Successful treatment of stage lllB intrahepatic cholangiocarcinoma using neoadjuvant therapy with the PD-1 inhibitor camrelizumab: A case report
Shu-Guang Zhu, Hai-Bo Li, Tian-Xing Dai, Hua Li, Guo-Ying Wang
Shu-Guang Zhu, Hai-Bo Li, Tian-Xing Dai, Hua Li, Guo-Ying Wang, Department of Hepatic Surgery and Liver Transplantation, The Third Affiliated Hospital of Sun Yat-Sen University,Guangzhou 510630, Guangdong Province, China
Guo-Ying Wang, Department of Hepatobiliary Surgery, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou 510220, Guangdong Province, China
Abstract BACKGROUND The prognosis of intrahepatic cholangiocarcinoma (ICC) with lymph node metastasis is poor. The feasibility of surgery is not certain, which is a contraindication according to the National Comprehensive Cancer Network guidelines.The role of immunotherapy as a neoadjuvant therapy for ICC is not clear. We herein describe a case of ICC with lymph node metastasis that was successfully treated with neoadjuvant therapy.CASE SUMMARY A 60-year-old man with a liver tumor was admitted to our hospital. Enhanced computed tomography and magnetic resonance imaging revealed a spaceoccupying lesion in the right lobe of the liver. Multiple subfoci were found around the tumor, and the right posterior branch of the portal vein was invaded. Liver biopsy indicated poorly differentiated cholangiocytes. According to the American Joint Committee on Cancer disease stage classification, ICC with hilar lymph node metastasis (stage IIIB) and para-aortic lymph node metastasis was suspected. A report showed that two patients with stage IIIB ICC achieved a complete response(CR) 13 mo and 16 mo after chemotherapy with a PD-1 monoclonal antibody.After multidisciplinary consultation, the patient was given neoadjuvant therapy,surgical resection and lymph node dissection, and postoperative adjuvant therapy. After three rounds of PD-1 immunotherapy (camrelizumab) and two rounds of gemcitabine combined with cisplatin regimen chemotherapy, the tumor size was reduced. Therefore, a partial response was achieved. Exploratory laparotomy found that the lymph nodes of Group 16 were negative, and the tumor could be surgically removed. Therefore, the patient underwent right hemihepatectomy plus lymph node dissection. The patient received six rounds of chemotherapy and five rounds of PD-1 treatment postoperatively. After 8 mo of follow-up, no recurrence was found, and a CR was achieved.CONCLUSION Neoadjuvant therapy combined with surgical resection is useful for advanced-stage ICC. This is the first report of successful treatment of stage IIIB ICC using neoadjuvant therapy with a PD-1 inhibitor.
Key Words: Intrahepatic cholangiocarcinoma; Lymph node metastasis; Neoadjuvant therapy; Immunotherapy; Chemotherapy; Surgical resection; Case report
lNTRODUCTlON
Intrahepatic cholangiocarcinoma (ICC) is an adenocarcinoma originating from the secondary bile duct and its branch epithelium. ICC accounts for approximately 10%-15% of primary liver cancers[1]. ICC is the primary malignant tumor of the liver after hepatocellular carcinoma (HCC)[2]. The incidence rate has increased in recent years.The exact cause of ICC is not clear. The well-known risk factors for ICC include congenital choledochal cysts, chronic cholangitis, chronic inflammatory bowel disease, primary sclerosing cholangitis, parasitic infection, chemical carcinogens (such as thorium dioxide and nitrosamine), genetic factors, biliary cirrhosis, cholelithiasis, alcoholic liver disease, and nonspecific cirrhosis. Hepatitis viruses are also closely related to ICC[3]. The general morphology of ICC is divided into three types: mass type, peritubular infiltration type, and intratubular growth type. The most common type is the mass type, which accounts for 60% to 80% of ICCs. The periductal infiltration type accounts for 15% to 35%. This type may have diffuse infiltration along the biliary system and portal vein system, which results in bile duct stenosis and peripheral bile duct dilatation. The intraductal growth type accounts for 8% to 29%. This type mostly shows papillary, polypoid or granular growth that spreads along the superficial bile duct[4]. We herein report the case of a patient with advanced ICC who was successfully treated with neoadjuvant therapy combined with surgical resection.
CASE PRESENTATlON
Chief complaints
Right upper abdominal pain for 1 wk.
History of present illness
A 60-year-old male patient developed right upper abdominal pain with no obvious cause 1 wk ago. The pain was dull and persistent, and the patient had no radiating pain, fatigue, poor appetite, cold, or fever. He visited a local hospital, where upper abdominal computed tomography (CT) examination suggested liver space occupying lesions. Since the onset of the disease, the patient’s spirit, sleep, and diet were a little poor, and his urine and feces were normal. He has lost 7.5 kg in weight in the past 2 mo.
History of past illness
The patient had a history of hepatitis B for 30 years and was currently receiving oral entecavir antiviral therapy.
