水热温度和时间对3D打印Ti-6Al-4V植入体表面理化性能的影响
纪振冰,万熠,赵梓贺,于明志,王宏卫,范世缘
水热温度和时间对3D打印Ti-6Al-4V植入体表面理化性能的影响
纪振冰1,万熠1,赵梓贺1,于明志1,王宏卫2,范世缘1
(1.山东大学 a.高效洁净机械制造教育部重点实验室 b.机械工程学院,济南 250061;2.山东大学齐鲁医院 a.急诊科 b.山东大学急危重症临床医学研究中心,济南 250012)
研究在具有微纳米双级结构的3D打印钛合金植入体表面制备聚多巴胺涂层的最佳工艺及参数。对酸蚀和阳极氧化处理后的3D打印Ti-6Al-4V样品进行水热处理,通过水热法将聚多巴胺添加到样品表面,并分析不同水热温度和时间的处理效果。使用扫描电子显微镜、三维共聚焦激光显微镜、X射线光电子能谱仪、接触角测量仪、电化学工作站对各组样品的表面形貌、粗糙度、元素组成、表面润湿性、耐腐蚀性等进行表征。经过酸蚀和阳极氧化处理后,在3D打印钛合金植入体表面成功制备了微纳米双级结构,表面纳米管的管径为80 nm左右,水热处理后各组表面均可以观察到有涂层附着。样品表面元素分析结果表明,各组样品表面的N/C值均与理论值0.125接近,证明水热处理成功在微纳米结构表面添加了聚多巴胺涂层。随着水热处理温度的升高和时间的延长,纳米管的管径逐渐减小,由80 nm减小至40 nm左右。随着反应时间的延长,样品表面粗糙度逐渐降低,各组粗糙度均保持在4~5 μm,且接触角逐渐减小,酸蚀后的表面接触角为52.1°,阳极氧化后,接触角降低至42.9°。经过水热处理,各组接触角均小于35°,表现出较好的亲水性。相比于酸蚀组和阳极氧化组,水热处理后的各组的耐腐蚀性均得到增强。在基本保留原有微纳米双级结构的前提下,37 ℃的反应温度和24 h的反应时间适用于聚多巴胺在钛合金植入体表面的沉积。研究结果为聚多巴胺在钛合金植入体表面自聚合的工艺参数优化提供了参考。
3D打印;钛合金;植入体;水热处理;微纳结构;聚多巴胺涂层
3D打印可以以计算机设计模型为基础,进行逐层打印,实现复杂模型的制备[1]。作为一种加工效率较高、能够用于复杂结构制造的生产方法,3D打印正在逐渐应用于植入体的制备中,并得到了众多学者的关注[2-4]。钛及其合金因具有良好的耐腐蚀性、较低的弹性模量和较好的力学性能,被广泛应用于临床植入手术中[5-7]。然而,3D打印制备的钛植入体具有生物惰性,表面质量较差,植入后难以与周围骨组织发生良好的骨结合[8]。为提高3D打印钛植入体的生物活性,需要进行表面改性处理,以增强钛与周围组织的骨整合[9-11]。
目前,钛植入体的表面改性处理主要集中在表面形貌改性和生物活性涂层改性[12-14]。3D打印制备的钛植入体表面具有微米级形貌,无需进行微米级表面改性[15],而钛植入体表面的微纳米双级结构可以促进成骨细胞的粘附、增殖和矿化[16-18]。研究表明,钛植入体表面构建的分级微纳米结构可以显著增强细胞成骨相关基因的表达,并促进动物体内新骨的形成[19]。聚多巴胺在促进细胞粘附、增殖[20-22]、降低细胞毒性[23]等方面具有较好的表现,已被广泛应用于提高钛植入体的生物相容性。Hu等[24]通过水热处理,在Ti-6Al-4V表面制备了PDA/GO/ZnO纳米复合涂层,样品具有良好的润湿性和抗菌活性。在钛植入体微纳米结构表面添加聚多巴胺涂层成为了新的研究热点。He等[25]通过碱热处理在钛表面构建了纳米结构,而后将植入体依次浸入多巴胺和硫酸铜溶液中,改性后的样品具有良好的抗菌性能。Yan等[26]将阳极氧化后的钛依次浸入多巴胺溶液和IL-4溶液中,成功在钛表面制备了TiO2/PDA/IL-4复合涂层,样品可以诱导巨噬细胞向抗炎M2表型极化。
