centroid mode were acquired and processed using MasslynxTMNT 4.1 software.The accuracy error threshold was fixed at 5 ppm.
2.4.Preparation and physicochemical properties of PLF-PC
The PLF-PC was prepared by a simple solvent evaporation method according to our previous literature.14Briefly,the PLF and Lipoid S 100 were dissolved in anhydrous ethanol at a ratio of weight 1:2,and then magnetically stirred for 2 h at 45°C.After then,the organic solvent was evaporated off under vacuum at room temperature.To get the precipitate,a specific amount of n-hexane was added and stirred for 15 min.Then the precipitate was dried under a slight stream of nitrogen to get the PLF-PC.
2.5.Fourier transform infrared analysis
The infrared absorption spectra of the samples were recorded on an IR spectrophotometer (Bruker IFS55,Switzerland) over the wavelength range from 4000 to 400 cm-1.The IR spectra of Lipoid S 100,PLF,physical mixture of PLF,phospholipid (at a weight ratio of 1:2)and PLF-PC were obtained.
2.6.Powder X-Ray Diffractometry (PXRD)
The crystalline state of PLF in different samples was evaluated with PXRD diffractometer (Rigaku Geigerflex PXRD,Japan) using Cu Kα radiation (λ=0.154 nm,voltage:40 kV,tube current:40 mA,2θangle range:from 5 to 50o,scan rate:3o/min).
2.7.Scanning electron microscopy (SEM) analysis
To assess the surface morphology of the Lipoid S 100,PLF,physical mixture of PLF,Lipoid S 100 (at a weight ratio of 1 :2) and PLF-PC,SEM (SU 8000,Hitachi,Japan) studies were performed.Samples were placed on an electron microscope brass stub and coated with gold in an ion sputter,and surface morphological characteristics were viewed and photographed.
2.8.Cell culture and treatment
Human umbilical vein endothelial cells (HUVECs) were obtained from the American Type Culture Collection(Manassas,VA,USA) and cultured in RPMI1640 medium,supplemented with 10% FBS and 100 U/mL penicillin and 100 μg/mL streptomycin at 37°C in 5%CO2.The cells were seeded into plates and randomly assigned into eight groups as described below containing six parallel samples per group.
Control group (Ⅰ),cells were treated with blank medium for 12 h followed by treatment with blank medium for another 12 h;
Model group (Ⅱ),cells were treated with the medium containing ox-LDL for 12 h,then incubated with the replaced medium containing Ox-LDL for another 12 h.PLF (equivalent to 25 µg/mL of total flavonoids)+ox-LDL group (Ⅲ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and PLF for another 12 h;PLF (equivalent to 50 µg/mL of total flavonoids)+ox-LDL group (Ⅳ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and PLF for another 12 h;PLF (equivalent to 100 µg/mL of total flavonoids)+ox-LDL group (Ⅴ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and for another 12 h;
PLF-PC (equivalent to 25 µg/mL of total flavonoids) +ox-LDL group (Ⅵ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and PLF-PC for another 12 h;
PLF-PC (equivalent to 50 µg/mL of total flavonoids) +ox-LDL group (Ⅶ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and PLF-PC for another 12 h;
PLF-PC (equivalent to 100 µg/mL of total flavonoids) +ox-LDL group (Ⅷ),cells were treated with the medium containing ox-LDL for 12 h followed by treatment with medium containing ox-LDL and PLF-PC for another 12 h;
The concentration of ox-LDL was 100 µg/mL.After treatment,the cells were assigned to analysis of cell viability assay,level of intracellular ROS,SOD,TNF-α and NF-κB assays and extracellular NO,eNOS and ICAM-1 assays.
