Rapid identification of volatile organic compounds and their isomers in the atmosphere
Xinglong ZHANG (张兴龙),Yifan GE (葛一凡),Enlai WAN (万恩来),Yuzhu LIU (刘玉柱),∗and Jinping YAO (姚金平)
1 Jiangsu Key Laboratory for Optoelectronic Detection of Atmosphere and Ocean,Jiangsu Collaborative Innovation Center on Atmospheric Environment and Equipment Technology (CICAEET),Nanjing University of Information Science &Technology,Nanjing 210044,People’s Republic of China
2 Jiangsu International Joint Laboratory on Meteorological Photonics and Optoelectronic Detection,Nanjing University of Information Science &Technology,Nanjing 210044,People’s Republic of China
3 State Key Laboratory of High Field Laser Physics,Shanghai Institute of Optics and Fine Mechanics,Chinese Academy of Sciences,Shanghai 201800,People’s Republic of China
Abstract Isomers are widely present in volatile organic compounds (VOCs),and it is a tremendous challenge to rapidly distinguish the isomers of VOCs in the atmosphere.In this work,laserinduced breakdown spectroscopy(LIBS)technology was developed to online distinguish VOCs and their isomers in the air.First,LIBS was used to directly detect halogenated hydrocarbons(a typical class of VOCs)and the characteristic peaks of the related halogens were observed in the LIBS spectra.Then,comparing the LIBS spectra of various samples,it was found that for VOCs with different molecular formulas,although the spectra are completely the same in elemental composition,there are still significant differences in the relative intensity of the spectral lines and other information.Finally,in light of the shortcomings of traditional LIBS technology in identifying isomers,machine learning algorithms were introduced to develop the LIBS technique to identify the isomers of atmospheric VOCs,and the recognition results were very good.It is proved that LIBS combined with machine learning algorithms is promising for online traceability of VOCs in the atmospheric environment.
Keywords: LIBS,VOCs,isomer,machine learning
1.Introduction
With the process of industrialization in many countries,a large number of volatile organic compounds(VOCs)are emitted from various emission sources.Atmospheric VOCs play a crucial role in the production of ozone,secondary organic aerosols and other pollutants [1,2],which can cause serious environmental pollution [3,4].Moreover,studies have shown that high VOC exposure can risk the health of humans,including the respiratory tract and vascular system,and can even cause cancer [5,6].Haloalkane,a typical class of VOCs,is a crucial source of halogen free radicals,and causes extremely serious damage to the ozone layer and the atmospheric environment [7].In addition,most halogenated hydrocarbon molecules can cause harm to human health [8].Hence,the governance of VOCs is very important.What is more,the processing technologies and governance schemes of different VOCs are distinct [9–11],which makes the online traceability of VOCs in the atmospheric environment particularly important.
To the best of our knowledge,there is almost no research on the online distinction between different VOCs in the atmosphere,especially the isomers of VOCs.Isomer is one of two or more compounds that have the same atoms,but in different arrangements,and is widely present in organic matter.At present,commonly used methods to distinguish isomers include nuclear magnetic resonance,mass spectrometry and colorimetric analysis as well as fluorescent responses,etc[12,13].However,most of the current researches on isomers are focused on the identification of isomeric carbohydrates [14–16],and research on the isomers of VOCs has received little attention.At present,the main techniques used in the analysis and identification of atmospheric VOCs and their isomers are mass spectrometry and synchrotron radiation [17,18].However,these techniques all require complex sample preparation and cannot achieve realtime monitoring.In addition,mass spectrometry cannot distinguish isomers.Therefore,research on the online identification of VOCs and their isomers is of great significance.