目前,多巴胺聚合的最佳时间并不确定,从几个小时[27]到24 h[28]不等。Zhou等[29]将纯钛分别在25、37、60 ℃下浸入聚多巴胺溶液中,以比较聚多巴胺在不同温度下在钛植入体表面的沉积效果。当前研究中,用于聚多巴胺在具有微纳米双级结构的3D打印钛合金植入体表面涂覆的最佳工艺参数没有得到深入讨论。水热法作为一种操作简单、对环境污染较小的处理方法,被广泛应用于表面改性处理[30-32]。文中研究首先对3D打印钛合金植入体进行酸蚀处理,而后进行阳极氧化,通过水热法在钛合金表面自聚合生成聚多巴胺涂层,研究在不同温度和时间下,聚多巴
胺与具有微纳米双级结构的3D打印钛合金植入体的结合效果及其对表面性能的影响。采用扫描电子显微镜、三维共聚焦激光显微镜、接触角测量仪、X射线光电子能谱仪、电化学工作站等对不同组样品的表面理化性能进行了表征。
1 试验
1.1 材料预处理
Ti-6Al-4V样品由3D Systems公司的MDP Prox320激光打印机制造,粉末直径为10~53 μm,激光功率为88 W,扫描速度为620 mm/s。钛合金样品尺寸为10 mm×10 mm×1 mm,打印后的样品在丙酮、乙醇、去离子水中依次超声清洗15 min。将干燥后的样品置于2%(质量分数)的氢氟酸溶液中腐蚀5 min,所得样品记为AE。将酸蚀后的样品置于NH4F-丙三醇的水溶液中(体积比为1∶1),钛片连接恒压电源的正极,铂片连接恒压电源的负极,在25 V的电压下阳极氧化1 h,实验装置如图1所示,所得样品记为AN。将阳极氧化后的样品分别在37、60 ℃的2 mg/mL盐酸多巴胺的Tris-HCl缓冲溶液(pH=8.5)中分别水热处理8、16、24 h,而后在去离子水中进行超声清洗,以去除表面结合较弱的聚多巴胺分子,并在空气中干燥,所得样品分别记为AL1、AL2、AL3、AH1、AH2、AH3,见表1。反应过程如图2所示。
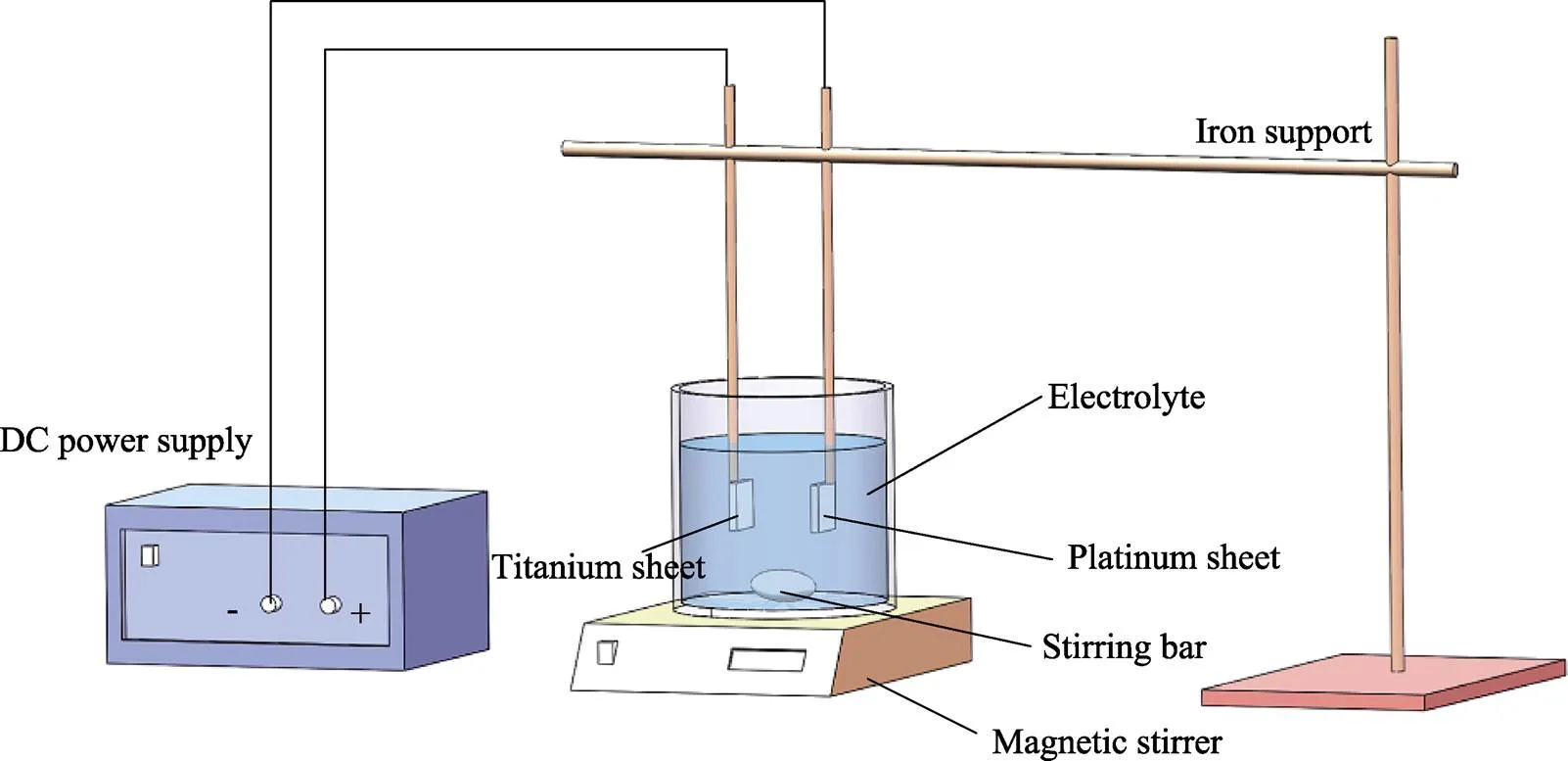
图1 阳极氧化实验装置
表1 样品缩写
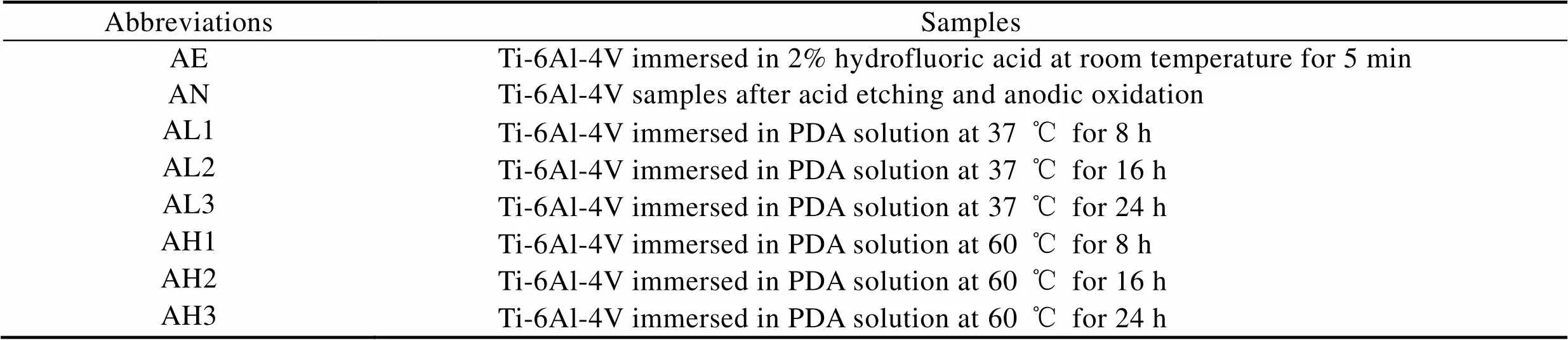
Tab.1 Abbreviations of samples
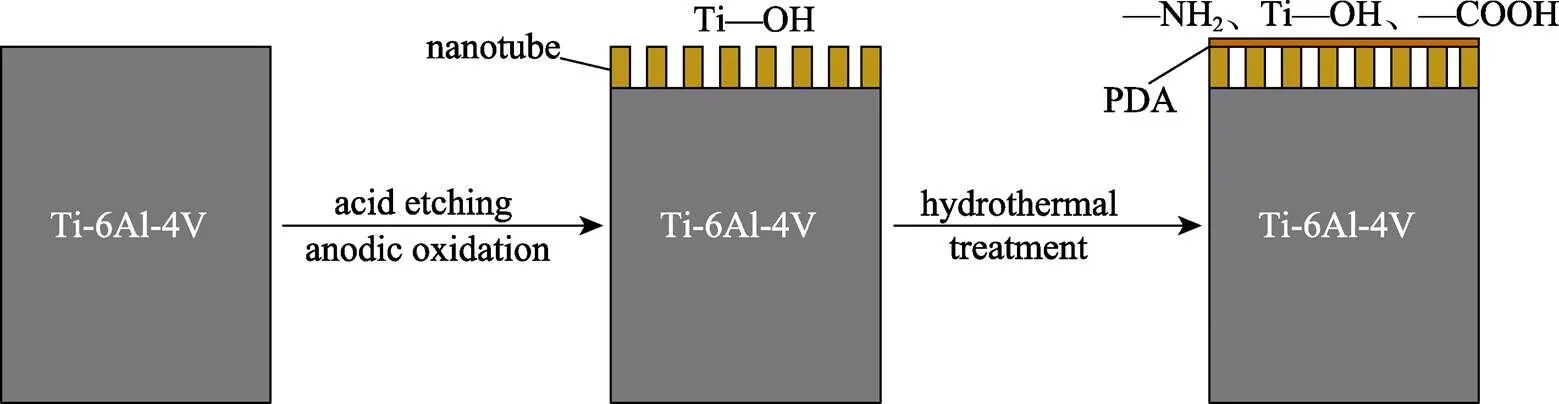
图2 反应过程
1.2 样品表面表征
使用扫描电子显微镜(SEM,JSM-7610F,日本)对样品表面形貌进行观察与表征。通过三维共聚焦激光显微镜(3D-LSM,VK-X200K,Keyence,日本)对样品三维形貌及表面粗糙度a进行测量及表征。通过X射线光电子能谱仪(XPS,AXIS Supra,英国)对样品表面元素进行定性及定量分析。
1.3 表面润湿性分析
通过接触角测量仪(SL200KS,美国)对样品表面的润湿性进行分析,将2 μL去离子水滴在样品表面,通过测量接触角的大小判断样品表面的亲水性。
1.4 电化学腐蚀试验
通过电化学工作站使用动电位扫描法对样品的耐腐蚀性进行检测。电化学工作站由饱和甘汞电极(参比电极)、铂电极(辅助电极)和试样(工作电极)构成三电极系统。样品通过铜线与工作电极相连接,样品的工作面积为1 cm2,非工作面积通过环氧树脂进行绝缘封装。电化学工作体系置于37 ℃恒温的0.9% NaCl溶液中,设置初始电位为–0.8 V,终止电位为2 V,扫描速率为2 mV/s。