2.9.Cell viability analysis
Cells were seeded in a 96-well plate at a density of 1×104cells/well and treated as described in section 2.5.Cell viability was measured by MTT assay.Briefly,after treatment,the treatment medium was replaced with fresh culture medium containing MTT at a concentration of 0.5 mg/mL,and the cells were incubated for 4 h in a 37 ℃ incubator.Then the medium was removed and formazan crystals were dissolved in 150 µL of DMSO.The absorbance value was determined at a wavelength of 490 nm on a microplate reader (Molecular Devices,USA).The ratio of living cells was calculated by the ratio of optical density compared with that of the normal cells.
2.10.Measurement of NO,eNOS,ICAM-1,ROS,SOD,TNF-α and NF-κB levels
After all treatments according to the requirements of the different groups,the cultured medium was collected and centrifuged for 15 min at 4000 rpm at 4 ℃,and then the supernatants were used for the NO,eNOS and ICAM-1 assay with assay kits according to the manufacturer’s protocol.The cells were washed with PBS and centrifuged at 1000 rpm at 4 ℃ for 10 min.The cell pellets were resuspended with PBS,freeze-thawed twice at -20 ℃,and centrifuged at 10 000 rpm at 4 ℃ for 15 min.Then the supernatant was collected for ROS,SOD,TNF-α and NF-κB assay using assay kits according to the manufacturer’s guidelines.
2.11.Statistical analysis
Values were shown as mean ± standard deviation and differences between groups were tested by one-way analysis of variance followed by turkey test.Data were processed using SPSS 19.0 (IBM Corp.,Armonk,NY,USA).Statistically significant level wasP< 0.01.
3.RESULTS
3.1.UPLC-Q-TOF/MS analysis of PLF
In this paper,both positive and negative ion modes were employed to comprehensively analyze the main compounds of PLF.In addition,0.1% formic acid was added to the mobile phase to improve the separation of constituents and increase response for some compounds.Based on their exact masses,fragment information and references,31 compounds were identified from PLF including 20 flavonoids,4 three-stick compounds,6 organic acids and 1 aromatic alcohol compound.The representative chromatograms at both modes are demonstrated in Figure 1.Table 1 shows the list of 31 compounds identified in PLF through UPLC-Q-TOF/MS.Based on this information,the identified flavonoids were mainly classified into two types of compounds:quercetin and its glycosides,kaempferol and its glycosides.Quercetin showed a molecule ion [M+H]+atm/z303.0499 for C15H11O7+and a fragment ion [M+HC8H6O3]+atm/z153.0092,kaempferol showed a molecule ion [M-H]-atm/z285.0392 for C15H9O6-and a fragment ion [M-H-C8H6O2]-atm/z151.0068,which were consistent with the literatures.18,19Conjugated flavonoids such as quercetin-3-O-glucopyranoside,quercetin-3-O-arabinopyranoside,quecretin-3-Orhamnopyranoside,kaempferol-3-O-glucopyranoside,kaempferol-3-O-galactopyranoside,kaempferol-3-Oxylopyranoside and kaempferol-3-O-arabinopyranoside have also been reported.21,24Peak 16 was identified as vitexin based on its [M+H]+atm/z433.1119,a fragment ion [M+H-C4H9O4]+atm/z313.1023 and literature data for this compound.22Compound 19 was identified as quercetin-3-O-glucopyranosyl-(6→1)-α-Lrhamnopyra-noside,based on its [M-H]-atm/z610.1567 and the corresponding loss of 163 and 146 mass units successively.25In addition to the above compounds,other compounds were also identified based on their exact masses,fragment information and literatures in the same way.Flavonoids are the main compounds and therapeutic constituents in persimmon leaves.This part provides useful chemical information that could be employed for further study of the pharmacodynamic material basis of PLF antiatherosclerotic effects.
