Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
Wang Chunfeng (王春峰), Li Jiansheng (李健生), Wang Lianjun (王连军), Sun Xiuyun (孙秀云) and Huang Jiajia (黄佳佳)
Adsorption of Dye from Wastewater by Zeolites Synthesized from Fly Ash: Kinetic and Equilibrium Studies*
Wang Chunfeng (王春峰), Li Jiansheng (李健生), Wang Lianjun (王连军)**, Sun Xiuyun (孙秀云) and Huang Jiajia (黄佳佳)
School of Chemical Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
The removal performance of a basic dye, methylene blue (MB), in aqueous solution was investigated by adsorption process on single-phase and high-crystalline zeolite A (FA-ZA) and X (FA-ZX). Both adsorbents FA-ZA and FA-ZX were synthesized from fly ash prepared aluminosilicate gel followed by the hydrothermal treatment at 100°C with the control of Si/Al molar ratio, respectively. The properties of the synthetic zeolites and commercial grade zeolites, such as thermal stability, elemental composition, and cation exchange capacity, were investigated for comparison. Batch method was used to study the influential parameters, such as initial pH value of the solution, temperatures, and adsorbents dosage, on the adsorption process. The experimental data were well fitted by Ho’ pseudo-second-order model and liquid film diffusion model. The suitability of Langmuir and Freundlich isotherms to the equilibrium data was investigated in the solid-liquid system while the Langmuir model produces the best results. Thermodynamic data (Δ, Δ, and Δ) corresponding to the MB uptake were evaluated from the Langmuir model. In all the adsorption experiments, the adsorption capacity followed the order as follows: FA-ZX > FA-ZA. In addition, attempts were also made to regenerate the adsorbents.
fly ash, zeolite, dye, adsorption, kinetic
1 INTRODUCTION
The wastewater containing dyes, generated by the textile, printing and papermaking industries, pose major hazard to the environment and the public health. Many of the dyes used in these industries are generally toxic, nondegradable, stable, and even carcinogenic [1]. Basic dyes are the brightest class of soluble dyes used in the textile industry [2]. Their tinctorial value is very high: less than 1×10-6(1 ppm) of the dye produces an obvious coloration. The coloration of water inhibits sunlight penetration into the stream so as to reduce the photosynthetic reaction. During the last few years, various methods, such as aerobic and anaerobic microbial degradations, electrochemical treatment, hydrogen peroxide catalysis, chemical oxidation, reverse osmosis, coagulation, and flocculation, have been investigated for the removal of these dyes. Most of these methods suffer from a number of disadvantages, such as high capital, operational costs, and problem of unsuccessful removal of the coloration from wastewater [3]. Adsorption techniques using solid absorbents are well established for treating industrial wastewater. Currently, activated carbon is the most popular adsorbent used for the removal of dyes, as well as other organic and inorganic pollutants from wastewater because of its significant effectiveness. Generally, the activated carbon can be regenerated by heat treatment and the cost of such a process naturally limits its application. Considering discharged volume, this has encouraged research into developing the low-cost and effective adsorbents to purify water contaminated with dyes.
Fly ash is a waste material generated from electric power plants. The generation rate of the fly ash is approximately 500 million tons per year for the whole world and is predicted to increase [4]. Presently, about 20% of the fly ash is used for building materials and related applications, whereas the remainder of the fly ash is disposed of for piles and in landfill, causing severe environmental problems, such as polluting soils and groundwater [5]. As the environmental friendly applications of fly ash, several investigations have been reported on using fly ash for adsorption of pollutants in an aqueous solution and flue gas [6-17]. However, these data showed that fly ash still exhibits less adsorption capacity so as to make it unattractive for widespread commercial applications. At present, there is growing interest in using the synthetic products (zeolites and amorphous geopolymers) from fly ash for removing various dyes [18-22]. The most common method used for zeolites or amorphous geopolymers synthesized from fly ash involves a hydrothermal treatment, whereby fly ash is mixed with an alkali solution at different conditions of temperature, concentration of alkali solution, reaction time, and pressure. However, these synthetic products still contain considerable amount of fly ash residue, and the obtained zeolitic phases, such as NaP1 and HS, usually has more condensed structure (low pore volume). This limits the cation exchange capacity (CEC) and adsorption capacity of the products and considerably hinders the potential applications in wastewater treatments. To overcome these problems, Hollman. [23] initiated a two-stage method that has the advantage of synthesizing pure-form zeolites, such as zeolite A and X (high pore volume) from high-Si solution extracted by NaOH solution from fly ash. However, such a method suffers from some drawbacks, such as the low yield of the products and problem of disposal of residual fly ash. To our knowledge, no previous reports have been made to investigate the adsorption performance of dyes on the synthetic products from fly ash after regeneration.