Laser-induced breakdown spectroscopy (LIBS) is based on using a high-energy pulsed laser to induce high-temperature plasma on the sample surface,and the emission signal of the high-temperature plasma provides the element information of the target.LIBS has many advantages including the ability to detect samples in various states (solid,liquid and gas),requires no sample preparation,is capable of real-time and in situ detection,etc[19,20].Based on these advantages,LIBS has a wide range of application in many fields.In particular,there have been extensive studies on the application of LIBS in the field of environmental monitoring,and the monitoring of the atmospheric environment has also made considerable progress [21–23].The online detection of atmospheric VOCs using LIBS technique has been achieved[24,25].Dibromoethane and fluorobromobenzene are typical VOCs that are highly toxic and carcinogenic,and also cause a great amount of environmental pollution.However,they are widely used in biological research,pesticide production,the chemical industry and other fields.At the same time,there are two isomers of dibromoethane,including 1,1 dibromoethane and 1,2 dibromoethane,while fluorobromobenzene has three isomers,2-fluorobromobenzene,4-fluorobromobenzene and 3-fluorobromobenzene.They are widely used in many ways.Dibromoethane and fluorobromobenzene are important commodity chemicals and indispensable synthetic intermediates in modern chemistry.However,halogenated pollutants are persistent in environmental pollution.In addition,studies have indicated that a high carcinogenic risk occurs from dibromoethane and fluorobromobenzene.Meanwhile,there are various treatment schemes for different VOCs due to their distinctive physical and chemical properties,and the same is true for isomers.Therefore,the identification of dibromoethane and fluorobromobenzene and their isomers is particularly important.
Nevertheless,as known,traditional LIBS technology was limited to the detection of the element information of the sample,and could not provide molecular structure information,which caused the online traceability of atmospheric VOCs using LIBS technique to be an extreme challenge.In this work,combined with principal component analysis(PCA)and BP-ANN[26–28],LIBS technique was developed to explore the online identification of VOCs and their isomers,which can provide a reference for the online traceability of VOCs in the atmospheric environment.
2.Experimental section
A schematic diagram of the experimental device is shown in figure 1.A Q-switched Nd:YAG laser (Continuum Co.,Ltd)functions as the excitation laser,which was operated at a fundamental wavelength of 1064 nm and produced a 6 ns duration pulse at a repetition frequency of 10 Hz.The maximum energy was 680 mJ in a single laser pulse,and the laser energy was set to 380 mJ in this experiment.The laser beam was focused by a 150 mm focal length plano-convex lens to ablate the aerosol samples.The emission signal of the high-temperature plasma was collected by the optical fiber probe and transmitted to the spectrometer system (AvaSpec-ULS2048-4 Channel-usb2.0,Avantes).The spectrometer has a wavelength range of 200–895 nm and a spectral resolution of about 0.08 nm.To control the time delay of signal collection,a digital delay generator was placed between the pulse laser system and spectrometer,and the delay time in this work was set at 2.5 μs.In order to simulate the state of VOCs in the atmosphere,the volatilization of the liquid sample is accelerated by the method of water-bath heating,and the generated aerosol is uniformly dispersed near the focus of the laser.Altogether,1000 spectral data were collected for each sample.The quantitative analysis of VOCs has been studied in detail in a previous work [29],and this work focused on the traceability research of large samples of VOCs.
3.Results and discussion
3.1.Spectral analysis of VOCs in the atmosphere
Halogen elements in halogenated VOCs are the main source of halogen free radicals in the air,and they do inestimable harm to the atmospheric environment.Therefore,two halogenated isomers were selected for LIBS testing.To make the data more credible,the spectrum used for analysis was obtained by averaging ten measurement results.As isomers,the spectra of 1,1 dibromoethane and 1,2 dibromoethane were basically the same in the whole band,so only the spectrum of 1,2 dibromoethane was given.Similarly,only the spectrum of 4-fluorobromobenzene (an isomer of fluorobromobenzene)was presented.Figures 2 and 3 are the LIBS spectra of 1,2 dibromoethane and 4-fluorobromobenzene,respectively.The spectral lines were identified and annotated with reference to the NIST atomic spectra database and some related studies[19,20,30].

Figure 1.Schematic of the LIBS experimental setup.

Figure 2.LIBS spectra of 1,2 dibromoethane.(a) 320–460 nm band,(b) 460–680 nm band,(c) 690–895 nm band.