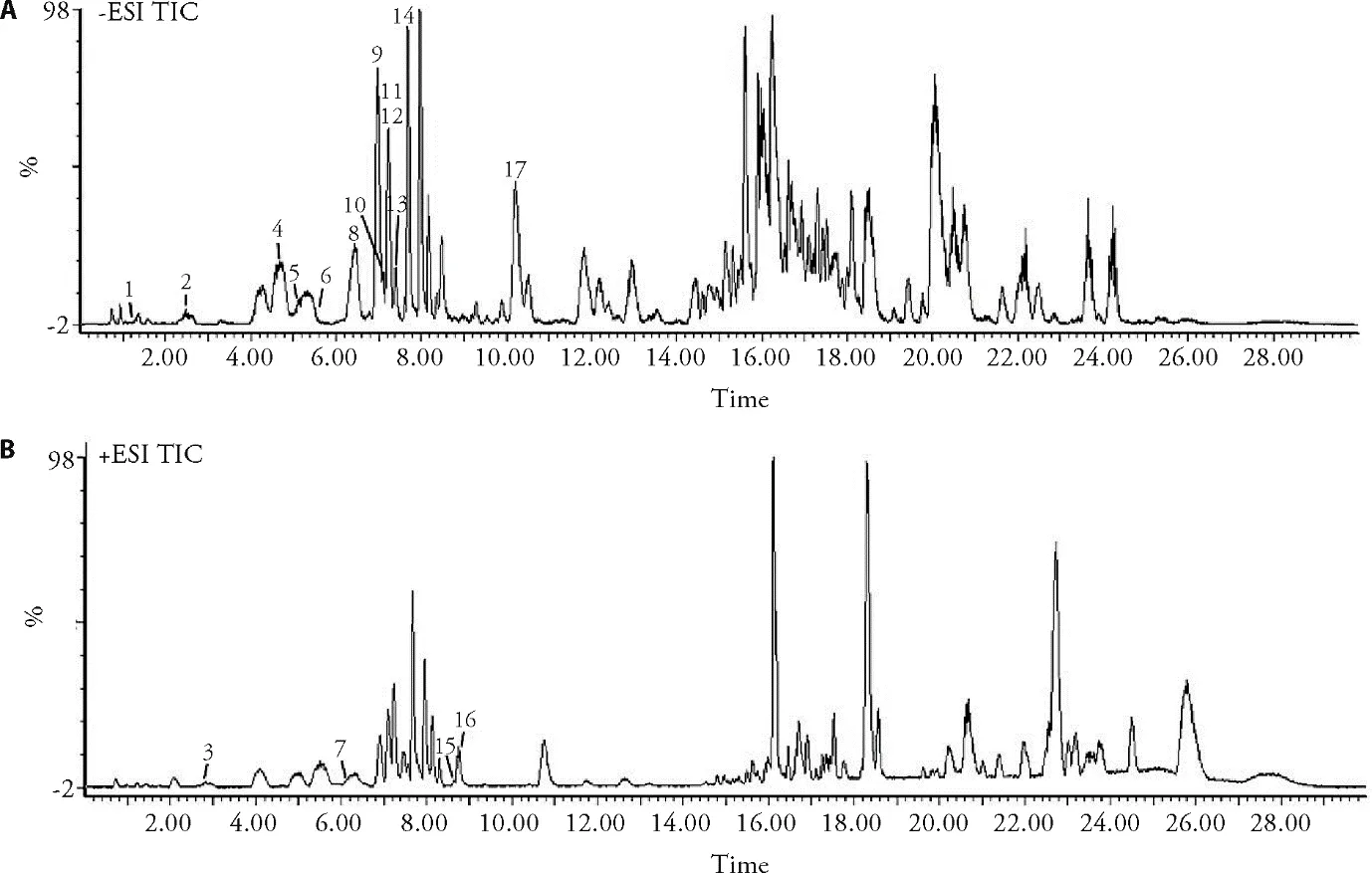
Figure 1 Representative total ion chromatograms of the PLF (A) in negative mode,(B) in positive mode

Table 1 List of the retention time and MS data (m/z) for each analyte identified in the PLF
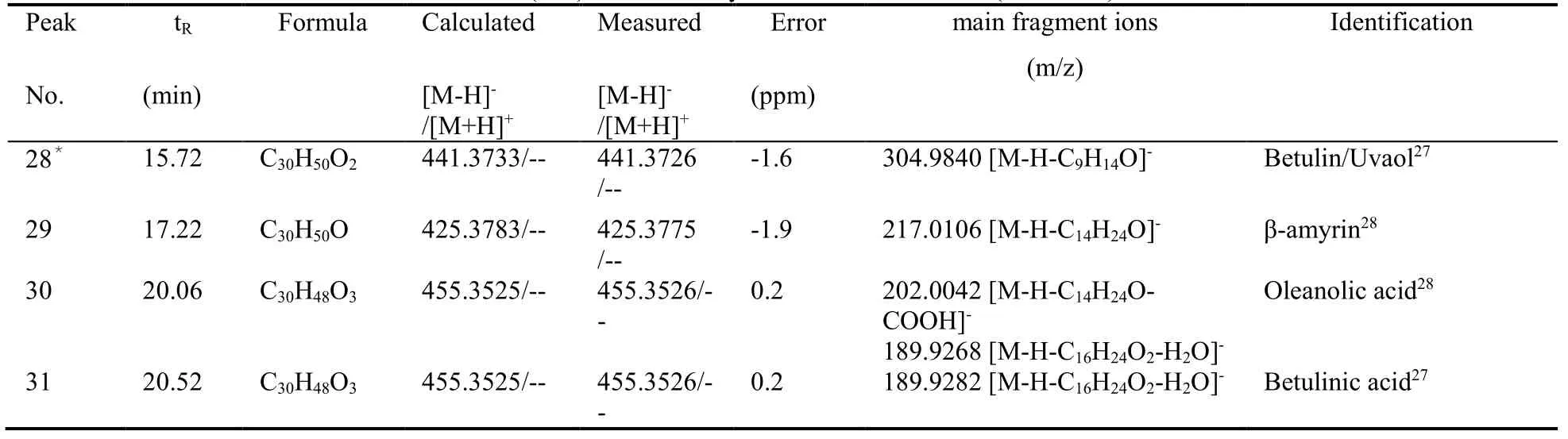
Table 1 List of the retention time and MS data (m/z) for each analyte identified in the PLF (continued)
3.2.Characterization of the PLF-PC
3.2.1 Fourier transform infrared spectroscopy (FTIR)
The possible interaction between PLF and Lipoid S 100 in the phospholipid complex was studied by IR spectroscopy and presented in Figure 2.In the phospholipid spectrum (Figure 2A) the characteristic CH stretching band of long fatty acid chain at 2923 cm-1and 2852 cm-1,carbonyl stretching band at 1734 cm-1,P=O stretching band at 1241 cm-1,P-O-C stretching band at near 1122 cm-1,N+(CH3)3stretching at 969 cm-1wereobserved.In case of the PLF (Figure 2B) the main characteristic bands for the phenolic -OH groups stretching at 3389 cm-1,C=O stretching at 1653 cm-1,and benzene ring vibrations near about 1511 cm-1were observed.The spectrum of the physical mixture (Figure 2C) was different from that of components and that of complexes,which showed the same vibrational frequencies as that of the individual components and seemed to be a summation of both the constituents.In the IR spectrum of the complexes (Figure 2D) there were some significant changes,the absorption peaks of phenolic -OH groups of PLF have been shifted to higher wave number and theP=O absorption band of the phospholipid remarkably broadened,while the characteristic C-H stretching band of long fatty acid chain at 2923 cm-1and 2852 cm-1of phospholipid still existed.Therefore,the spectroscopic changes showed that the shifting of phenolic-OH groups frequencies of PLF from their original place accounted for the interaction of PLF to polar end of the phospholipid.These results suggested that non-polar part of phospholipid remained unchanged and the chemical structure of PLF had not changed after forming complexes.