2 结果和讨论
2.1 样品表面表征
样品表面形貌对植入体的生物相容性和与周围组织结合的能力有着十分重要的作用。在不同倍数扫描电镜下观察到的样品表面形貌如图3所示。在低倍镜下,AE组样品表面可以观察到峰–谷状的微结构,AN组表面在酸蚀后,表面锐边变得更平滑,AL1—AH3各组样品的表面形貌大致相同,具有微米级的沟壑结构,同时表面观察到有涂层附着。在高倍镜下可以发现,AE组表面出现了微米级的凹坑,这些微米级结构被认为有利于提高植入体与周围组织的剪切强度[33],阳极氧化后,AN组表面在原凹凸不平的表面上生成了有序排列的TiO2纳米管阵列,形成了微纳米双级结构。对于AL1—AH3组,当反应时间相同时,随着温度的升高,试件表面物质团聚的现象越来越明显。AL1组纳米管直径在80 nm左右,而AH各组相比于AL组,纳米管的管径明显减小。随着时间的延长,涂层的厚度逐渐增大,AL各组之间和AH各组之间均发现纳米管的管径随时间的延长而逐渐变小,AH3组纳米管管径减小至40 nm左右,且纳米管之间的间隙逐渐减小,被涂层所填充,甚至可以观察到有些纳米管出现被堵塞的情况。以上结果表明,反应时间的延长和温度的升高会使反应更加充分,生成的涂层也更加致密,在温度较高和反应时间较长时,甚至可以覆盖试件表面原有的微纳米结构。由图3可知,AL3组表面形成的涂层较均匀,并较大程度地保留了表面原有的微纳米双级结构。
阳极氧化是在基于氟化物的电解液中,将钛作为阳极,并施加恒定电压,在钛表面生成二氧化钛纳米管的过程[34],与其他方法相比,该方法效率较高,且纳米管管径和长度可控[16]。在阳极氧化的过程中,钛与电解液在电场的作用下发生反应,生成二氧化钛层,二氧化钛层会阻碍电化学反应的进行。此时,二氧化钛会与水中的F–发生反应,生成水溶性的[TiF6]2–,暴露出原有的钛基底,在溶解和生成的动态循环中形成紧密排列的纳米管,并随着时间的延长,纳米管逐渐向基体延伸,从而使纳米管的长度逐渐增大,反应方程式见式(1)、(2)[35-37]:
Ti+2H2O→TiO2+4H++4e–(1)
TiO2+6HF→[TiF6]2-+2H2O+2H+(2)
各组试件的三维形貌如图4所示。从图4可以看出,试件表面均可以观察到3D打印过程中激光熔融的痕迹,阳极氧化后,AN组表面的棱角变得更平滑。采用不同参数进行水热处理后的样品表面未观察到明显差异,这与低倍镜下扫描电镜观察结果相一致。
经过处理之后样品的粗糙度如图5所示,各组粗糙度均保持在4~5 μm。其中,酸蚀组的表面粗糙度为4.905 μm,阳极氧化后样品的表面粗糙度降低至4.422 μm,AL1组由于在37 ℃的条件下进行,且反应时间较短,表面仍较大程度地保留着原有的微纳米形貌,表面粗糙度为4.633 μm,而AL2、AL3组由于反应时间较长,在样品表面形成了较均匀的涂层,粗糙度分别降低至4.438、4.130 μm。同时,在60 ℃的反应温度下,样品表面粗糙度略有提升,分析原因是反应速率加快使表面涂层出现团聚现象。AH1组经过8 h的反应后,粗糙度为4.758 μm,且随着反应时间的延长,粗糙度逐渐降低。AH2、AH3组的粗糙度分别为4.601、4.239 μm。这种现象可以归因于表面自组装方法的特性,随着时间的延长,表面分子将呈现更加均匀的分布,可以降低表面的粗糙度[38]。有研究表明,粗糙度a为3~5 μm的表面可以增加骨与植入体之间的接触面积[39-40],有利于细胞的粘附、矿化、相关基因的表达及骨整合[41-43]。
