Arteriovenous thrombotic events in a patient with advanced lung cancer following bevacizumab plus chemotherapy: A case report
Ying Kong,Xiao-Cheng Xu, Liang Hong
Abstract
Key Words: Cerebral infarction; Pulmonary embolism; Bevacizumab; Chemotherapy; Non-small cell lung cancer; Case report
lNTRODUCTlON
Bevacizumab is a monoclonal antibody that acts against vascular endothelial growth factor (VEGF). A wide range of drugs targeting the VEGF signaling pathway may be used in the treatment of various solid tumors; however, a plethora of toxic effects have increasingly been discovered, which have been shown to be fatal in a small number of cases[1,2]. The increased incidence of potentially fatal arterial thromboembolism (ATE), including transient ischemic attack, stroke, angina pectoris, and myocardial infarction, has been initially reported in advanced colorectal cancer treated with a chemotherapy regimen containing bevacizumab, with the incidence of severe ATE being 4%-5%[3]. Some studies have revealed that bevacizumab can increase the risk of venous thromboembolism (VTE) in patients with cancer; however, inconsistent conclusions have been reported. Two meta-analyses conducted at the patient level have shown that, compared to patients solely receiving chemotherapy, the risk of VTE did not increase in patients who received bevacizumab plus chemotherapy[4]. Here, we describe a 55-yearold man who suffered from arteriovenous thrombosis after receiving bevacizumab in conjunction with intravenous chemotherapy.
CASE PRESENTATlON
Chief complaints
A 55-year-old man presented to our department with hemoptysis.
History of present illness
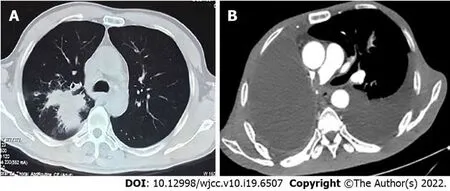
Figure 1 Chest computed tomography and enhanced computed tomography. A: Computed tomography (CT) revealed a mass at the right hilum prior to performing radical resection of right lung cancer; B: Enhanced CT revealed pulmonary embolism after bevacizumab combined with chemotherapy therapy.

Figure 2 Positron emission tomography-computed tomography before radical resection of right lung cancer. A: Irregular soft tissue mass in the right lung hilum; B: Posterior wall cavity with abnormal concentration of radioactivity in the mass; C: Consolidation and thick-walled voids in the posterior and dorsal lobes of the right lung; D: Mediastinal lymph node metastasis with no distant metastases.
The patient’s chest computed tomography (CT) and positron emission tomography-CT demonstrated consolidation and thick-walled voids in the posterior and dorsal lobes of the right lung (Figures 1 and 2). Ultrasound bronchoscopy revealed the presence of adenocarcinoma. The patient then underwent radical resection for right lung cancer, after which postoperative pathology revealed central invasive adenocarcinoma (pT4N1M0, IIIA). Moreover, genetic testing revealed the following: Epidermal growth factor receptor (-), anaplastic lymphoma kinase (-), ROS1 (-), Kirsten rat sarcoma/murine sarcoma viral oncogene homolog B1 (-), and programmed death 1 (-). Adjuvant chemotherapy was not provided following surgery in light of the patient’s poor physical condition. Six months postoperatively, the patient’s condition worsened, and chest enhanced CT demonstrated enlarged mediastinal lymph nodes, diffuse nodules in the left lung, and left pleural effusion. Endobronchial ultrasound-guided transbronchial lung biopsy was difficult to obtain and was not performed due to the presence of diffuse small lesions within the left lung. The patient subsequently received regimen chemotherapy with bevacizumab 400 mg d1 + pemetrexed 750 mg d1 + carboplatin 500 mg d1, q3w for six cycles. The curative effect was evaluated as partial relief during chemotherapy according to RECIST 1.1 standards. The patient had no chemotherapy-related adverse reactions, including nausea and vomiting, and no bone marrow suppression, increased blood pressure, proteinuria, or bleeding. Bevacizumab 400 mg d1 + pemetrexed 750 mg d1, q3w was then maintained for three cycles. Afterward, the patient developed dizziness with nausea and vomiting, for which magnetic resonance imaging (MRI) of the head displayed acute cerebral infarction in the right lateral ventricle (Figure 3A), and CT angiography (CTA) of the head demonstrated local occlusion of the right superior cerebellar artery following bevacizumab combined with chemotherapy (Figure 3B). In addition, the patient experienced symptoms, such as progressive dyspnea and decreased blood oxygen saturation. D-dimer increased 10-fold, and other blood coagulation function indexes were within normal range. Routine blood tests and C-reactive protein and procalcitonin levels were also within normal range. Chest enhanced CT revealed a filling defect of the right pulmonary artery (Figure 1), after which pulmonary embolism was diagnosed.