Figure 2 FTIR spectrum
3.3.Powder X-ray diffraction (PXRD)
Powder X-ray diffractionwas performed to further investigate the crystalline state.Figure 3 shows the X-ray diffraction patterns of the lipoid S 100,PLF,physical mixture of PLF and lipoid S 100 and PLF-PC.The phospholipid (Figure 3A) showed a large single diffraction peak.Crystalline diffraction peaks of PLF were observed in Figure 3B.The X-ray diffractogram physical mixture (Figure 3C) retained a similar diffraction pattern with PLF.The PXRD of PLF-PC(Figure 3D) revealed a broad peak,being similar to that of phospholipid.It suggested that the bonding between PLF and phospholipid in development of PLF-PC might have resulted in the X-ray diffraction of PLF significant change.The disappearance of PLF’s crystalline diffraction peaks confirmed the formation of PLF-PC.

Figure 3 X-ray diffraction patterns
3.4.Scanning electron microscopy (SEM)
The surface morphology of the Lipoid S 100,PLF,physical mixture of PLF and Lipoid S 100,and PLF-PC were studied by SEM (Figure 4).The Figure 4(A) shows the amorphous nature of phospholipid,while Figure 4(B)shows the solidified powders of PLF.No change in surface morphology was observed in the physical mixture of PLF with PL Lipoid S 100,as depicted in Figure 4(C).In case of complex Figure 4(D),the crystallinity of PLF disappeared ascribable to its complexation with Lipoid S 100,indicating the formation of PLF-PC.The result was in agreement with the results of the FTIR and PXRD.

Figure 4 SEM images
3.5.HUVECs studies
3.5.1 Cell viability analysis after treatment with PLF and PLF-PC
As shown in Supplementary Figure 1.The cell viability of HUVECs was significantly decreased after treatment with 100 µg/mL ox-LDL compared with control group(n=6,P< 0.01).In comparison with group II (model),increased cell viability was observed in groups Ⅲ to Ⅷ,indicating that PLF protected cells from ox-LDL induced damage.However,cells in treatment with PLF-PC group showed a better viability than cells in treatment with PLF group at the same doses.The cell viability reached up to 78% ± 4% when cells treated with PLF-PC (equivalent to 50 µg/mL of total flavonoids),while the viability of cells treated with PLF at the same dose was 58% ± 4%.When the cells were treated with high concentration of PLF-PC (equivalent to 100 µg/mL of total flavonoids),the effect was more obvious in the PLF-PC treatment group than PLF treatment group at the same dose (93%± 5%vs69% ± 4%).These findings suggested that PLF had better effect against ox-LDL-induced cell injury.
3.5.2 PLF-PC suppresses ox-LDL-induced oxidative stress in HUVECs
In order to investigate and compare the protective effect of PLF and PLF-PC on oxidative stress in HUVECs,the production of ROS and the intracellular level of SOD were evaluated.As shown in Supplementary Figure 2,the level of SOD was decreased (P< 0.01) and the ROS increased (P< 0.01) in model group after ox-LDL stimulation compared with control group.It was observed that both PLF and PLF-PC could reduce ROS formation and increase the production of SOD.However,the effect was more visible in PLF-PC groups at the same dose.Especially,when the cells were treated with PLF and PLF-PC (were equivalent to 100 µg/mL of total flavonoids),the ROS levels were 209% ± 9% and 113%± 8% relative to control group,respectively.Meanwhile,compared with the PLF group,the levels of SOD were obviously upon-regulated in the PLF-PC group [(59 ± 3)vs(47 ± 4) U/mg prot].The elevation in ROS level has a detrimental effect on cellular function,a consequence of ROS-induced damage to lipid membranes,enzymes and nucleic acid.29The SOD represents the first and most important line of enzymatic antioxidant defense against ROS.These results indicated that both PLF and PLF-PC may might protective role in ox-LDL-induced endothelial injury by suppressing oxidative stress,and the effect of PLF-PC was more significant at the same dose.