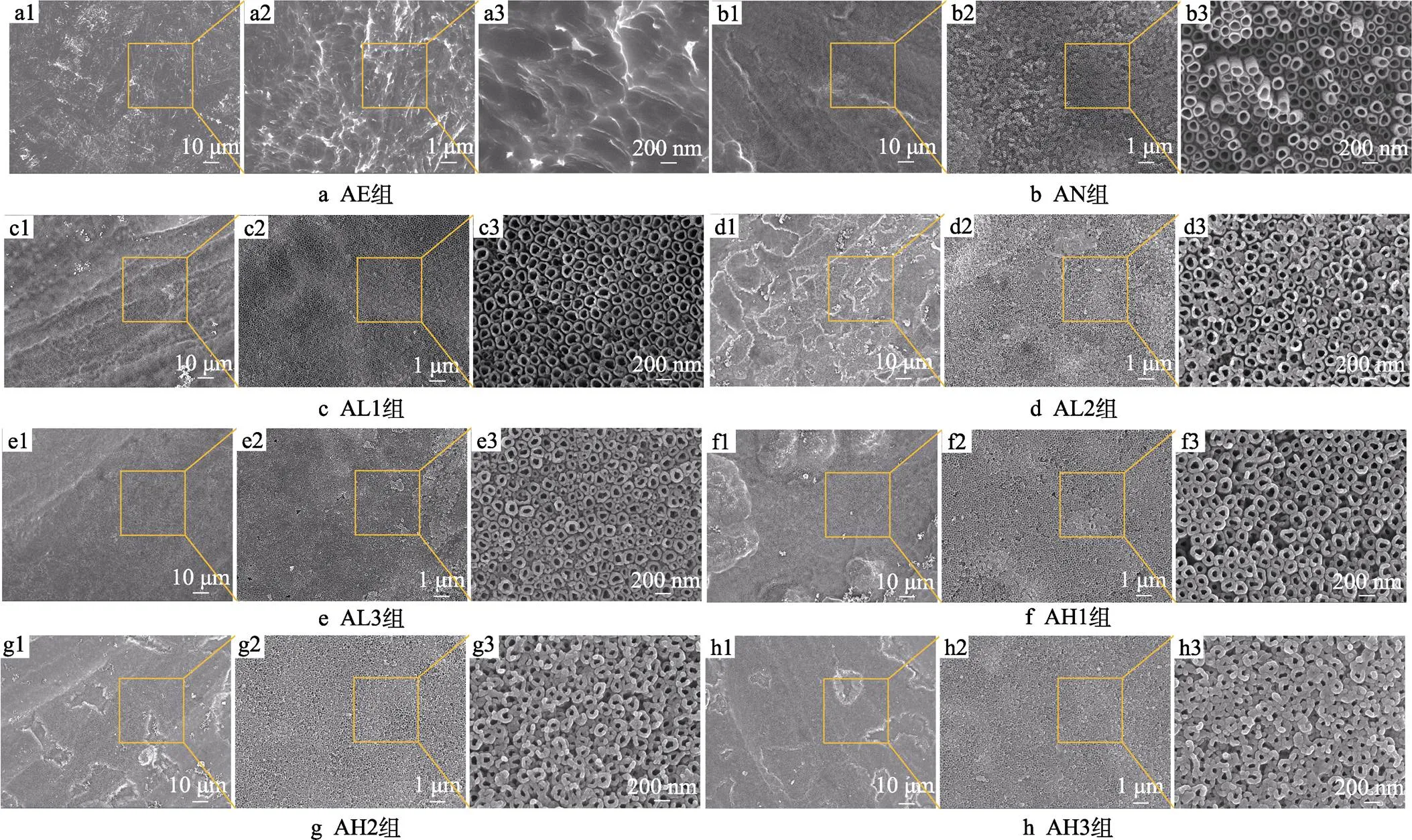
图3 各组试件的表面SEM形貌
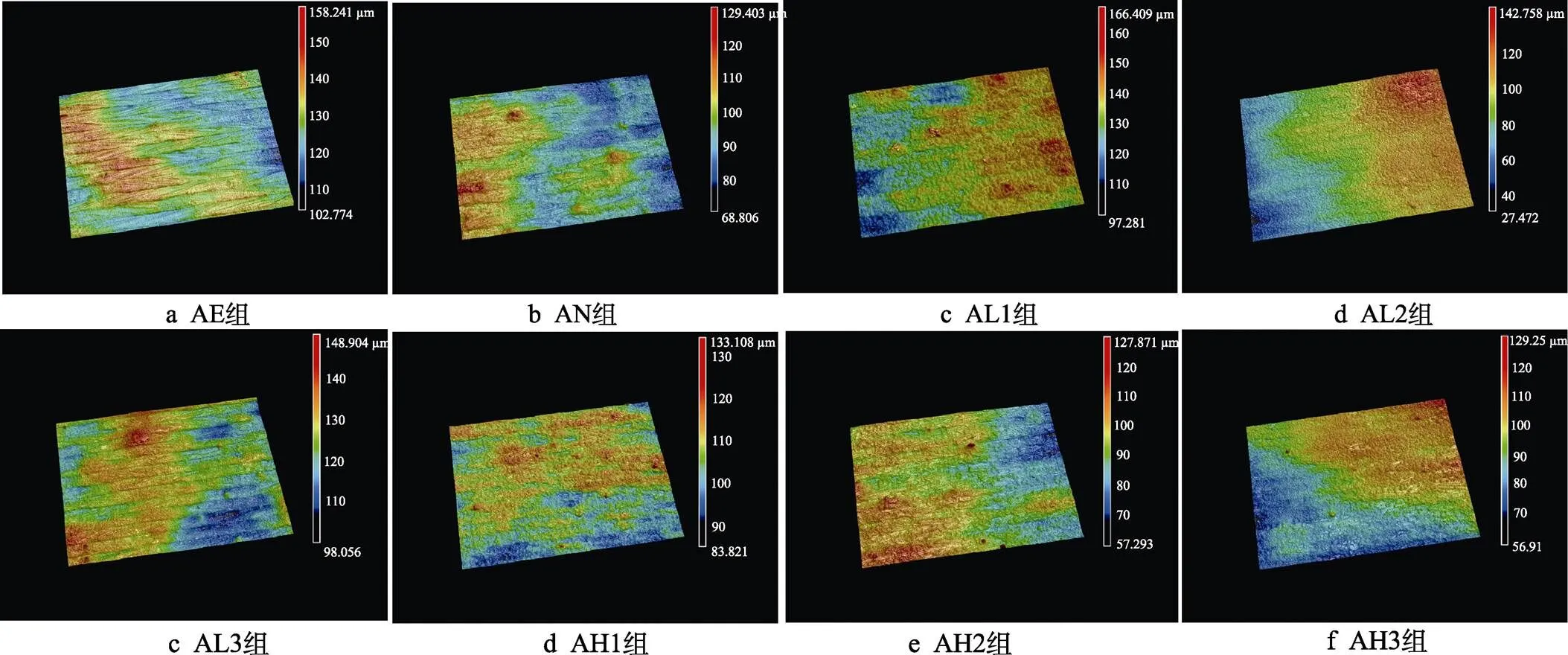
图4 各组样品表面的三维形貌
2.2 表面元素分析
使用XPS分析样品表面元素组成,各组样品的XPS全谱图如图6所示。由全谱图可以看出,由于钛的化学性质较活泼,极易与空气中的氧气反应,因此酸蚀组表面可以检测到较强的Ti2p峰和O1s峰。阳极氧化后,表面可以检测到酸蚀组表面没有的F1s峰。经过水热处理之后,各组在400.1 eV处均出现了N1s峰,且样品表面碳和氧的含量较高,证明在钛基底表面添加了聚多巴胺涂层。在37 ℃下对样品进行处理时,样品表面可以检测到较弱的F1s峰和Ti2p峰,证明经过阳极氧化,表面仍然有残留的[TiF6]2–。在60 ℃下,随着反应温度的升高,多巴胺聚合的速率加快,样品表面的聚多巴胺涂层较致密,使得原有的F1s峰和Ti2p峰消失。通过表2数据计算可知,各组的N/C值均与理论值0.125接近,表明经过水热处理后在样品表面成功添加了聚多巴胺涂层[44-45]。随着温度的升高,N/C值逐渐降低,且AL3组与AH3组大致相同,随着时间的延长,AL各组的N/C值先增大、后减小,AH各组的N/C值先减小、后增大,而AH各组N/C值相比于AL组,距离理论值差距较大。温度较高时,在反应过程中可能存在溶液中的杂质等参与反应的现象,造成其他非目标产物的生成。
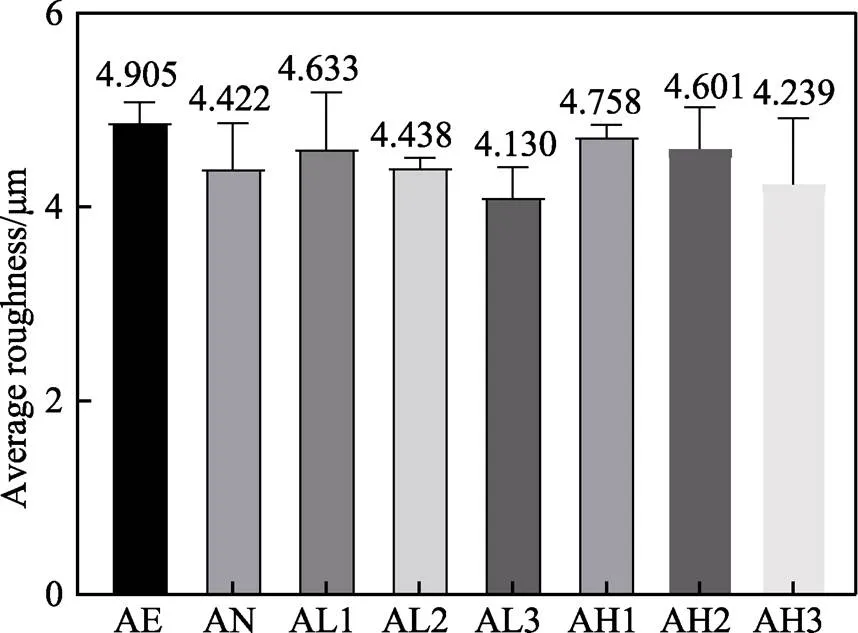
图5 不同样品表面的粗糙度平均值(n=3)
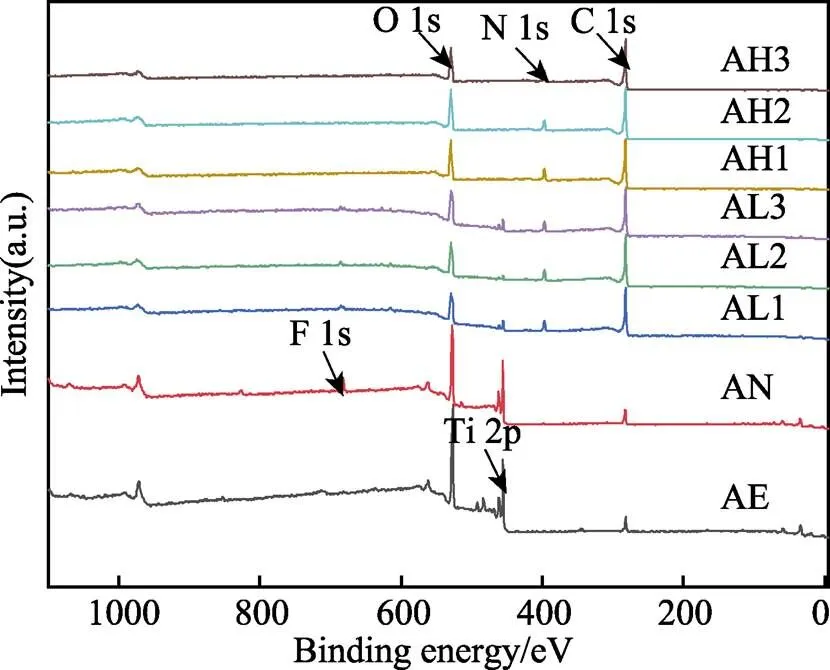
图6 各组样品表面的XPS全谱图
表2 各组样品表面元素含量分析结果
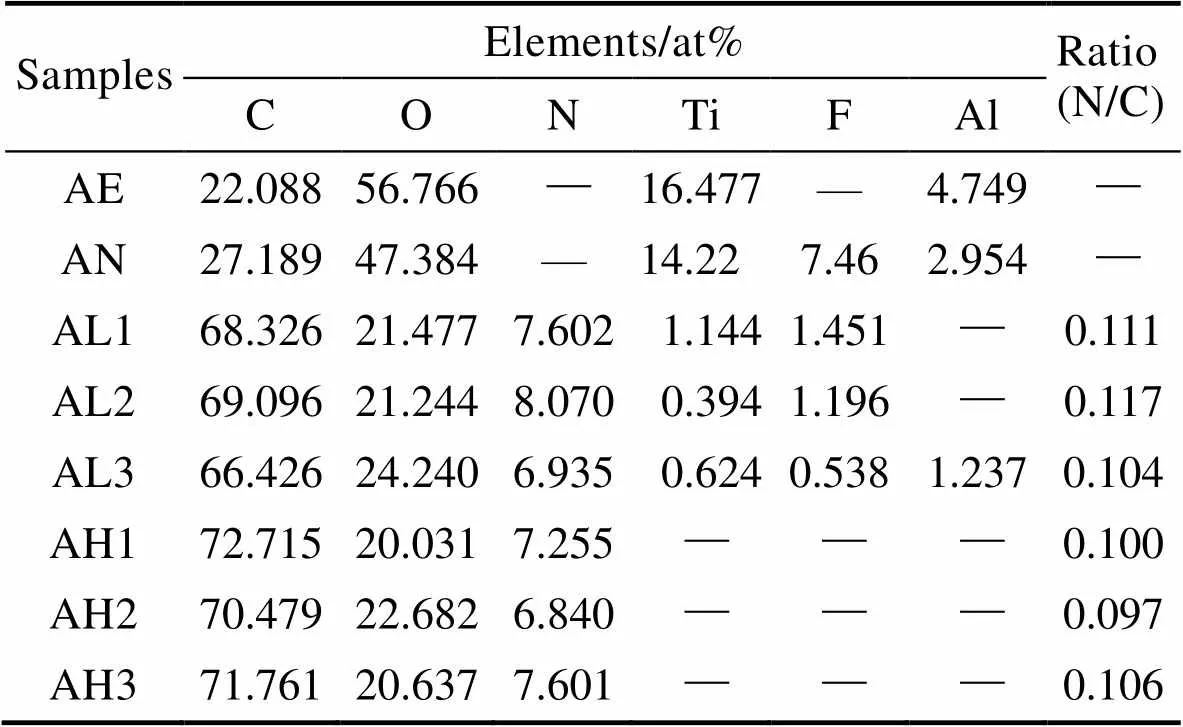
Tab.2 Surface element content analysis results of different groups
目前,因为聚多巴胺的形成过程复杂,其形成机理尚未明确。有学者认为,PDA的形成为单纯的化学聚合,即直接氧化聚合。多巴胺首先被氧化生成多巴胺醌,分子内环化,氧化成无色多巴色素,形成5,6–二羟基吲哚,并进一步氧化成5,6–二羟基吲哚醌。之后发生分支反应,产生二聚体的多种异构体。随后在更高的低聚物上发生分支反应,然后这些低聚物进一步发生自聚合反应,生成小于100 nm的聚多巴胺颗粒,氧化过程如图7所示[46-48]。
由于PDA的形成机制较为复杂,不同文献对PDA聚合的机理提出了不同的解释。其中,Hong等[49]从物理和化学的角度对PDA的聚合过程提出了解释,得到了许多学者的认可,反应过程如图8所示。首先,多巴胺在氧气的参与下被氧化为多巴胺醌,这一过程已得到学者们的公认。另外,结合多巴胺在聚合过程中膜层的颜色和状态的变化,推测多巴胺在聚合过程中发生了类似黑色素的聚合过程,同时表面形成的膜层阻碍了氧气的进入,从而内部未被氧化的多巴胺存在物理自聚合的可能。
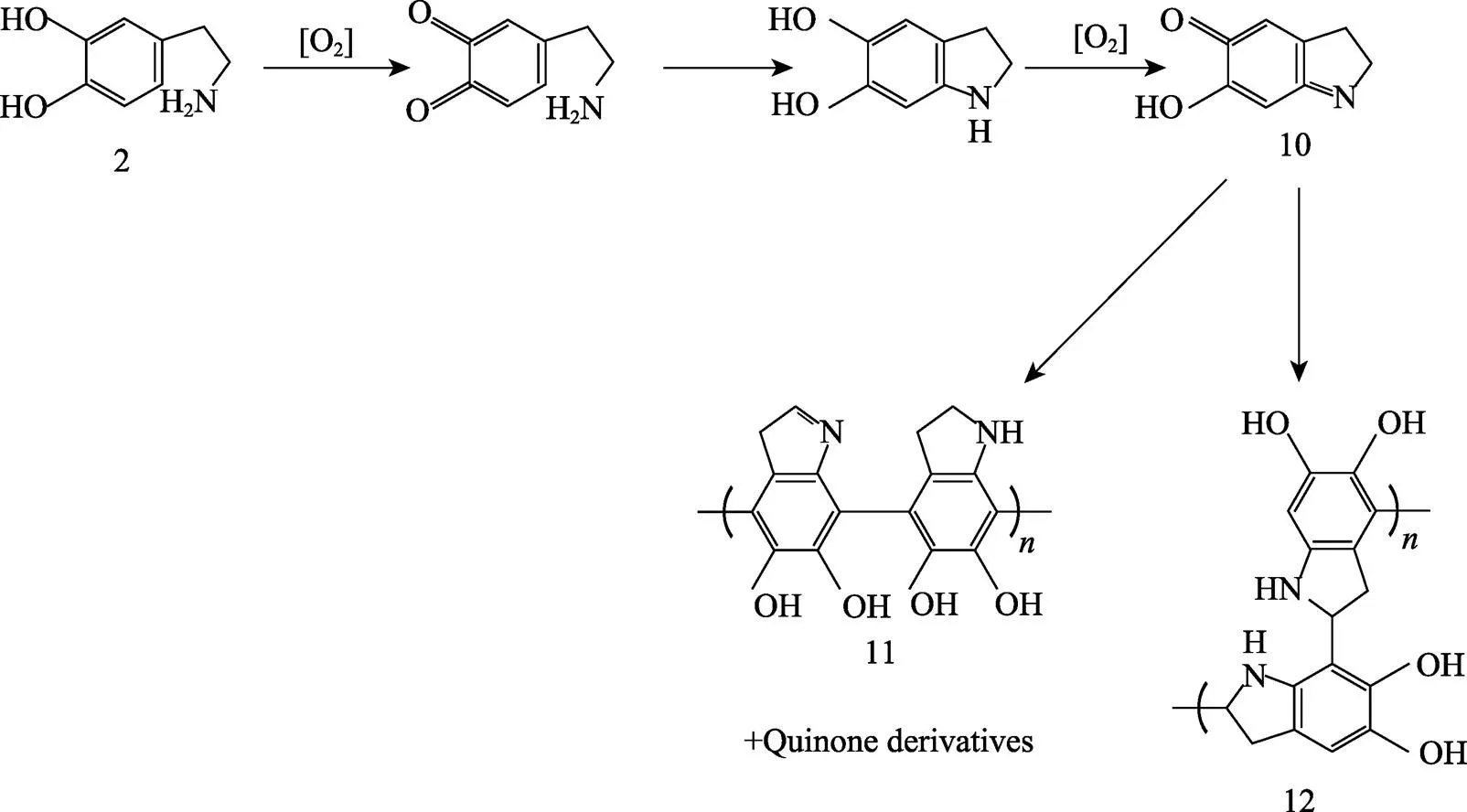
图7 直接氧化交联聚合生成PDA的反应过程[46-48]