Figure 3.LIBS spectra of fluorobromobenzene.(a) 320–460 nm band,(b) 460–680 nm band,(c) 690–895 nm band.
It is found from figure 3 that halogen bromine was found in the detection of fluorobromobenzene.However,another halogen element,fluorine,was not detected.Considering the difference in ionization energies between the two halogen elements (F: 1681 kJ·mol−1,Br: 1139.9 kJ·mol−1),this phenomenon can be explained.The element bromine with lower ionization energy was detected in VOCs,while the element fluorine with higher ionization energy was not detected in fluorobromobenzene.Comparing figures 2 and 3,it is found that the failure to detect fluorine in the fluorobromobenzene resulted in the same elemental composition of the spectra of dibromoethane and fluorobromobenzene,and the spectra in the whole band were very similar.
However,through careful observation of the spectra of the two VOCs,it is found that although the spectra of the two samples were very similar,the difference between them can be identified by the LIBS spectrum.In the band of 320–460 nm,the main spectral lines of the two targets in this band were CN molecular spectra.However,unlike fluorobromobenzene,the intensity of the CN line in the dibromoethane spectrum was low and there are many other elements,such as oxygen,which were the elemental components in the air.The reason for this phenomenon may be the different molecular composition of the two VOCs.The benzene ring contains more carbon than alkanes.When a high-energy pulsed laser ablates the target sample and generates plasma,more carbon can combine with nitrogen in the air to form CN radicals,resulting in a higher intensity of CN radicals.At the same time,in the third and fourth channels(460–680 nm band and 690–895 nm band),the intensity of each line of dibromoethane,especially the intensity of nitrogen,is stronger,which further verified the formation mechanism of the CN radical mentioned above.The content of N in the air is stable and none of the samples used in the experiments contain N.Therefore,the weakening of the N spectral line intensity and the enhanced CN spectral line intensity is due to the formation of CN radicals by C and N.It is worth noting that although characteristic lines of bromine were observed in the LIBS spectra of the two samples,the relative intensity and number of bromine lines in the dibromoethane were much higher than in the fluorobromobenzene sample.Not only were there seven additional atomic lines(635.07,700.51,734.85,780.30,881.99,882.52,889.76 nm)but also three ion lines of bromine were observed in dibromoethane and only one in fluorobromobenzene.
3.2.Rapid identification of different VOCs
According to the previous spectroscopic analysis of two VOCs with different molecular formulas,it is known that although the spectral information of the two is very similar,the samples with different molecular formulas could be directly distinguished by carefully observing the spectra.However,the original artificial identification of the difference in spectral information inevitably lacked identification speed.Therefore,artificial intelligence algorithms were introduced for rapid analysis and identification of spectra data.
First,a PCA algorithm was applied to reduce the dimensionality of the original spectral data,and a series of principal component data that carry a large amount of information on the spectral data was obtained.According to the detected spectral data,five atomic lines (635.07,780.30,881.99,882.52,889.76 nm) and five ion lines (470.49,478.54,481.66,518.23,523.82 nm)of bromine were selected as a spectral feature.Figures 4 and 5 display the distributions of principal components of fluorobromobenzene and dibromoethane and the cumulative contribution of the top 10 principal components,respectively.As shown in figure 5,the cumulative contribution of the first two principal components exceeded 98%,that is,the first two principal components carried a large amount of information of the original data,which can express the relevant characteristics of the original spectrum to a large extent.Therefore,we draw a 2D scatterpoint distribution diagram according to the scores of the first two principal components.As shown in figure 4,the point clusters of the fluorobromobenzene sample are concentrated in the areas where the scores of the two principal components were negative.The data points of dibromoethane were basically distributed in the area where the score of the first principal component was positive,and the overall distribution was relatively scattered.The two VOCs presented obvious differences in the distribution of principal components,and their rapid identification was realized.

Figure 4.Distribution of the scores of the first two principal components of dibromoethane and fluorobromobenzene samples.