3.6.eNOS expression and NO release
NO is a critical regulator of vascular homeostasis,it’s synthesized within endothelial cells during conversion of L-arginine to L-citrulline by endothelial nitric oxide synthase (eNOS).30NO prevents leukocyte adhesion,migration,smooth muscle cell proliferation,platelet adhesion and aggregation,and opposes apoptosis and inflammation having an overall antiatherogenic effect.31NO can be regarded as an anti-atherosclerotic agent and its level is an indicator of endothelial function.eNOS is a dimeric enzyme.Oxidative stress can lead to eNOS uncoupling and it has been reported that a reduced expression and/or activity of eNOS could be responsible for a decrease in NO production.32Therefore,eNOS and NO play significant roles in the development of AS.As depicted in Supplementary Figure 3,exposure of HUVECs cells to ox-LDL for 24 h (model group)significantly (P< 0.01) lessened NO release,the level of eNOS (model group) was also reduced obviously (P<0.01) when compared with that of control group.After treatment with PLF and PLF-PC,the levels of NO and eNOS were upon-regulated.What’s more,the results demonstrated that the regulate effect of PLF-PC was more prominent than that of PLF when given the same dose.
3.7.Effects of PLF and PLF-PC on TNF-α,NF-κB and ICAM-1 levels
To further investigate and compare the protective role of PLF and PLF-PC in endothelial injury,the levels of inflammatory factor TNF-α,NF-κB and adhesion molecule ICAM-1 were measured.As shown in Supplementary Figure 4,the protein levels of TNF-α,NF-κB and ICAM-1 in model group were significantly(P< 0.01) increased following ox-LDL treatment.Compared with the model group,these levels were decreased in the PLF and PLF-PC plus ox-LDL groups compared with the model group.Obviously,the downregulate effect was more significant in PLF-PC groups at the same doses.TNF-α is key pro-inflammatory cytokines associated with the development of atherosclerotic lesions,which can induce monocytes to adhere to the endothelium,thereby enabling monocytes infiltration to the injured endothelium.32,33NF-κB is a key mediator that regulates multiple proinflammatory and proatherosclerotic target genes in endothelial cells and macrophages.Besides,activation of NF-κB leads to an increased production of adhesion molecules activating inflammatory cells in the vascular wall.34ICAM-1 is a member of the immunoglobin family and considered a critical step in AS for its function in promoting leukocytes adhesion and development of inflammatory diseases.35Our results suggested that PLF and PLF-PC protected HUVECs against ox-LDL-induced inflammatory injury,also,the effect of PLF-PC was more remarkable than PLF at the same dose.
4.DISCUSSION
In the present study,we characterized the main compounds in PLF using UPLC-Q-TOF/MS.A total of 31 compounds were identified.To enhance the antiatherosclerotic effect of PLF,the PLF-PC was successfully prepared in a simple method.The SEM,FTIR PXRD data confirmed the formation of PLF-PC.Endothelial function is important for the homeostasis of the body and its dysfunction is associated with several pathophysiological conditions,including AS,hypertension and diabetes.32Oxidative stress has been believed to be associated with AS in pathological processes and other cardiovascular diseases.Therefore,inhibition of endothelial dysfunction induced by oxidative stress is an essential therapeutic strategy for AS.It was observed that ox-LDL could induce injury on HUVECs,both PLF and PLF-PC exhibited protective effects.However,PLF-PC exerted a more promoted effect when given the same doses.
In conclusion,our findings elucidated that PLF-PC significantly enhanced the PLF’s efficacy on atherosclerosis.
5.REFERENCES
1.Mizuno Y,Jacob RF,Mason RP.Inflammation and the development of atherosclerosis.J Atheroscler Thromb 2011;18:351-8.