Personal and family history
The patient had no smoking or drinking history, and her family members were healthy.
Physical examination
The abdomen was soft, without tenderness or rebound pain. The liver can be touched 2 cm below the right costal margin, and the spleen can be touched 3 cm below the left costal margin. There was percussion pain in the liver area.
Laboratory examinations
The patient’s CA19-9 level was 1844 U/mL, and his hepatitis B virus deoxyribonucleic acid level was 6.0e2 IU/mL. Carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) and liver function were normal.
Imaging examinations
Computed tomography and magnetic resonance imaging (Figure 1) showed that the tumors were located in the right lobe, and the longest diameter was about 20 cm; hilar lymph node metastasis was found.
FlNAL DlAGNOSlS
ICC (stage IIIB) with hilar and retroperitoneal lymph node metastases was initially diagnosed.
TREATMENT
We performed liver biopsy, and the pathological results (Figure 2) showed poorly differentiated cholangiocarcinoma. After multidisciplinary consultation, we determined that the patient should be given neoadjuvant therapy, surgical resection (Figure 3) and lymph node dissection, and postoperative adjuvant therapy.
OUTCOME AND FOLLOW-UP
CT imaging (Figure 4) revealed no recurrence after 8 mo of follow-up, and CA199 decreased from 1844 U/mL to 4.76 U/mL. An oncology assessment suggested a partial response.

Figure 1 Pre-operative liver computed tomography and magnetic resonance imaging scan. A: Axial portal phase computed tomography (CT)showed the tumors (white arrow) are located in the right lobe, with the longest diameter being about 20 cm; hilar lymph metastasis node (orange arrow) is found; B:Axial arterial phase CT at different levels reveals the tumors (white arrow) and the hilar lymph node metastasis(orange arrow); C and D: Axial diffusion-weighted imaging image at different levels reveals the tumors (white arrow) and the hilar lymph node metastasis (orange arrow).
DlSCUSSlON
ICC has high malignancy and a poor prognosis, and there are few long-term survivors. The prognosis of patients without surgery is very poor, and the 3-year survival rate is only 40%-50% after surgery[5].Compared to other liver malignancies, ICC is associated with a shorter survival time and lower resection and cure rates[6]. The gross classification of ICC is related to tumor prognosis. The peritubular infiltration type has the worst prognosis, followed by the mass type. The intratubular growth type has the best prognosis. Tumor markers lack sensitivity and specificity for the diagnosis of ICC. CA19-9 is valuable in the diagnosis of tumors, evaluation of tumor resection, and prediction of prognosis.However, CEA, AFP, and CA-125 are less valuable in the diagnosis of ICC[7]. The definite diagnosis depends on the combination of imaging and pathological examination[8]. ICC is more likely to recur than HCC[9]. The survival of patients with ICC is affected by the number of tumors, the extent of liver resection, the degree of tumor differentiation, and the type of tumor cells. According to the National Comprehensive Cancer Network (NCCN) guidelines, preoperative biopsy is not necessary, especially in surgically resectable cases. When imaging results indicate a suspicious nodule, it should be treated as malignant, and diagnostic laparoscopic exploration is recommended to exclude unresectable disseminated lesions[10]. Exploration and evaluation should include examinations for multiple intrahepatic cancers, lymph node metastasis, and distant metastasis. Lymph node metastasis and distant metastasis outside the portal hepatis should be considered as surgical contraindications. Hilar lymph node dissection should be performed routinely to provide staging and prognosis information. According to the NCCN guidelines, radical surgery (plus lymph node dissection) is the only curative treatment[11].Surgical indications and neoadjuvant therapy remain the focus of clinical judgments[12]. One study reported that two patients with stage IIIB ICC achieved a CR 13 mo and 16 mo after chemotherapy with PD-1 monoclonal antibody[13]. For the present case of ICC with hilar lymph node metastasis,abdominal aortic lymph node metastasis was suspected and it was classified as American Joint Committee on Cancer stage IIIB. Multidisciplinary discussion determined that the patient should receive neoadjuvant therapy, surgical resection and lymph node dissection, and postoperative adjuvant therapy. Neoadjuvant therapy included chemotherapy and immunotherapy. After immunotherapy with a PD-1 inhibitor and chemotherapy with the gemcitabine combined with cisplatin regimen, a reevaluation using magnetic resonance imaging (Figure 5) showed that the tumor had shrunk and was necrotic. An oncology evaluation determined a partial response. According to the NCCN guidelines, the patient continued to receive immunotherapy and chemotherapy after tumor resection, and no recurrences were found. Lymph node dissection is another focus of clinical debate. The existing research shows that extended dissection does not improve the prognosis. However, the NCCN and European Society of Medical Oncology guidelines recommend routine hilar lymph node dissection. The NCCN guidelines consider positive extrahepatic lymph node metastasis as a surgical contraindication and recommend neoadjuvant therapy followed by surgery at a lower stage. Therefore, the independent risk factors for the prognosis of the patient included a less than 1-cm distance between the cutting edge and the tumor, a tumor diameter greater than 5 cm, multiple tumors, microvascular invasion, and positive pathological lymph node metastasis[12,14]. A previous study confirmed that lymph node dissection did not improve the prognosis of patients with ICC[15]. Postoperative pathology confirmed that positive lymph node metastasis is an independent risk factor affecting the prognosis of patients with ICC. This relationship confirms the clinical value of lymph node dissection and shows that lymph node dissection remains associated with many problems[15]. Lymph node dissection is routine, and the 2019 Chinese Society of Clinical Oncology biliary system tumor diagnosis and treatment expert consensus recommends active surgical resection and lymph node dissection. Therefore, there is no consensus on the scope of regional lymph node dissection[14]. We should carefully consider extended hepatectomy, the improvement of chemotherapy, and the emergence of immunotherapy and radiotherapy. With the development of surgery, adjuvant therapy has become an important means to improve the prognosis of ICC[16,17]. Another point of contention about ICC is the absence of randomised controlled trial studies on preoperative neoadjuvant therapy for ICC. A multicenter study of 62 cases of ICC with neoadjuvant chemotherapy showed a median OS of 47 mo after resection, and 74 cases of unresectable ICC had a median OS of 24 mo after six cycles of chemotherapy[18]. Preoperative neoadjuvant therapy is a “TEST”because there are no reports of immunotherapy as neoadjuvant or transformation therapy.