The aim of this study is to examine the effectiveness of both synthetic zeolitic adsorbents (FA-ZA and FA-ZX) from fly ash in removing a basic dye from aqueous solution. A basic dye, methylene blue (MB), was chosen for the adsorption studies because of its toxicity to the environment and to the human health. The kinetic of the process and the adsorption capacity of the adsorbents were determined on the adsorption process. The influences of various parameters on the dye removal performance by original and regenerative adsorbents were investigated and also studied for comparison. The parameters studied in this study included initial pH value, temperature, and adsorbent dosage.
2 MATERIALS AND METHODS
2.1 Fly ash
Raw fly ash was obtained from a Chinese power plant located in Henan province using electrostatic precipitators. The chemical composition of the fly ash is SiO2︰49.29%, Al2O3︰33.07%, Fe2O3︰5.76%, K2O︰2.58%, TiO2︰1.18%, CaO︰2.05%, MgO︰0.27%, Na2O︰2.22%, and MnO︰0.13%. Before synthesis, raw fly ash was pretreated by acid-washing process. Raw fly ash is mixed with HCl solution (10%) at the liquid/solid ratio of 25 ml HCl solution per gram of solid, then the mixture was stirred and kept in a water bath at 80°C for 1 h. Afterward, fly ash was filtered off, washed repeatedly with distilled water, and finally dried at 100°C for 24 h for further use. The chemical composition of the pretreated fly ash is SiO2︰59.62%, Al2O3︰30.95%, and Fe2O3︰1.40% (involved composition for zeolites synthesis).
2.2 Adsorbent
9 g of pretreated fly ash was mixed and grounded with NaOH powder (Guangdong, analytical reagents) to obtain a homogeneous mixture. The mass ratio of the fly ash to NaOH powder was 1︰1.3. Then, the homogeneous mixture was heated in a nickel crucible in air at 600°C for 90 min. The fusion products were grounded and poured into a plastic bottle followed by the addition of distilled water to form a mixture. The mass ratio of the fusion products to water was 0.1725.
For zeolite X synthesis, the mixture was stirred intensely at 25°C for 24 h to form the aluminosilicate gel. For zeolite A synthesis, 2.823 g of NaAlO2(Shanghai, chemical reagents) was added to the mixture followed by intense stir at 25°C for 24 h to form the aluminosilicate gel. Then, both aluminosilicate gels were poured into the specially designed stainless alloy autoclave and kept in conventional air oven at 100°C at autogenous pressure for 24 h (zeolite X) and 5 h (zeolite A), respectively. After hydrothermal treatment, the precipitated samples were extracted from the mixed solution and then washed with distilled water until the pH was about 7. The samples were dried at 100°C for 12 h and were kept in powder form for further use. Hereafter, labeling of the synthetic zeolite A and X from fly ash was denoted as FA-ZA and FA-ZX, respectively.
2.3 Adsorbate
Methylene blue was obtained from Shanghai SAS Chemical Co. Ltd., Shanghai, China. Different concentrations of dye solutions (20, 30, 40, 50, 60, and 70 mg·L-1) were prepared by dissolving a weighed quantity of MB in deionized water. The initial pH of the dye solution was adjusted to the desired value by adding 2% HNO3and 1 mol·L-1NaOH solution and measured with a pH meter (PHS-3B, Shanghai).