History of past illness
The patient had no history of hypertension, cardiovascular or cerebrovascular disease, and chronic kidney disease. He was a smoker for 30 years.
Personal and family history
There was no family history of lung cancer.
Physical examination
There were no breath sounds in his right lung and low breath sounds in his left lung. His heart examination showed no significant abnormality.
Laboratory examinations
Routine blood tests and C-reactive protein and procalcitonin levels were within normal range. D-dimer increased 10-fold, and other blood coagulation function indexes were within normal range.
Imaging examinations
MRI of the head displayed acute cerebral infarction in the right lateral ventricle (Figure 3A). CTA of the head demonstrated local occlusion of the right superior cerebellar artery following bevacizumab combined with chemotherapy (Figure 3B). Chest enhanced CT revealed a filling defect of the right pulmonary artery (Figure 1). Electrocardiogram (ECG) and color Doppler echocardiography showed no obvious abnormalities.
FlNAL DlAGNOSlS
Cerebral infarction and pulmonary embolism.
TREATMENT
The patient developed distant metastatic disease and was unable to be cured; therefore, systemic treatment was the goal in his palliative care plan. Accordingly, treatment with bevacizumab was terminated, and aspirin (100 mg/d) + clopidogrel bisulfate tablets (75 mg/d) + atorvastatin (20 mg/d) + low-molecular-weight heparin (LMWH) (4100 IU/12 h) anticoagulation were provided. In addition, diuresis, drainage of pleural fluid, and non-invasive ventilator therapy were performed.
OUTCOME AND FOLLOW-UP
The patient died of respiratory failure 1 mo later.
DlSCUSSlON
Patients suffering from lung cancer are known to have the highest incidence of cerebral infarction among all existing cancer types. Previous studies have shown that cancer-related cerebral infarction may give rise to unique clinical manifestations, including increased D-dimer levels and multiple cerebral infarctions distributed in the blood supply area of various arteries in the brain[5]. Its pathophysiological mechanism may be related to cancer cells directly invading the left atrium, leading to infiltrating endocarditis and hypercoagulable status[6]. ECG and color Doppler echocardiography of the discussed patient showed no obvious abnormalities. The pathogenesis of more cancer-related cerebral infarctions remains unknown. MRI of the head is advantageous in detecting early cerebral infarction as it can accurately assess brain tissues at risk of infarction and exclude certain diseases that exhibit strokelike characteristics, such as tumors. MRI of the head using diffusion imaging is better than CT, and neurovascular imaging is very important for acute ischemic stroke. Magnetic resonance angiography and CTA can be used to assess extracranial and intracranial large vessels in order to determine the presence of embolism or hypoemia in ischemic stroke as well as the potential source of the flow.

Figure 3 Magnetic resonance imaging and computed tomography angiography of the head. A: Magnetic resonance imaging revealed cerebral infarction following treatment using bevacizumab combined with chemotherapy; B: Computed tomography angiography revealed local occlusion of the right superior cerebellar artery.
A variety of anti-angiogenic drugs, such as bevacizumab, are known to be associated with an increased risk of ATE[7]. However, it remains unclear whether these patients have an increased risk of VTE due to inconsistent research data. Patients receiving angiogenesis inhibitors have an increased risk of thromboembolism, but its pathophysiology remains unclear[8]. The common hypothesis posits that tumor-associated endothelial cells are disturbed, which then transforms the endothelium from a natural anticoagulant state to a pro-thrombotic state. Afterward, systemic coagulation is activated, where such cancer patients are more prone to thromboembolism for underlying diseases. In addition, the VEGF pathway can regulate and protect endothelial cell function by inhibiting apoptosis and inflammation pathways. Endothelial dysfunction may expose phospholipids that can promote thrombosis and underlying stroma[9]. Recent studies have shown that the preventive application of antiplatelet and anticoagulant drugs can effectively reduce the incidence of thrombotic events in cancer patients[10]. Lung cancer has a higher risk of VTE compared to other malignancies[11,12]. The mechanism of lung cancer with pulmonary embolism is complicated. Adenocarcinoma, advanced lung cancer, surgery, chemotherapy, and underlying co-morbidities serve as important high-risk factors. International guidelines recommend LMWH as the first-line treatment in the management of tumor-related pulmonary embolism[13]. If patients with lung cancer and pulmonary embolism have no bleeding manifestations and are at a low risk of bleeding, novel oral anticoagulants can be considered instead of LMWH[14]. The anticoagulation time should be at least 6 mo, while anticoagulation time should be prolonged or even given long-term depending on the situation[15].