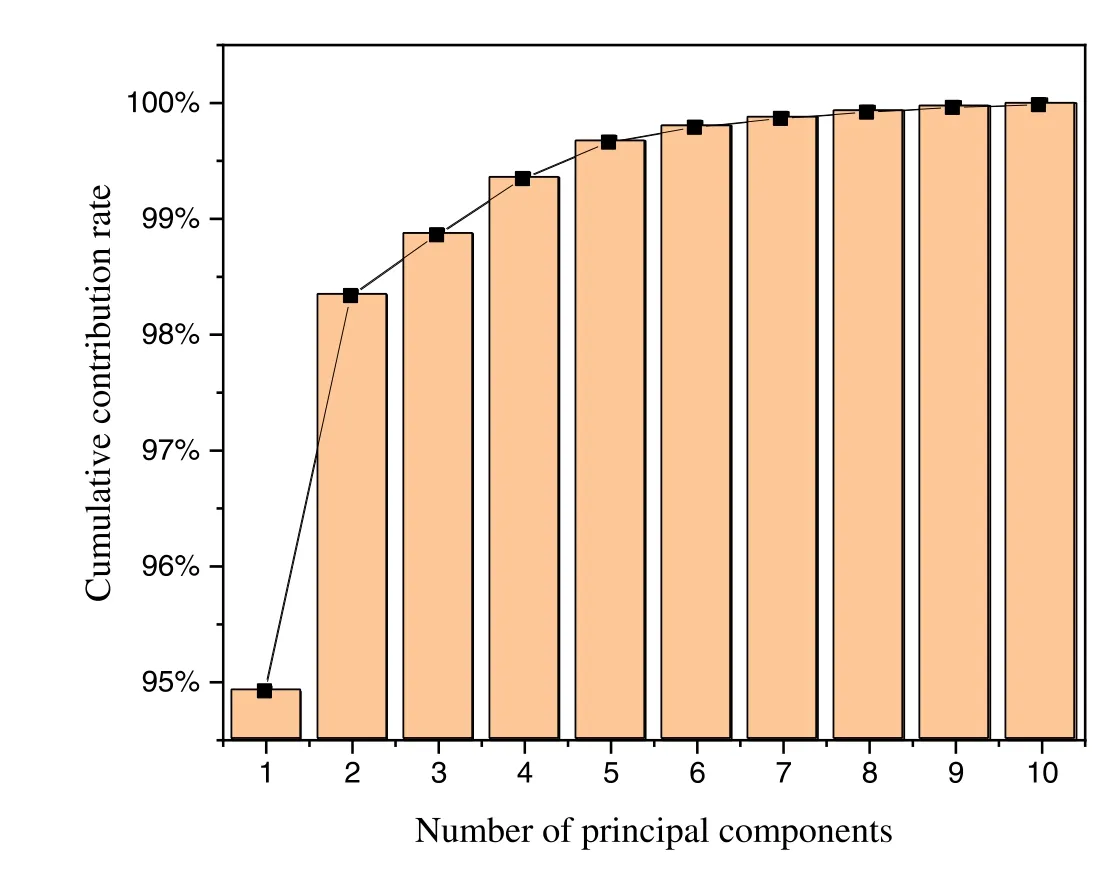
Figure 5.Cumulative contribution of the top ten principal components.

Figure 6.Scatter-point distribution diagram of the first two main components of the two isomers of dibromoethane.

Figure 7.Cumulative contribution of the top ten principal components.

Figure 8.Distribution of the first two main components of the two isomers of fluorobromobenzene.

Figure 9.Cumulative contribution of the top ten principal components.

Figure 10.Optimized molecular configuration diagram of the four VOCs.