2.4 Characterization
Powder X-ray diffraction (XRD) patterns of the adsorbents were taken on a Bruker D8 Advance XRD instrument (Cu, Kα). Scattering patterns were collected from 2° to 50° with a scan time of 1 min per two steps. Various crystalline phases in the adsorbents were identified with the help of XRD JCPDS codes [24]. The morphological structure of the adsorbents was obtained using the electron microscope (JEOLJSM-6380LV). The elemental composition of the adsorbents was determined by X-ray fluorescence vacuum spectrograph (ARL9800XP+). The CEC values were determined using the ammonium acetate method [25]. In thermal stability analysis of adsorbents, DTA-50 (differential thermal analysis) thermal analyzer was used at a heating rate of 20°C·min-1in air atmosphere over the range 100-1000°C with Al2O3as reference. All MB concentrations were spectroscopically analyzed using spectrophotometer (TU-1901 instrument, China) atmaxof 665 nm. The calibration standards were prepared using five concentrations of dye solutions (2, 4, 6, 8, and 10 mg·L-1), and the blank solution was used to calibrate the equipment. A linear calibration curve was obtained after calibration and the correlation coefficient2was 0.999. The samples were automatically measured three times in one aspiration. If the standard deviation of the test results were greater than 1%, the samples were measured again until the test results fulfilled the analysis requirement. All the instrumental conditions were optimized for the maximum sensitivity as indicated by the manufacturer’s manual.
2.5 Batch adsorption experiments
The study of adsorption kinetics and equilibrium is essential in supplying the fundamental information required for the design and the operation of adsorption equipments for wastewater treatment. All adsorption experiments were performed in a batch-stoppered conical flask (250 ml) in a temperature-controlled shaker with continuous stirring of 300 r·min-1.
2.5.1
The 0.1 g of adsorbents was left in contact with 100 ml of the dye solutions (50 mg·g-1) at the initial pH of 11.25 at a constant temperature of 30°C. Kinetic of adsorption was determined by analyzing the adsorptive uptake of MB from aqueous solution at different time intervals (10-1440 min).
2.5.2
At three temperatures (20, 30, and 40°C), 100 ml of MB solutions in the range of 20-70 mg·L-1were agitated with adsorbents (0.1 g) at the initial pH of 6.58 until the equilibrium was reached (48 h). The amount of MB adsorbed on the adsorbentse(mg·g-1) was calculated using the following expression:
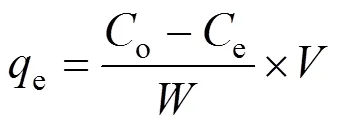
whereoandeare the initial and equilibrium MB concentrations of the test solution (mg·L-1), respectively,is the test solution volume (L), andis the amount of adsorbents (g). Removal efficiency (%) of MB on the adsorbents is considered in percentage as follows:
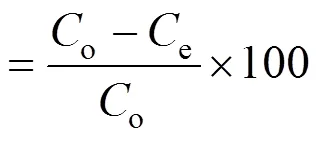
2.6 Adsorbents regeneration
In wastewater treatment, reuse and recovery of the spent adsorbents could play important role. Keeping this in view, experiments were carried out using the heat treatment to regenerate the dye-saturated adsorbents at 300°C for 3 h. The effect of experimental parameters, such as the initial pH (3.48-11.18), temperature (20-50°C), and adsorbents dosage (0.1-0.3 g), on the adsorption capacity of regenerative and original adsorbents was investigated in batch experiments for comparison.
3 RESULTS AND DISCUSSION
3.1 Characterization of adsorbents
The XRD patterns in Fig. 1 illustrates that adsorbents FA-ZA and FA-ZX from fly ash are identified as single-phase and high-crystalline zeolites A (JCDPDS 43-0142) and X (JCDPDS 39-0218), respectively.