Certain lung cancer patients that are at a high risk of pulmonary embolism can benefit from preventive anticoagulation, which should be carefully evaluated in combination with the thrombosis risk assessment model and D-dimer levels. The prediction models used for VTE risk assessment of cancer patients include the Khorana score, PROTECHT score, COMPASS-CAT score, and CONKO score. These models have been developed and validated in patients with ambulatory lung cancer[16]. Khorana score is currently the most validated chemotherapy-related VTE risk assessment model, which is a reliable model based on five clinical and laboratory parameters. It is suitable for the VTE risk assessment of patients undergoing lung cancer chemotherapy and can be used to customize anticoagulant thrombosis prevention in such patients[17]. However, Khorana score may not be sensitive enough to identify high-risk lung cancer patients. Versoet al[18] have shown that PROTECHT score is more advantageous than Khorana score in distinguishing patients at high risk of VTE. Meanwhile, COMPASS-CAT score is able to accurately distinguish high-risk and low-risk VTE patients[19]. It is more sensitive in predicting the VTE risk of lung cancer patients compared to Khorana, PROTECHT, and CONKO models and may be more suitable for thrombosis prevention in lung cancer patients receiving anti-tumor therapy; however, further verification is needed.
The patient reported in this study was suffering from advanced lung cancer and benefited from treatment with bevacizumab in conjunction with chemotherapy. The patient was in good physical condition and did not have hypertension or a history of cardiovascular or cerebrovascular diseases. Risk factors for deep vein thrombosis include hospitalization, indwelling deep vein catheters, and aggressive chemotherapy. Unfortunately, fatal arteriovenous thrombosis occurred during maintenance treatment with bevacizumab. Moreover, pleural effusion of the left lung increased during maintenance therapy, indicating that the patient had disease progression at the time of these thromboembolic events. Therefore, LMWH anticoagulation, diuresis, drainage of pleural fluid, and non-invasive ventilator therapy were administered for the patient; however, the patient still developed shock and experienced a progressive decrease in blood oxygen saturation. The patient continued to suffer from uncontrollable respiratory failure and died after 1 mo.
CONCLUSlON
This report is the first to discuss cerebral infarction and pulmonary embolism occurring in a patient with non-small cell lung cancer following treatment using bevacizumab combined with chemotherapy. Accordingly, the above drug combination may increase the risk of arteriovenous thromboembolism. However, its mechanism of occurrence remains unclear, and future studies are required to investigate the prevention and management of ATE and VTE associated with bevacizumab. In light of these findings, patients who receive angiogenesis inhibitor therapy should be carefully selected according to certain parameters, such as reasonable daily physical status, controlled blood pressure, and no severe cardiovascular events within 6-12 mo. Furthermore, close monitoring and timely intervention are necessary to reduce risk of toxicity.
FOOTNOTES
Author contributions:Kong Y and Hong L were the patient’s oncologists, reviewed the literature, and contributed to manuscript drafting; Hong L and Xu XC analyzed and interpreted the imaging findings; Kong Y, Xu XC, and Hong L were responsible for the revision of the manuscript for important intellectual content; and all authors issued final approval for the version to be submitted.
lnformed consent statement:Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement:The authors declare that they have no conflict of interest to report.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Ying Kong 0000-0001-5153-3235; Xiao-Cheng Xu 0000-0001-6268-1225; Liang Hong 0000-0002-7327-0322.
S-Editor:Wang JJ
L-Editor:Wang TQ
P-Editor:Wang JJ
 World Journal of Clinical Cases2022年19期
World Journal of Clinical Cases2022年19期
- World Journal of Clinical Cases的其它文章
- Current guidelines for Helicobacter pylori treatment in East Asia 2022: Differences among China, Japan, and South Korea
- Review of epidermal growth factor receptor-tyrosine kinase inhibitors administration to non-small-cell lung cancer patients undergoing hemodialysis
- Endoscopic ultrasound radiofrequency ablation of pancreatic insulinoma in elderly patients: Three case reports
- Acute choroidal involvement in lupus nephritis: A case report and review of literature
- Choroidal thickening with serous retinal detachment in BRAF/MEK inhibitor-induced uveitis: A case report
- Esophageal granular cell tumor: A case report