研究发现,海洋贻贝足腺分泌的粘附蛋白具有超强的粘附性能,这些粘附蛋白中的主要物质为DOPA和部分赖氨酸残基,DOPA中含有丰富的儿茶酚官能团,其具有极强的化学多功能性和高亲和力,其粘附能力和自身的快速化学交联固化可以实现在多种材料上的粘附,而多巴胺因具有DOPA中的儿茶酚官能团和赖氨酸中的端氨基,也被证明也具有较强的粘附性能[50,51]。多巴胺在碱性溶液中可以实现氧化自聚合,从而形成具有较强粘附性能的聚多巴胺,实现在钛基底表面的粘附。其中,类似于海洋贻贝,PDA的超强粘附性能是由于含有丰富的儿茶酚官能团,其可与基底形成牢固的共价键或非共价键(氢键、范德华力或堆积作用力)[46,52]。
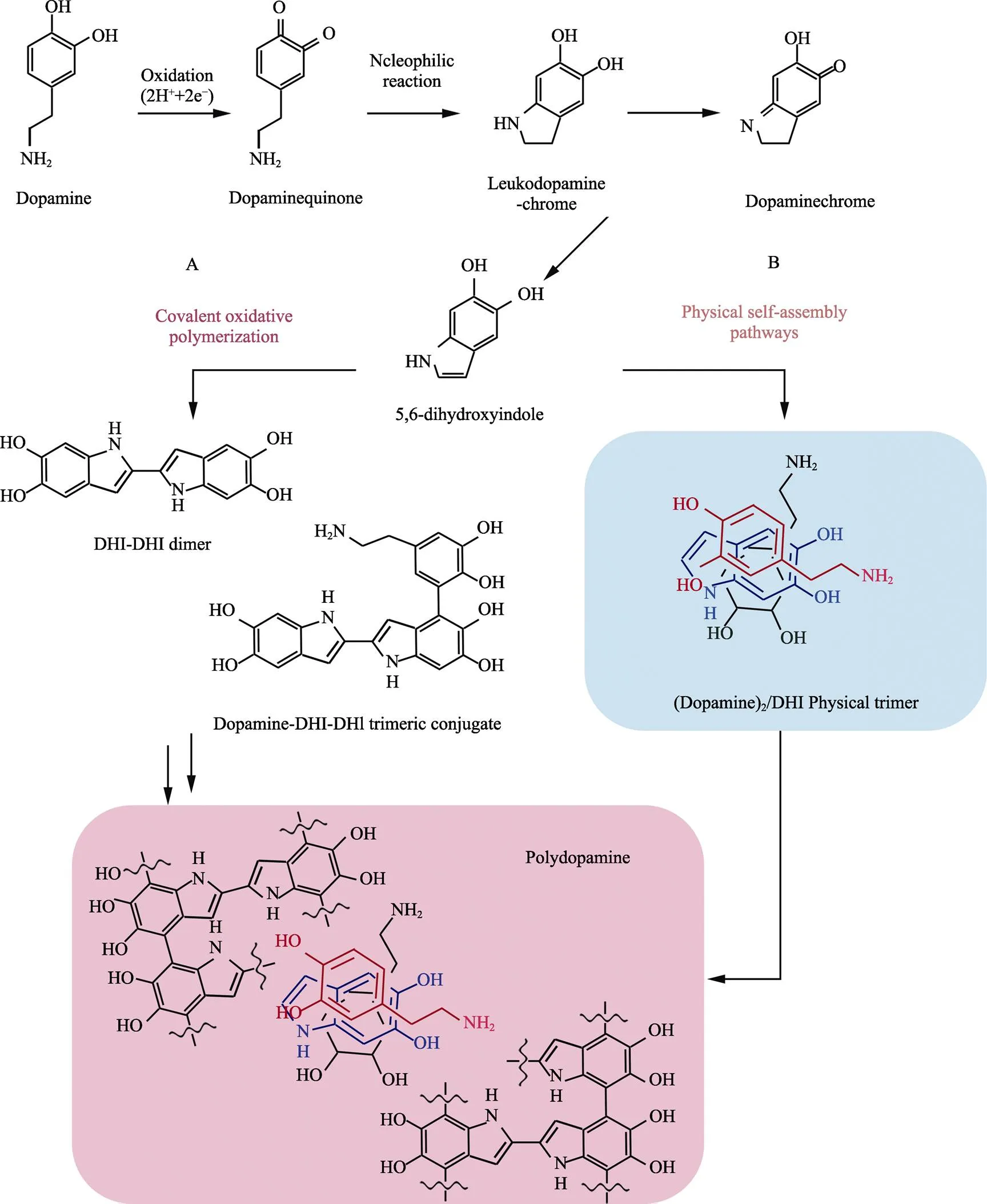
图8 物理和化学聚合生成PDA的反应过程[49]
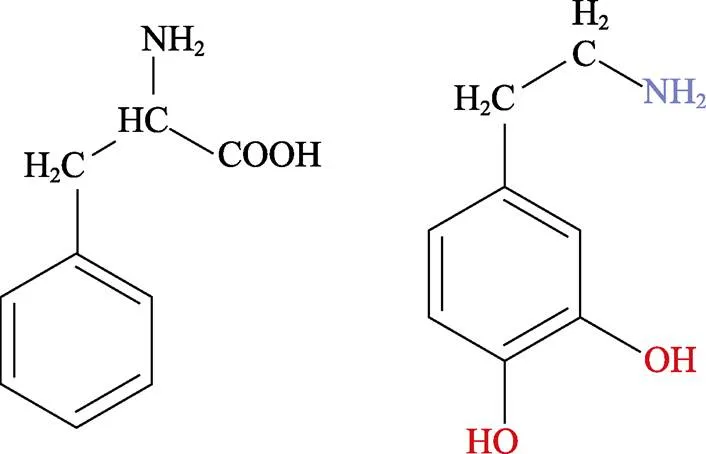
图9 多巴(DOPA)和多巴胺(Dopamine,DA)的结构式
为更好理解表面的化学成分,对各组样品表面的C1s峰及N1s峰进行分峰拟合处理,如图10所示。各组均只有一个主要的C1s峰,这可以归因于存在的C—C/C==C及C==O/O—C==O[53]。在37 ℃下,各组样品表面醌基—C==O的峰面积由46.66%减小至16.68%后,又增大至39.29%,且峰位先减小、后增大。60 ℃下,各组样品表面醌基—C==O的峰面积逐渐增大,由21.65%增大至25.75%和29.19%,各组峰的位置大致相同。由图11可知,样品表面只有一个主要的N1s峰,表面峰面积为100%的吡咯型氮,其来自于聚多巴胺的氨基[54]。有研究表明,吡咯型氮可氧化为吡啶型氮,利于表面多巴胺的—C—OH向—C==O转变[45]。
2.3 表面润湿性
2 μL去离子水滴在各组试样上的图像如图12所示。水滴滴下后,迅速在样品表面铺展开。从图12中可以看出,AE组表面的接触角为52.1°。阳极氧化后,由于表面纳米管存在毛细效应,且表面存在丰富的羟基,接触角降低至42.9°。水热处理之后,各组均表现出良好的亲水性,且随着反应时间的延长,接触角逐渐降低,AL3组和AH3组的接触角最低,分别为23.9°和22.8°。根据Wenzel模型[55],当将液滴滴在样品表面时,水滴会完全渗透进样品的微结构中,从而导致样品表面接触角降低。同时,由于阳极氧化后形成的纳米管具有毛细效应和丰富的Ti—OH基团[35],且水热处理后表面聚多巴胺涂层含有丰富的酚羟基、羧酸和氨基[56-57],使接触角进一步降低。有研究表明,表面较好的亲水性能够促进蛋白质和细胞的粘附,对后期的细胞增殖、分化及植入体内后的骨整合具有促进作用[58-60]。
2.4 耐腐蚀性测试
生物医用金属材料植入体内后,在使用过程中不可避免地会发生腐蚀,导致植入体不稳定,甚至松动等。生物材料一旦发生腐蚀,表面的金属离子会从表面释放出来,造成溶骨等并发症[61]。试验结果如图13所示。相比于AE组,阳极氧化和通过水热处理添加聚多巴胺涂层后的各组的腐蚀电位逐渐增大,代表腐蚀倾向减小,表明阳极氧化和聚多巴胺涂层能够有效阻止腐蚀离子与Ti-6Al-4V基底的接触。其中,AL1、AL3、AH1组的腐蚀电位最大,说明其腐蚀倾向最小。除此之外,AL1、AL3、AH1组的腐蚀电流较小,说明其腐蚀效率较低,这也与之前的研究结果相一致。因此,AL1、AL3、AH1组的耐腐蚀性相比于其他各组得到了较明显的增强,猜想可能原因是形成的涂层较均匀,对基底形成了较好的保护作用,减弱了腐蚀离子对钛基底的侵蚀作用。
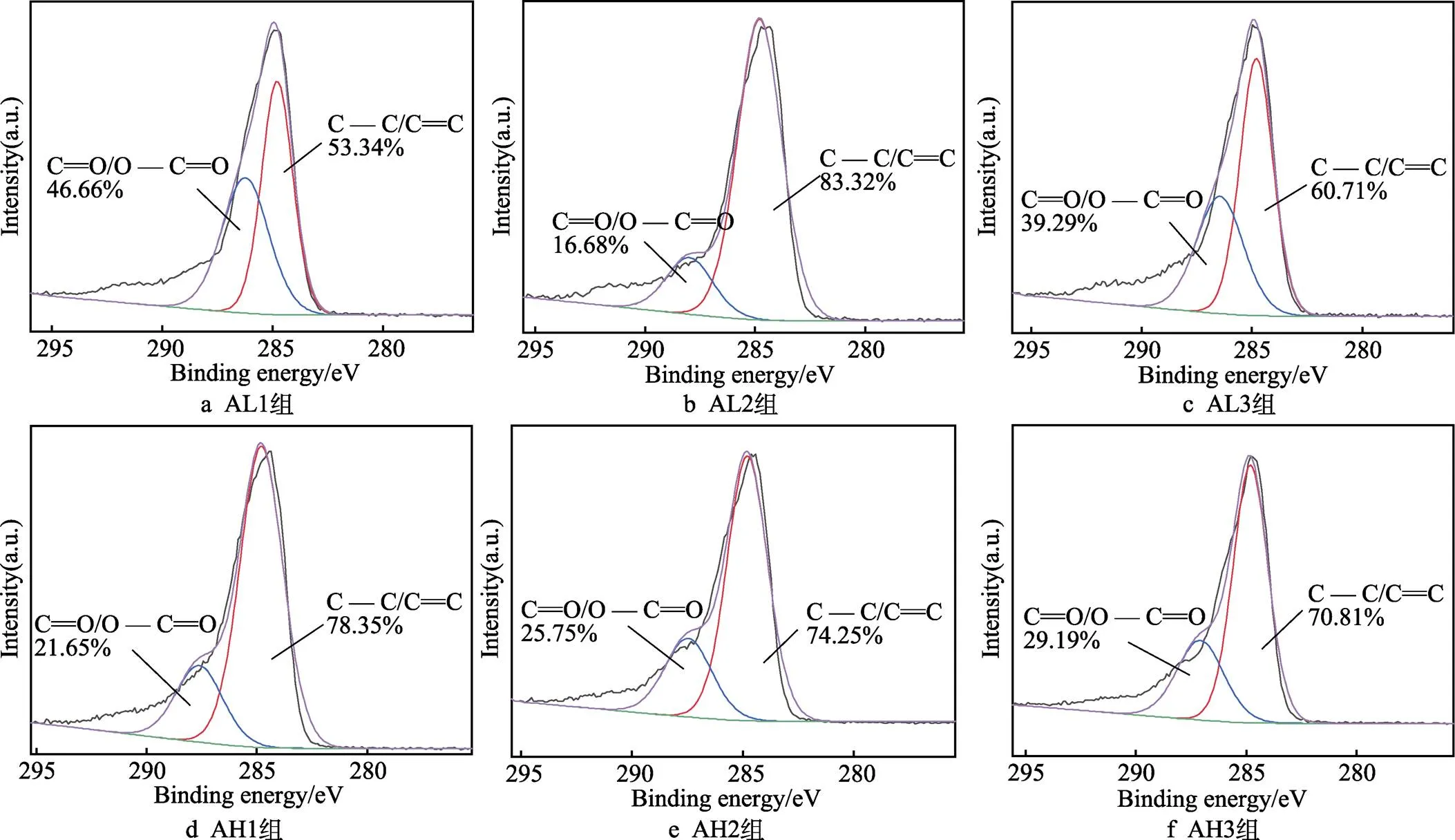