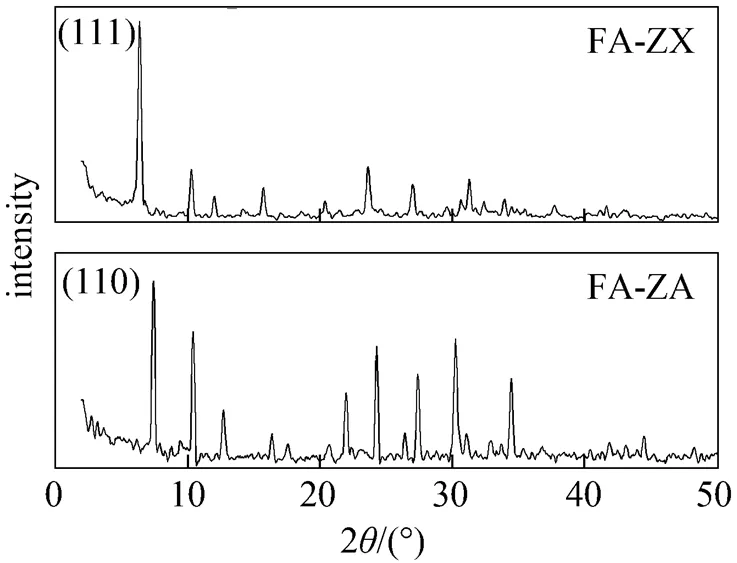
Figure 1 XRD patterns of adsorbents FA-ZA and FA-ZX
It has been known that the SiO2/Al2O3molar ratio of the prepared gel can determine the final framework of particular zeolites [26]. The addition of Al source can result in the decrease of the solubility of Si source in the gel. The lower Si concentration in the gel promotes the formation of small silicate species, such as double four member rings (D4R) [27]. It is also worth noting the difference in the synthesis time required for both zeolites synthesis. The structural formation of zeolite X requires longer synthesis time because of its more complex and larger polymeric silicate units (D6R) and sparser structure [28]. Morphological analysis of the adsorbents FA-ZA and FA-ZX performed by SEM (scanning electronic microscopy) is shown in Fig. 2.
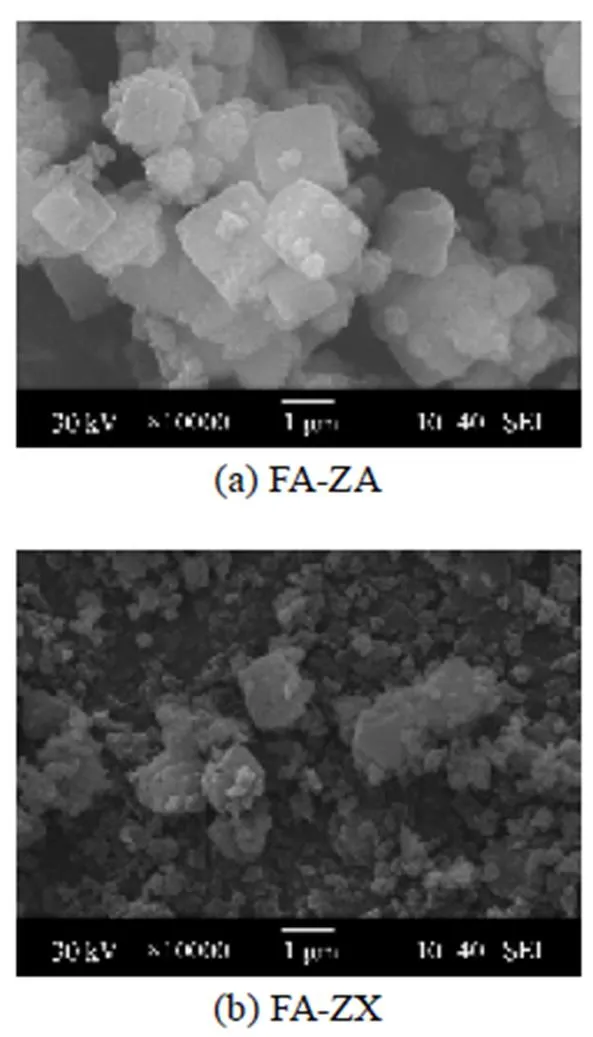
Figure 2 SEM images of adsorbents
It is observed that two samples contain a cluster of zeolite A and X crystals, respectively. Moreover, the XRD patterns in Fig. 1 prove that the crystalline particles with lattice fringes that can be assigned to the {110} faces of the cubic structure of zeolite A are synthesized from fly ash [29] and the crystallographic planes {111} is reflected to the structure of zeolite X [30]. Fig. 3 shows the DTA curves of the synthetic (FA-ZA and FA-ZX) and commercial grade zeolites (The commercial grade zeolites A and X were obtained from Luoyang Jianlong Chemical Industrial Co., Ltd., China).