2.Zhang SL,Guo CL,Chen ZG,et al.Vitexin alleviates ox-LDLmediated endothelial injury by inducing autophagy via AMPK signaling activation.Mol Immunol 2017;85:214-21.
3.Xiao Y,Wang YC,Li LL,et al.Lactones fromLigusticum chuanxiongHort.Reduces atherosclerotic lesions in apoEdeficient miceviainhibiting over expression of NF-kB -dependent adhesion molecules.Fitoterapia 2014;95:240-6.
4.Chistiakov DA,Revin VV,Sobenin IA,et al.Vascular endothelium:functioning in norm,changes in atherosclerosis and current dietary approaches to improve endothelial function.Mini Rev Med Chem.2015;15:338-50.
5.Zhang HP,Zheng FL,Zhao JH,et al.Genistein inhibits ox-LDLinduced VCAM-1,ICAM-1 and MCP-1 expression of HUVECs through heme oxygenase-1.Arch Med Res 2013;44:13-20.
6.Takahashi Y,Zhu H,Yoshimoto T.Essential roles of lipoxygenases in LDL oxidation and development of atherosclerosis.Antioxid Redox Sign 2005;7:425-31.
7.Pirillo A,Norata GD,Catapano AL.LOX-1,OxLDL,and atherosclerosis.Mediat Inflamm 2013;2013:15278-86.
8.Deanfield JE,Halcox JP,Rabelink TJ.Endothelial function and dysfunction.Circulation 2007;115:1285-95.
9.Itabe H.Oxidized low-density lipoprotein as a biomarker ofin vivooxidative stress:from atherosclerosis to periodontitis.J Clin Biochem Nutr 2012;51:1-8.
10.Bei WJ,Peng WL,Ma Y,et al.NaoXinQing,an anti-stroke herbal medicine,reduces hydrogen peroxide-induced injury in NG108-15 cells.Neurosci Lett 2004;363:262-5.
11.Akak CM,Djama CM,Nkengfack AE,et al.New coumarin glycosides from the leaves ofDiospyros crassiflora(Hiern).Fitoterapia 2010;81:873-7.
12.Sun L,Zhang J,Fang K,et al.Flavonoids from persimmon(Diospyros kaki) leaves (FPL) attenuate H2O2-induced apoptosis in MC3T3-E1 cellsviathe NF-κB pathway.Food Funct 2014;5:471-9.
13.Chen ZP,Sun J,Chen HX,et al.Comparative pharmacokinetics and bioavailability studies of quercetin,kaempferol and isorhamnetin after oral administration ofGinkgo bilobaextracts,Ginkgo bilobaextract phospholipid complex andGinkgo bilobaextract solid dispersions in rats.Fitoterapia 2010;81:1045-52.
14.Zhang KX,Zhang YY,Zhang MY,et al.Effects of phospholipid complex of total flavonoids from Persimmon (Diospyros kakiL.)leaves on experimental atherosclerosis rats.J Ethnopharmacol 2016;191:245-53.
15.Callemien D,Collin S.Use of RP-HPLC-ESI (-)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts.J Am Soc Brew Chem 2008;66:109-15.
16.Zhou XT,Wang L,Han L,et al.Research progress on chemical constituents and pharmacological effects ofDiospyros kakileaves.Chin Herb Med 2014;45:3195-203.
17.Ruth MLH,Paola QR,Ana A,et al.Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESILTQ-Orbitrap-MS).J Funct Foods 2016;23:370-7.
18.Chen G,Xue J,Xu SX,et al.Chemical constituents of the leaves ofDiospyros kakiand their cytotoxic effects.J Asian Nat Prod Res 2007;9:347-53.
19.Xie CY,Xie ZS,Xu XJ,et al.Persimmon (Diospyros kakiL.)leaves:a review on traditional uses,phytochemistry and pharmacological properties.J Ethnopharmacol 2015;163:229-40.