图10 各组样品表面C1s光谱分峰结果
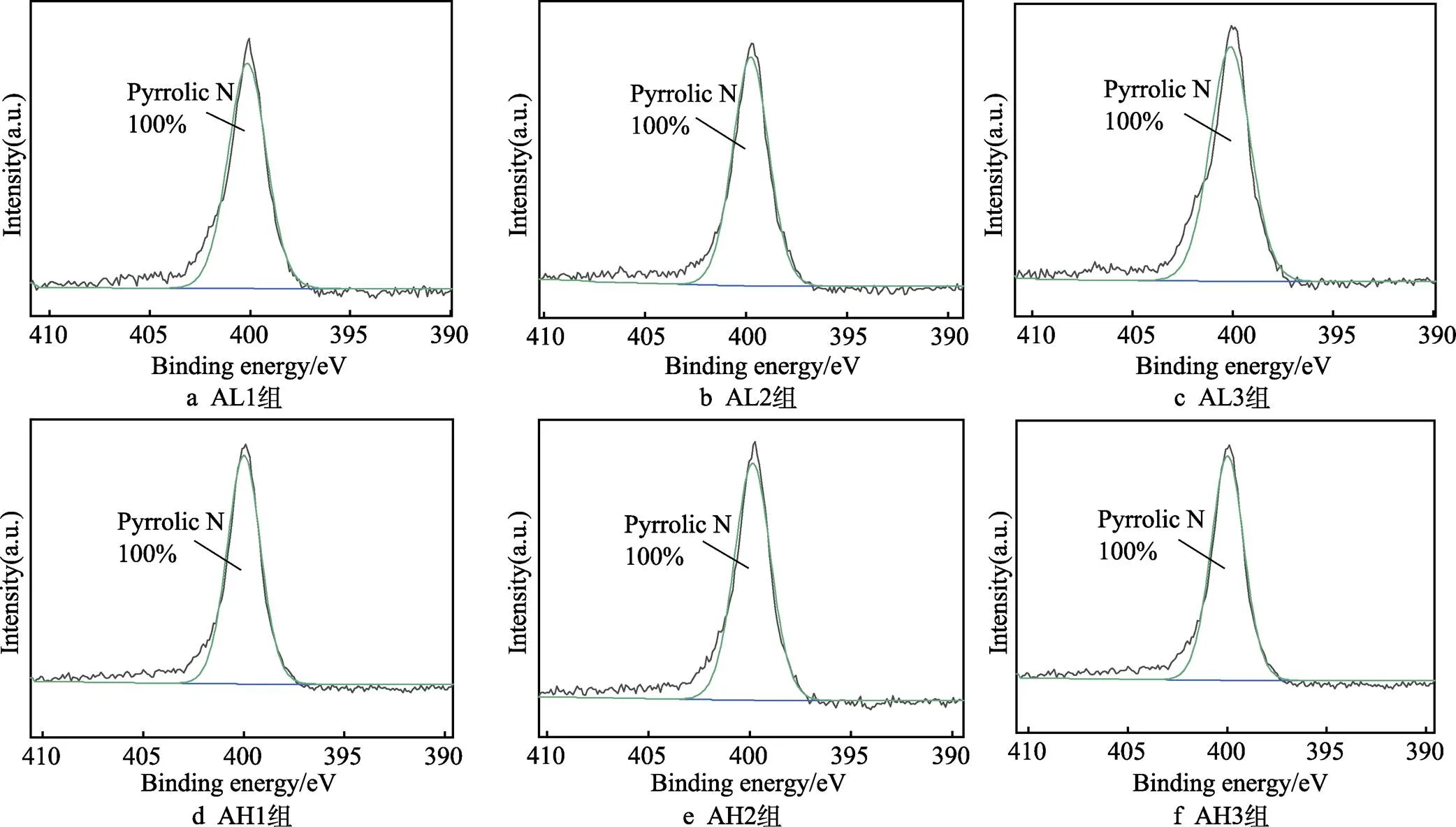
图11 各组样品表面N1s光谱分峰结果
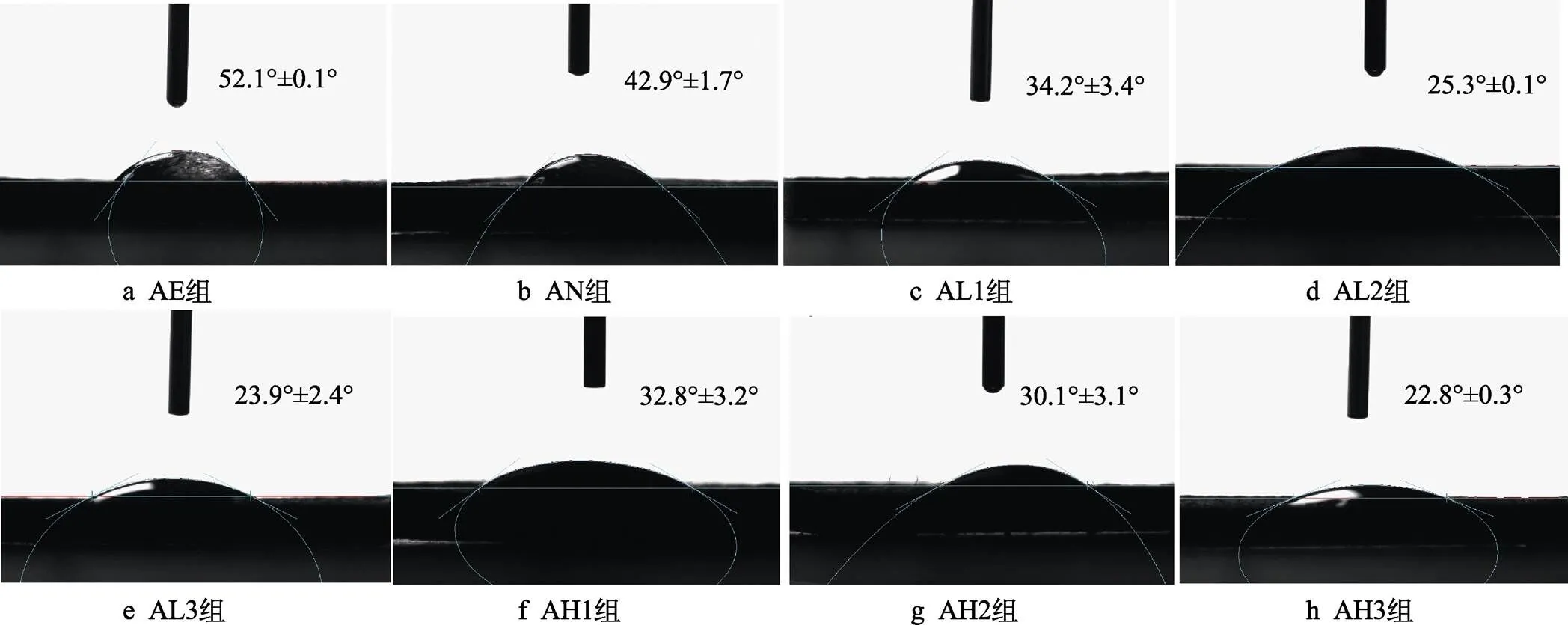
图12 样品的表面润湿性

图13 各组样品在0.9% NaCl溶液中的动电位极化曲线
3 结论
1)经过酸蚀和阳极氧化处理,在3D打印制备的钛合金基底上成功制备了微米和亚微米级形貌,并构建了有序排列的TiO2纳米管阵列,水热处理之后,各组表面均成功添加了聚多巴胺涂层。
2)随着水热处理温度的升高和时间的延长,纳米管的管径逐渐减小,在温度较高、时间较长时,聚多巴胺出现了较明显的团聚现象。表面粗糙度与反应温度成正相关,与反应时间成负相关。
3)表面元素分析结果表明,各组N/C值均与理论值接近,进一步证明聚多巴胺已经成功添加在样品表面。表面润湿性试验结果表明,各组均表现出较好的亲水性。电化学腐蚀试验结果表明,阳极氧化和聚多巴胺涂层均能够提高样品的耐腐蚀性。
4)在基本保留基底表面原有的微纳米双级结构的前提下,将3D打印钛合金植入体在37 ℃下浸入聚多巴胺溶液中24 h后获得的样品具有最优的综合性能。
[1] ZHANG Jin-kai, ZHOU Wen-hui, WANG Hui, et al. 3D- Printed Surface Promoting Osteogenic Differentiation and Angiogenetic Factor Expression of BMSCS on Ti6Al4V Implants and Early Osseointegration in Vivo[J]. Journal of Materials Science & Technology, 2019, 35(2): 336-343.
[2] DENG Fu-yuan, LIU Lin-lin, LI Zhong, et al. 3D Printed Ti6Al4V Bone Scaffolds with Different Pore Structure Effects on Bone Ingrowth[J]. Journal of Biological Engineering, 2021, 15(1): 1-13.
[3] PEI Xuan, WU Li-na, ZHOU Chang-chun, et al. 3D Printed Titanium Scaffolds with Homogeneous Diamond-Like Structures Mimicking that of the Osteocyte Microenvironment and Its Bone Regeneration Study[J]. Biofabrication, 2020, 13(1): 1-15.
[4] ZHAO Peng-yu, LIU Ya-qin, LI Tian, et al. 3D Printed Titanium Scaffolds with Ordered TiO2Nanotubular Surface and Mesoporous Bioactive Glass for Bone Repair[J]. Progress in Natural Science: Materials International, 2020, 30(4): 502-509.