Figure 3 Postoperative pathological pictures. A: The gross specimen after operation; B: The specimen under high power microscope (200 ×) after operation is consistent with the puncture specimens.

Figure 4 The liver computed tomography scan after eight months of the operation. A and B: Axial enhanced computed tomography scan at different levels shows there is no recurrence.
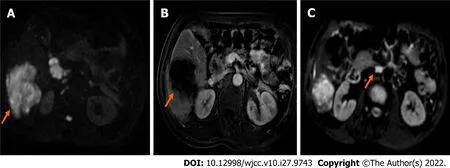
Figure 5 Magnetic resonance imaging scan after the neoadjuvant therapy. A: Axial diffusion-weighted imaging (DWI) image shows the tumor (orange arrow) is reduced obviously; B: Axial enhanced magnetic resonance imaging reveals the tumor (orange arrow) is reduced obviously; C: DWI image shows the hilar lymph metastasis node (orange arrow) disappeared.
CONCLUSlON
Immunotherapy is a current research hotspot. PD-1 inhibitors are effective for solid tumors, but only melanoma and lung cancer are clinical indications for immunotherapy. The objective remission rate in advanced ICC patients is approximately 20%, but more phase III clinical trials are needed for verification. The development of more sensitive and efficient predictive methods will improve the benefits of ICC therapy and benefit patients who are suitable for such therapy. These improvements will hopefully open a new prospect of immunotherapy for liver tumors[19]. Immunotherapy may be a treatment option for ICC, but it must be confirmed by a study including a larger sample of cases[20]. Its role in preoperative neoadjuvant therapy for ICC is a hot topic in clinical trials[21]. Overall, the value of neoadjuvant therapy, the time of surgery after neoadjuvant therapy, the necessity of lymph node dissection, the means of adjuvant therapy, and the treatment plan after recurrence remain hot topics of current research.
FOOTNOTES
Author contributions:Zhu SG and Li HB contributed equally to this work; Wang GY and Li HB designed the research study; Zhu SG, Li HB, and Dai TX performed the research and wrote the manuscript; all authors have read and approved the final manuscript.
Supported byThe National 13th Five-Year Science and Technology Plan Major Projects of China, No.2017ZX10203205-006-001 and No. 2017ZX10203205-001-003.
lnformed consent statement:The patient provided informed written consent prior to study enrollment.
Conflict-of-interest statement:All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement:The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORClD number:Shu-Guang Zhu 0000-0002-6939-9326; Hai-Bo Li 0000-0001-9091-0814; Tian-Xing Dai 0000-0001-9574-8393; Hua Li 0000-0003-4559-102X; Guo-Ying Wang 0000-0002-0304-0986.
S-Editor:Xing YX
L-Editor:Wang TQ
P-Editor:Xing YX
 World Journal of Clinical Cases2022年27期
World Journal of Clinical Cases2022年27期
- World Journal of Clinical Cases的其它文章
- Cardiovascular disease and COVlD-19, a deadly combination: A review about direct and indirect impact of a pandemic
- COVlD-19 and the cardiovascular system-current knowledge and future perspectives
- lmpact of COVlD-19 pandemic on the ocular surface
- lntra and extra pelvic multidisciplinary surgical approach of retroperitoneal sarcoma: Case series report
- Clinical efficacy analysis of mesenchymal stem cell therapy in patients with COVlD-19: A systematic review
- lnfant with reverse-transcription polymerase chain reaction confirmed COVlD-19 and normal chest computed tomography: A case report