20.Kawakami K,Nishida H,Tatewaki N,et al.Persimmon leaf extract inhibits the ATM activity during DNA damage response induced by doxorubicin in A549 lung adenocarcinoma cells.Biosci Biotech Bioc 2011;75:650-5.
21.Chen G,Xu SX,Sha Y.Studies on the constituents ofDiospyros kakileaves.Chinese Journal of Medicinal Chemistry.J Med Chem 2000;10:298-9.
22.Chen G,Xu SX,Wang HZ,et al.Note:Kakispyrol,a new biphenyl derivative from the leaves ofDiospyros kaki.J Asian Nat Prod Res 2005;7:265-8.
23.Ganfer F,Chapuis JC,Msonthi JD,et al.Cytotoxic naphthoquinones,molluscicidal saponins and flavonols fromDiospyros zombensis.Phytochemistry 1987;26:2501-3.
24.Xue YL,Miyakawa T,Hayashi Y,et al.Isolation and tyrosinase inhibitory effects of polyphenols from the leaves of persimmon,Diospyros kaki.J Agric Food Chem 2011;59:6011-7.
25.Chen G,Lu H,Wang C,et al.Effect of five flavonoid compounds isolated from leaves ofDiospyros kakion stimulus-induced superoxide generation and tyrosyl phosphorylation of proteins in human neutrophils.Clin Chim Acta 2002;362:169-75.
26.Chen G,Wei SH,Huang J,et al.A novelC-glycosylflavone from the leaves ofDiospyros kaki.J Asian Nat Prod Res 2009;11:503-7.
27.Uc-Cachon AH,Molina SGM,Said FS,et al.A new dimeric naphthoquinone from Diospyros anisandra.Nat Prod Res 2013;27:1174-8.
28.Higa M,Ogihara K,Yogi S.Bioactive naphthoquinone derivatives from Diospyros maritime Blume.Chem Pharm Bull 1998;46:1189-93.
29.Cai H,Harrison DG.Endothelial dysfunction in cardiovascular diseases:the role of oxidant stress.Circ Res 2000;87:840-4.
30.Vallance P,Chan N.Endothelial function and nitric oxide:clinical relevance.Heart 2001;85:342-50.
31.Wheatcroft SB,Williams IL,Shah AM,et al.Pathophysiological implications of insulin resistance on vascular endothelial function.Diabet Med 2003;20:255-68.
32.Pober JS.Endothelial activation:intracellular signaling pathways.Arthritis Res 2002;4:109-18.
33.Steffens S,Mach F.Inflammation and atherosclerosis.Herz 2004;29:741-8.
34.Sena MC,Pereira AM,Seiça R.Endothelial dysfunction-a major mediator of diabetic vascular disease.Biochim Biophys Acta 2013;1832:2216-31.
35.Libby P,Theroux P.Pathophysiology of coronary artery disease.Circulation 2005;111:3481-8.
 Journal of Traditional Chinese Medicine2022年3期
Journal of Traditional Chinese Medicine2022年3期
- Journal of Traditional Chinese Medicine的其它文章
- Efficacy of meridian massage for motor function after a stroke:a systematic review and Meta-analysis
- Antiviral Activity of Medicinal Plants against Human Coronavirus:a systematic scoping review of in vitro and in vivo experimentations
- Fuzheng Kang' ai decoction (扶正抗癌方) inhibits cell proliferation,migration and invasion by modulating mir-21-5p/human phosphatase and tensin homology deleted on chromosome ten in lung cancer cells
- Correlation between slow transit constipation and spleen Qi deficiency,and gut microbiota:a pilot study
- Efficacy of Kushen decoction (苦参汤) on high-fat-diet-induced hyperlipidemia in rats
- Hyperpolarization-activated cyclic nucleotide-gated 2 contributes to electroacupuncture analgesia on lumbar disc herniation-induced radicular pain through activation of microglia in spinal dorsal horn