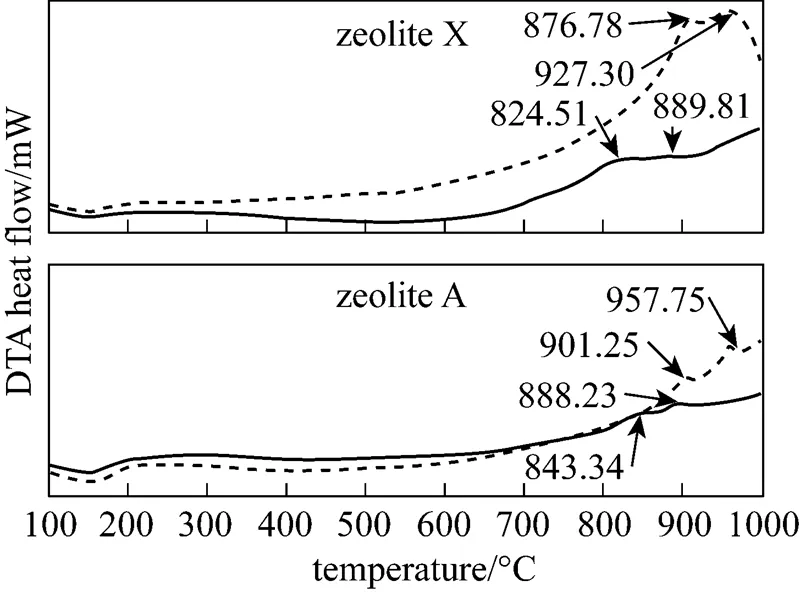

As shown in Fig. 3, the endothermic peak occurs in the region of 100-200°C for these samples. The endothermic reaction may be attributed to the presence of zeolitic water into zeolitic surface and pore before 500°C. With an increase of temperature, two exothermic peaks may be observed for each DTA curves. The former peak is associated with collapse of zeolitic framework and the latter peak implies the transformation of zeolitic phases. DTA analysis also shows that the crystallinity of the adsorbents FA-ZA and FA-ZX is near to the corresponding commercial grade zeolites. As such, DTA curves provide the important information for adsorbents regeneration. Synthetic zeolites from pure chemicals have undergone intensive toxicological studies and have been shown to be nontoxic to living organisms [31, 32]. To investigate if there was any toxic element existing in the synthetic zeolites from fly ash, a comparative study of elemental composition between the synthetic (FA-ZA and FA-ZX) and the commercial grade zeolites was carried out. Table 1 lists various trace elements of the synthetic zeolites and commercial grade zeolites.

Table 1 A comparison of elemental composition of the synthetic and commercial grade zeolites (%)
Apparently, the presence of these elements, such as Fe, Mn, Mg, Ti, Ca, and K, may be resulted from metal oxides of pretreated fly ash itself. The results show that the elemental composition of the synthetic zeolites does not pose any serious threat to the environment and public health when compared with commercial grade zeolites.
Figure 4 illustrates that the CEC values of both synthetic zeolites from fly ash with raw and pretreated fly ash as starting materials and the commercial grade zeolites.