[5] REN Bing, WAN Yi, LIU Chao, et al. Improved Osseointegration of 3D Printed Ti-6Al-4V Implant with a Hierarchical Micro/Nano Surface Topography: An in Vitro and in Vivo Study[J]. Materials Science and Engineering: C, 2021, 118: 111505.
[6] JIA Zhao-jun, XIU Peng, LI Ming, et al. Bioinspired Anchoring AgNPs Onto Micro-Nanoporous TiO2Orthopedic Coatings: Trap-Killing of Bacteria, Surface-Regulated Osteoblast Functions and Host Responses[J]. Biomaterials, 2016, 75: 203-222.
[7] TRZCIŃSKA Z, BRUGGEMAN M, IJAKIPOUR H, et al. Polydopamine Linking Substrate for AMPs: Characterisation and Stability on Ti6Al4V[J]. Materials (Basel, Switzerland), 2020, 13(17): 3714.
[8] WEI Yong-jie, HU Yang, LI Ming-rui, et al. Fabrication of Sr-Functionalized Micro/Nano-Hierarchical Structure Ceramic Coatings on 3D Printing Titanium[J]. Surface Engineering, 2021, 37(3): 373-380.
[9] 孙玉洁, 章露娇, 赵玉清, 等. 基于表面引发聚合构建表面抗菌功能化牙科植入体[J]. 表面技术, 2019, 48(7): 237-246.
SUN Yu-jie, ZHANG Lu-jiao, ZHAO Yu-qing, et al. Functionalized Dental Implants with Antibacterial Surface Based on Surface Initiated Polymerization[J]. Surface Technology, 2019, 48(7): 237-246.
[10] MENDONÇA G, MENDONÇA D B S, ARAGÃO F J L, et al. Advancing Dental Implant Surface Technology from Micron- to Nanotopography[J]. Biomaterials, 2008, 29(28): 3822-3835.
[11] TENG Fu-yuan, TAI I C, HO M L, et al. Controlled Release of BMP-2 from Titanium with Electrodeposition Modification Enhancing Critical Size Bone Formation[J]. Materials Science and Engineering: C, 2019, 105: 109879.
[12] ZHU Yi, LIU Dan-dan, WANG Xiu-li, et al. Polydopamine- Mediated Covalent Functionalization of Collagen on a Titanium Alloy to Promote Biocompatibility with Soft Tissues[J]. Journal of Materials Chemistry B, 2019, 7(12): 2019-2031.
[13] DIAS-NETIPANYJ M F, SOPCHENSKI L, GRADOWSKI T, et al. Crystallinity of TiO2Nanotubes and Its Effects on Fibroblast Viability, Adhesion, and Proliferation[J]. Journal of Materials Science Materials in Medicine, 2020, 31(11): 94.
[14] ALBU A M, DRAGHICESCU W, MUNTEANU T, et al. Nitrodopamine Vs Dopamine as an Intermediate Layer for Bone Regeneration Applications[J]. Materials Science and Engineering: C, 2019, 98: 461-471.
[15] GULATI K, PRIDEAUX M, KOGAWA M, et al. Anodized 3D-Printed Titanium Implants with Dual Micro- and Nano-Scale Topography Promote Interaction with Human Osteoblasts and Osteocyte-Like Cells[J]. Journal of Tissue Engineering and Regenerative Medicine, 2017, 11(12): 3313-3325.
[16] KHUDHAIR D, BHATTI A, LI Y, et al. Anodization Parameters Influencing the Morphology and Electrical Properties of TiO2Nanotubes for Living Cell Interfacing and Investigations[J]. Materials Science and Engineering: C, 2016, 59: 1125-1142.
[17] QIN Jie, YANG Dong-qing, MAHER S, et al. Micro- and Nano-Structured 3D Printed Titanium Implants with a Hydroxyapatite Coating for Improved Osseointegration [J]. Journal of Materials Chemistry B, 2018, 6(19): 3136-3144.
[18] 王桂森, 万熠, 王滕, 等. 植入体微纳结构表面制备及生物相容性研究综述[J]. 表面技术, 2016, 45(5): 8-18.
WANG Gui-sen, WAN Yi, WANG Teng, et al. Review on Preparation of Micro-Nano Structure on Implant Surface and Its Biocompatibility[J]. Surface Technology, 2016, 45(5): 8-18.
[19] DING Xiang-long, ZHOU Lei, WANG Jing-xu, et al. The Effects of Hierarchical Micro/Nanosurfaces Decorated with TiO2Nanotubes on the Bioactivity of Titanium Implants in Vitro and in Vivo[J]. International Journal of Nanomedicine, 2015, 10: 6955-6973.
[20] STEEVES A J, ATWAL A, SCHOCK S C, et al. Evaluation of the Direct Effects of Poly(Dopamine) on the in Vitro Response of Human Osteoblastic Cells[J]. Journal of Materials Chemistry B, 2016, 4(18): 3145-3156.
[21] JIA Luan-luan, HAN Feng-xuan, WANG Huan, et al. Polydopamine-Assisted Surface Modification for Orthopaedic Implants[J]. Journal of Orthopaedic Translation, 2019, 17: 82-95.
[22] WANG Yu-lan, QI Hao-ning, MIRON R J, et al. Modulating Macrophage Polarization on Titanium Implant Surface by Poly(Dopamine)-Assisted Immobilization of IL4[J]. Clinical Implant Dentistry and Related Research, 2019, 21(5): 977-986.
[23] [23] LEE J J, PARK I S, SHIN G S, et al. Effects of Polydopamine Coating on the Bioactivity of Titanium for Dental Implants[J]. International Journal of Precision Engineering and Manufacturing, 2014, 15(8): 1647-1655.
[24] HU Yong, LI Shen-shen, KANG Wen-jiang, et al. Surface Modification of Ti6Al4V Alloy by Polydopamine Grafted GO/ZnO Nanocomposite Coating[J]. Surface and Coatings Technology, 2021, 422: 127534.
[25] HE T, ZHU W, WANG X, et al. Polydopamine Assisted Immobilisation of Copper(II) on Titanium for Antibacterial Applications[J]. Materials Technology, 2015, 30(sup6): B68-B72.
[26] YAN Xu-feng, SHEN Ke, TANG Qiang, et al. IL-4 Functionalized Titanium Dioxide Nanotubes Modulate the Inflammatory Response of Macrophages[J]. Journal of Biomaterials Science, Polymer Edition, 2020, 31(17): 2238-2251.
[27] ZHENG Long-zhen, LIU Qiang, XIONG Le-yan, et al. Controlled Preparation of Titania Nanofilm by a Template of Polydopamine Film and Its Reversible Wettability[J]. Thin Solid Films, 2012, 520(7): 2776-2780.
[28] BALL V, FRARI D D, TONIAZZO V, et al. Kinetics of Polydopamine Film Deposition as a Function of pH and Dopamine Concentration: Insights in the Polydopamine Deposition Mechanism[J]. Journal of Colloid and Interface Science, 2012, 386(1): 366-372.
[29] ZHOU Ping, DENG Yi, LYU Bei-er, et al. Rapidly- Deposited Polydopamine Coating via High Temperature and Vigorous Stirring: Formation, Characterization and Biofunctional Evaluation[J]. PLoS One, 2014, 9(11): e113087.
[30] MELO-FONSECA F, GASIK M, MADEIRA S, et al. Surface Characterization of Titanium-Based Substrates for Orthopaedic Applications[J]. Materials Characterization, 2021, 177: 111161.
[31] ELLIOTT D T, WIGGINS R J, DUA R. Bioinspired Antibacterial Surface for Orthopedic and Dental Implants [J]. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2021, 109(7): 973-981.
[32] NAKAGAWA M, ZHANG L, UDOH K, et al. Effects of Hydrothermal Treatment with CaCl2Solution on Surface Property and Cell Response of Titanium Implants[J]. Journal of Materials Science Materials in Medicine, 2005, 16(11): 985-991.
[33] HANSSON S, NORTON M. The Relation between Surface Roughness and Interfacial Shear Strength for Bone- Anchored Implants. a Mathematical Model[J]. Journal of Biomechanics, 1999, 32(8): 829-836.
[34] WANG Lu-ning, JIN Ming, ZHENG Yu-dong, et al. Nanotubular Surface Modification of Metallic Implants via Electrochemical Anodization Technique[J]. International Journal of Nanomedicine, 2014, 9: 4421-4435.
[35] HAN Ying, LIU Si-zhe, SUN Yu-long, et al. Bioinspired Surface Functionalization of Titanium for Enhanced Lubrication and Sustained Drug Release[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2019, 35(20): 6735-6741.
[36] MINAGAR S, BERNDT C C, WANG J, et al. A Review of the Application of Anodization for the Fabrication of Nanotubes on Metal Implant Surfaces[J]. Acta Biomaterialia, 2012, 8(8): 2875-2888.
[37] XU Hao, ZHANG Qian, ZHENG Chun-li, et al. Application of Ultrasonic Wave to Clean the Surface of the TiO2Nanotubes Prepared by the Electrochemical Anodization[J]. Applied Surface Science, 2011, 257(20): 8478-8480.
[38] CHEN Li, LI Jin-gan, CHANG Jia-wei, et al. Mg-Zn-Y- Nd Coated with Citric Acid and Dopamine by Layer- by-Layer Self-Assembly to Improve Surface Biocompatibility[J]. Science China Technological Sciences, 2018, 61(8): 1228-1237.
[39] MATOS G R M. Surface Roughness of Dental Implant and Osseointegration[J]. Journal of Maxillofacial and Oral Surgery, 2021, 20(1): 1-4.
[40] SHAOKI A, XU Jia-yun, SUN Hai-peng, et al. Osseointegration of Three-Dimensional Designed Titanium ImplantsManufactured by Selective Laser Melting[J]. Biofabrication, 2016, 8(4): 045014.
[41] SCHNEIDER G B, PERINPANAYAGAM H, CLEGG M, et al. Implant Surface Roughness Affects Osteoblast Gene Expression[J]. Journal of Dental Research, 2003, 82(5): 372-376.
[42] GITTENS R A, OLIVARES-NAVARRETE R, SCHWARTZ Z, et al. Implant Osseointegration and the Role of Microroughness and Nanostructures: Lessons for Spine Implants[J]. Acta Biomaterialia, 2014, 10(8): 3363-3371.