The rapid identification of VOCs with different molecular formulas have been achieved by PCA.In the selection of machine learning algorithms,the neural network algorithm was more advantageous in various performances according to some studies on the comparison of the results of different model training [31].Therefore,the typical BP-ANN algorithm was selected for deep learning in this work.In order to further obtain identification accuracy,on the basis of PCA,BP-ANN was applied to conduct deep learning of principal component information.In this work,the input variable of the BP-ANN was the original features.A total of 400 sample spectra were used as input,and each sample had ten characteristic spectral lines.Then,200 sets of experimental data were taken for each of the two VOCs,80% of which were used as the training set and 20% as the test set,and the number of hidden nodes was optimized from 1 to 30.In this work,a simple attempt is made to determine the number of hidden layer neurons.The number of hidden neurons is set from 1 to 30 one by one,and the number of hidden neurons is obtained when the recognition accuracy reaches the maximum.First,the hidden neuron was determined through the training set,and finally we obtained the best recognition accuracy of 99.75% of the test set when the hidden neuron was 1.To further demonstrate the efficiency of the classification,parameters such as precision and recall were also calculated.The precision and recall rate parameters of the algorithm are calculated according to the formulas given by relevant researches [31],and the average value of multiple calculations functions as the final precision and recall rate.The final classification precision was 99.58%and the recall rate was as high as 100%.It was worth mentioning that the results obtained were also quite good when all the information of the spectrum,that is,the spectrum data in the full band of 200–895 nm were used to identify molecules,and the total recognition accuracy exceeded 90%.In particular,when the Δν=0 band of the CN molecule was used,the accuracy was as high as 98%.However,in order to achieve the goal of rapid identification,the bromine spectrum with a small amount of data was finally selected as the characteristic spectrum for classification.
3.3.Identification of isomers of VOCs
Although the spectra of dibromoethane and fluorobromobenzene were very similar,the spectral lines were quite distinct in terms of relative intensity.In particular,the difference in the bromine spectral lines was very obvious,and the two samples can be easily distinguished.The two isomers of dibromoethane have almost the same elemental composition and spectral line intensity,which could not be distinguished directly.Although their spectral information was very similar,there were still obvious differences between the two isomers with regard to various properties and other aspects,which led to some differences in their spectra that could not be distinguished through careful analysis.Therefore,the machine learning algorithm was used directly to process and analyze its spectrum,and then realized the identification of isomers.
In the same way,first the principal component information of the original spectrum was obtained.Unlike the classification of molecules with different molecular formulas,isomers could not be distinguished from a single spectral datum.After several attempts,the 470–525 nm band was chosen to extract the principal component information.The reason for this phenomenon may be that there are multiple ion lines of Br in this band,which carry some characteristic information of VOC samples.The spatial distribution map of the two principal components and the cumulative contribution of the first 10 principal components are shown in figures 6 and 7,respectively.According to figure 7,the cumulative contribution rate of the first two principal components exceeded 98%.That is,the first two principal components carried a large amount of information of the original data,which can express the relevant characteristics of the original spectrum to a large extent.After the second principal component,the contribution rate of each principal component was less than 1%.Consequently,a 2D scatter-point distribution map was constructed based on the scores of the first two principal components.From figure 6,it is found that the sample points of the two isomers of dibromoethane on the first principal component were overlapped and could not be distinguished.In the direction of the second principal component,1,1 dibromoethane was originally distributed in areas with higher scores.In contrast,1,2 dibromoethane was mostly distributed in areas with lower scores and even negative scores.
After obtaining the principal component information of the two isomers of dibromoethane,BP-ANN was used for accurate identification and classification.When the number of hidden neurons was 1,the best recognition accuracy of the test set was 98.25%,and the precision and recall were 96.08%and 97.5%,respectively.This result was perfect,and showed that the use of LIBS technology combined with BP-ANN to achieve classification of isomers is extremely promising.