[43] GITTENS R A, MCLACHLAN T, OLIVARES-NAVARRETE R, et al. The Effects of Combined Micron-/ Submicron-Scale Surface Roughness and Nanoscale Features on Cell Proliferation and Differentiation[J]. Biomaterials, 2011, 32(13): 3395-3403.
[44] WU Yun-tao, LIU Xiao-qiu, LI Yi, et al. Surface-AdhesiveLayer-by-Layer Assembled Hydroxyapatite for BioinspiredFunctionalization of Titanium Surfaces[J]. RSC Adv, 2014, 4(84): 44427-44433.
[45] 谭帼馨, 欧阳孔友, 周蕾, 等. 聚多巴胺螯合钙离子对钛表面的修饰及修饰后的细胞相容性[J]. 无机材料学报, 2015, 30(10): 1075-1080.
TAN Guo-xin, OUYANG Kong-you, ZHOU Lei, et al. Titanium Modification by Calcium Ion Chelated Polydopamine and Its Cytocompatibility[J]. Journal of Inorganic Materials, 2015, 30(10): 1075-1080.
[46] FAURE E, FALENTIN-DAUDRÉ C, JÉRÔME C, et al. Catechols as Versatile Platforms in Polymer Chemistry [J]. Progress in Polymer Science, 2013, 38(1): 236-270.
[47] HERLINGER E, JAMESON R F, LINERT W. Spontaneous Autoxidation of Dopamine[J]. Journal of the Chemical Society, Perkin Transactions, 1995, 2: 259-63.
[48] BERNSMANN F, BALL V, ADDIEGO F, et al. Dopamine-Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2011, 27(6): 2819-2825.
[49] HONG S, NA Y S, CHOI S, et al. Non-Covalent Self- Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation[J]. Advanced Functional Materials, 2012, 22(22): 4711-4717.
[50] LEE H, DELLATORE S M, MILLER W M, et al. Mussel-Inspired Surface Chemistry for Multifunctional Coatings[J]. Science, 2007, 318(5849): 426-430.
[51] WAITE J. Mussel Power[J]. Nature Materials, 2008, 7(1): 8-9.
[52] 刘宗光, 屈树新, 翁杰. 聚多巴胺在生物材料表面改性中的应用[J]. 化学进展, 2015, 27(S1): 212-219.
LIU Zong-guang, QU Shu-xin, WENG Jie. Application of Polydopamine in Surface Modification of Biomaterials[J]. Progress in Chemistry, 2015, 27(S1): 212-219.
[53] SU Jie-hua, DU Zhi-bin, XIAO Lan, et al. Graphene Oxide Coated Titanium Surfaces with Osteoimmunomodulatory Role to Enhance Osteogenesis[J]. Materials Science and Engineering: C, 2020, 113: 110983.
[54] HE Ye, MU Cai-yun, SHEN Xin-kun, et al. Peptide LL-37 Coating on Micro-Structured Titanium Implants to Facilitate Bone Formation in Vivo via Mesenchymal Stem Cell Recruitment[J]. Acta Biomaterialia, 2018, 80: 412- 424.
[55] WENZEL R N. Resistance of Solid Surfaces to Wetting by Water[J]. Industrial & Engineering Chemistry, 1936, 28(8): 988-994.
[56] CHENG Shi, WANG Dong-hui, KE Jin, et al. Improved in Vitro Angiogenic Behavior of Human Umbilical Vein Endothelial Cells with Oxidized Polydopamine Coating[J].Colloids and Surfaces B: Biointerfaces, 2020, 194: 111176.
[57] YANG Xiao-ling, LIU Xue, WU Qian-qian, et al. Dopamine-Assisted Immobilization of Peptide Arginine-Glycine- Aspartic Acid to Enhance the Cellular Performances of MC3T3-E1 Cells of Carbon-Carbon Composites[J]. Journal of Biomaterials Applications, 2019, 34(2): 284-296.
[58] GITTENS R A, SCHEIDELER L, RUPP F, et al. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects[J]. Acta Biomaterialia, 2014, 10(7): 2907-2918.
[59] ERIKSSON C, NYGREN H, OHLSON K. Implantation of Hydrophilic and Hydrophobic Titanium Discs in Rat Tibia: Cellular Reactions on the Surfaces during the First 3Weeks in Bone[J]. Biomaterials, 2004, 25(19): 4759-4766.
[60] BORNSTEIN M M, VALDERRAMA P, JONES A A, et al. Bone Apposition around Two Different Sandblasted and Acid-Etched Titanium Implant Surfaces: A Histomorphometric Study in Canine Mandibles[J]. Clinical Oral Implants Research, 2008, 19(3): 233-241.
[61] BULY R L, HUO M H, SALVATI E, et al. Titanium Wear Debris in Failed Cemented Total Hip Arthroplasty: An Analysis of 71 Cases[J]. The Journal of Arthroplasty, 1992, 7(3): 315-323.
[62] WANG Gui-sen, WAN Yi, REN Bing, et al. Bioactivity of Micropatterned TiO2Nanotubes Fabricated by Micro- Milling and Anodic Oxidation[J]. Materials Science and Engineering: C, 2019, 95: 114-121.
JI Zhen-bing (1998-), Male, Postgraduate, Research focus: surface engineering of biomaterials.
Effects of Hydrothermal Temperature and Time on Surface Physical and Chemical Properties of 3D Printed Ti-6Al-4V Implants
1,1,1,1,2,1
(1. a. Key Laboratory of Ministry of Education for High-efficiency and Clean Mechanical Manufacture, b. School of Mechanical Engineering, Shandong University, Jinan 250061, China; 2. a. Department of Emergency Medicine, b. Shandong University Emergency and Critical Care Clinical Medicine Research Center, Qilu Hospital of Shandong University, Jinan 250012, China)
This study aims to explore the optimal process and parameters for preparing polydopamine coating on the surface of 3D printed Ti-6Al-4V implants with micro-nano structure. 3D printing is a promising method for preparing Ti-6Al-4V implants. However, the biological inertness of 3D-printed titanium alloys limits their ability to bind to bone tissue. The micro-nano structure on the surface of titanium alloy implants can promote cell adhesion, proliferation and bone integration. In addition, polydopamine has been shown to promote cell proliferation and reduce cytotoxicity. Therefore, hydrothermal treatment was performed on the 3D printed Ti-6Al-4V implants treated by acid etching and anodic oxidation, and polydopamine was coated on the surface of samples by hydrothermal treatment. The effects of different hydrothermal treatment temperatures and time were analyzed. Moreover, the surface morphology, roughness, elemental composition, surface wettability and corrosion resistance of each sample were characterized by scanning electron microscope, three-dimensional confocal laser microscope, X-ray photoelectron spectroscopy, contact angle measurement instrument and electrochemical workstation. The results showed that a micron-scale pit structure was constructed on the surface by acid etching. Through anodic oxidation, an ordered array of TiO2nanotubes with a diameter of about 80 nm was constructed on the basis of the original micron-scale structure. Micro-nano structures were successfully prepared on the surface of the implant. With the increase of hydrothermal treatment temperature and time, the diameter of nanotubes gradually decreased from 80 nm to about 40 nm, and even blocked. The three-dimensional topography indicated that the traces of laser scanning during the printing process could be observed on the surface of samples. Through anodic oxidation, the edges and corners became smoother than the surface after acid etching. Going forward, the results of surface element analysis suggested that the N/C value of each sample was close to the theoretical value of 0.125, indicating that the hydrothermal treatment successfully coated polydopamine on the surface based on remaining the micro-nano structure. The carbon and nitrogen elements on the surface of samples after hydrothermal treatment were subjected to peak fitting processing. In addition, the carbon elements were composed of C==O/O—C==O and C—C/C==C, and the nitrogen elements were composed of pyrrolic N, further demonstrating that the hydrothermal treatment successfully added polydopamine to the sample surface. With the increase of reaction time, the surface roughness and contact angle gradually decreased, and the roughness of each group remained between 4-5 μm. The surface contact angle after acid etching was 52.1°. After anodic oxidation, the contact angle decreased to 42.9°. Through hydrothermal treatment, the contact angle was less than 35°, showing excellent hydrophilicity. Compared with the samples after acid etching and anodic oxidation, the corrosion resistance was enhanced through hydrothermal treatment. In addition, the principles of anodic oxidation and dopamine polymerization were discussed in depth. In conclusion, the reaction temperature of 37 ℃ and the reaction time of 24 hours were suitable for the deposition of polydopamine on the surface of titanium alloy implants under the premise that the original micro-nano structure was basically retained. Furthermore, the results of this study provide a reference for the optimization of process parameters of polydopamine self-polymerization on the surface of titanium alloy implants.
3D printing; titanium alloy; implant; hydrothermal treatment; micro-nano structure; polydopamine coating
TG146;R318
A
1001-3660(2022)09-0288-12
10.16490/j.cnki.issn.1001-3660.2022.09.000
2021–09–01;
2022–06–10
2021-09-01;
2022-06-10
国家自然科学基金(51975336);山东省重点研发计划(2020JMRH0202);山东省新旧动能转换重大产业攻关项目(2021-13);济宁市重点研发计划项目(2021DZP005);山东大学教育教学改革研究项目(2022Y133,2022Y124,2022Y312)
Fund:The National Nature Science Foundation of China (51975336); Key Research and Development Program of Shandong Province (2020JMRH0202); Major Industrial Research Projects in Shandong Province for the Conversion of Old and New Kinetic Energy (2021-13); Key Research and Development Project of Jining City (2021DZP005) and Education and Teaching Reform Research Project of Shandong University (2022Y133, 2022Y124, 2022Y312).
纪振冰(1998—),男,硕士研究生,主要研究方向为生物医学材料表面工程。
万熠(1977—),男,博士,教授,主要研究方向为生物材料加工制造理论与技术。
WAN Yi (1977-), Male, Doctor, Professor, Research focus: theory and technology of biomaterial processing and manufacturing.
纪振冰, 万熠, 赵梓贺, 等.水热温度和时间对3D打印Ti-6Al-4V植入体表面理化性能的影响[J]. 表面技术, 2022, 51(9): 288-299.
JI Zhen-bing, WAN Yi, ZHAO Zi-he, et al. Effects of Hydrothermal Temperature and Time on Surface Physical and Chemical Properties of 3D Printed Ti-6Al-4V Implants[J]. Surface Technology, 2022, 51(9): 288-299.
责任编辑:刘世忠