For the two isomers of fluorobromobenzene,4-fluorobromobenzene and 2-fluorobromobenzene,the same treatment method as the isomers of dibromoethane was used.After comparative analysis,spectral data in the 470–525 nm band were used as an input to PCA.The spatial distribution map of the two principal components and the cumulative contribution of the first ten principal components are shown in figures 8 and 9,respectively.It can be seen from figure 9 that the cumulative contribution rate of the first two reached 98.7%,while the contribution rate of the first principal component was as high as 97.9%,which carried most of the information of the original spectrum.Consequently,the distribution maps of the two samples are drawn based on the first two principal components.From figure 8,it can be observed that the spectra of the two isomers of fluorobromobenzene are highly overlapped.There is no difference between the two samples in the direction of the first principal component,and they are both very scattered.It should be noted that in the direction of the second principal component,unlike 2-fluorobromobenzene,the distribution of 4-fluorobromobenzene was more concentrated.In terms of the overall distribution trend,2-fluorobromobenzene was dispersed in the entire space,while 4-fluorobromobenzene formed linear clusters along the direction of the first principal component.For the principal component information of the two isomers of fluorobromobenzene,the best recognition accuracy of the test set was 81.25% when the hidden neuron is 5 by using the BP-ANN.Although the PCA distributions of the two isomers of fluorobromobenzene were highly overlapped,the identification accuracy after combining with BP-ANN was still more than 80%,and the precision and recall was 74.63% and 71.25%,respectively.In addition,the principal component information of the two spectra showed different trends in spatial distribution,which can also be used as a basis for identification.
The recognition accuracy of the two isomers of dibromoethane was significantly better than the classification of the isomers of fluorobromobenzene.This phenomenon may be explained by their molecular structure.With the help of the structure of the Gaussian09 program,the optimized molecular geometries of the chosen VOCs were optimized via densityfunctional theory on the basis of B3LYP/6-311G++(d,p)and is shown in figure 10.The structure of the two isomers of dibromoethane was that two bromine atoms replaced two hydrogens on the same carbon bond and two bromine atoms replaced hydrogen on different carbon bonds,respectively,which is a huge difference in molecular structure.For 2-fluorobromobenzene and 4-fluorobromobenzene,both fluorine and bromine replace the two hydrogens on the benzene ring,but one is adjacent to hydrogen,and the other is opposite hydrogen substitution,which led to the fact that 2-fluorobromobenzene and 4-fluorobromobenzene are very similar in molecular structure.What is more,fluorine,an important element that characterizes molecular information failed to be detected in the spectrum due to the high energy of the excited electronic levels[32].All the above factors can be the reason for the unsatisfactory classification results of the isomers of fluorobromobenzene.
4.Conclusion
In the present work,LIBS technology was developed to combine machine learning to identify VOCs and their isomers in the atmospheric environment.First,LIBS was applied to the online detection of atmospheric VOCs,and the characteristic line of halogen element bromine of VOCs was observed.Then,it was found that the spectral information was quite diverse for the VOCs with different molecular formulas,and various VOCs could be directly distinguished.In order to solve the difficulty of identifying isomers,machine learning algorithms (PCA and BP-ANN) were introduced to process and analyze the spectral data.LIBS technique,combining PCA and BP-ANN,had a very good classification effect on isomers with large differences in molecular configuration,and the recognition accuracy of isomers with similar molecular configurations exceeded 80%.In conclusion,all results demonstrate that LIBS technology combined with classification algorithms can quickly identify the isomers of VOCs in the atmospheric environment.This has provided a reference for the traceability and tracking of VOCs in the atmosphere.The research in this work has explored the use of LIBS for molecular identification,which is of great significance to the application of LIBS technique.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No.U1932149),the Natural Science Foundation of Jiangsu Province (No.BK20191395) and the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province of China(No.18KJA140002).The authors are grateful to Dr Chaochao Qin for assistance with the structure optimization on the Gaussian09 program performed at Henan Normal University.
 Plasma Science and Technology2022年8期
Plasma Science and Technology2022年8期
- Plasma Science and Technology的其它文章
- Image-based plasma morphology determination and LIBS spectra correction in combustion environments
- Fast identification of mural pigments at Mogao Grottoes using a LIBS-based spectral matching algorithm
- Aqueous ruthenium detection by microwaveassisted laser-induced breakdown spectroscopy
- Effect of lens-to-sample distance on spatial uniformity and emission spectrum of flat-top laser-induced plasma
- Quantitative analysis of the main components in ceramic raw materials based on the desktop LIBS analyzer
- Elemental and proximate analysis of coal by x-ray fluorescence assisted laser-induced breakdown spectroscopy