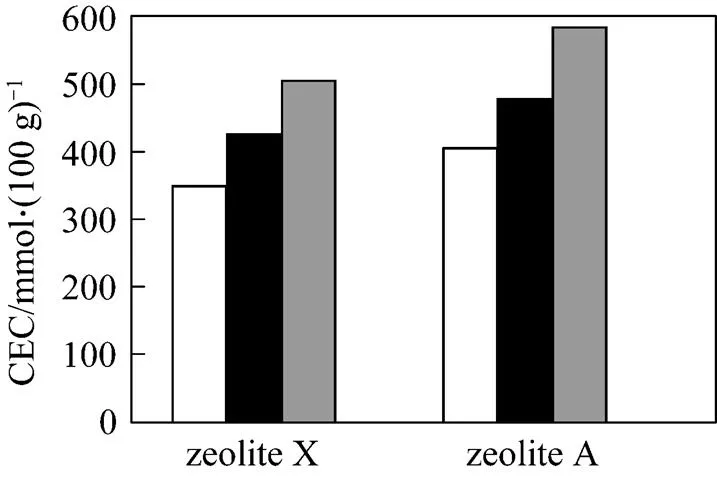


Since acid-washing process clearly demonstrated a benefit effect on synthesis, only the synthetic zeolitesfrom pretreated fly ash were used for subsequent studies.
3.2 Adsorption kinetics
There are essentially three steps in the adsorption process by porous adsorbents [34]: (1) solute transfer from the bulk solution to the external surface of the adsorbent through a liquid boundary layer (liquid film diffusion), (2) solute transfer from the adsorbent surface to the intraparticle active sites (intraparticle diffusion), and (3) interactions of the solute with the available sites on both the external and internal surfaces of the adsorbent (chemical reaction). One or more of the above-mentioned steps may control the rate at which the solute is adsorbed and the amount of solute that is adsorbed onto the adsorbents. To identify the step governing the overall removal rate of the adsorption process, the models given by Boyd. [35] and Reichenbery [36] were applied. Liquid film diffusion, intraparticle diffusion, and chemical reaction are described in the following equations, respectively:
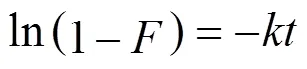

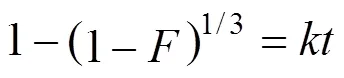
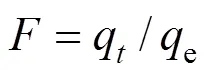
Figure 5 shows liquid film diffusion, intraparticle diffusion, and chemical reaction models fitting to the experimental data, respectively.
Correlation coefficient (2) between the experimental data and the three models indicates that liquid film diffusion can be considered as a rate determining step for adsorbents FA-ZA and FA-ZX. The negative charge density on the surface of the zeolitic adsorbents would attract the positively charged functional groups located on the dye. As a basic dye, MB on dissolution produces colored cation (M+) in aqueous solution. Thus, the adsorption mechanism of MB is the association of the cationic dye with zeolites by overcoming liquid film resistance.
Lagergren first-order model [37] and Ho’ pseudo- second-order model [38] are two important models applied for solid-liquid adsorption. The following expressions were used to describe two models, respectively:
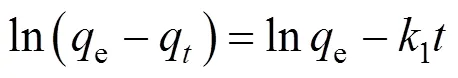

whereqandeare the amount of MB adsorbed on the adsorbents (mg·g-1) at timeand at equilibrium, respectively.1(min-1) and2(g·mg-1·min-1) are the rate constants of first order and second order, respectively.
Figure 6 shows the kinetics of MB adsorption on adsorbents FA-ZA and FA-ZX, and the kinetic parameters are also provided in this figure.
For the Lagergren first-order model, the values of2are lower than those obtained using the Ho’ pseudo-second-order model. This confirms the second- order nature of MB adsorption on adsorbents FA-ZA and FA-ZX. From Fig. 5 (b), it is shown that the calculated values ofe,cal(FA-ZA, 38.64 mg·g-1; FA-ZX, 44.07 mg·g-1) agree well with the experimental values ofe,exp(FA-ZA, 37.81 mg·g-1; FA-ZX, 43.02 mg·g-1).
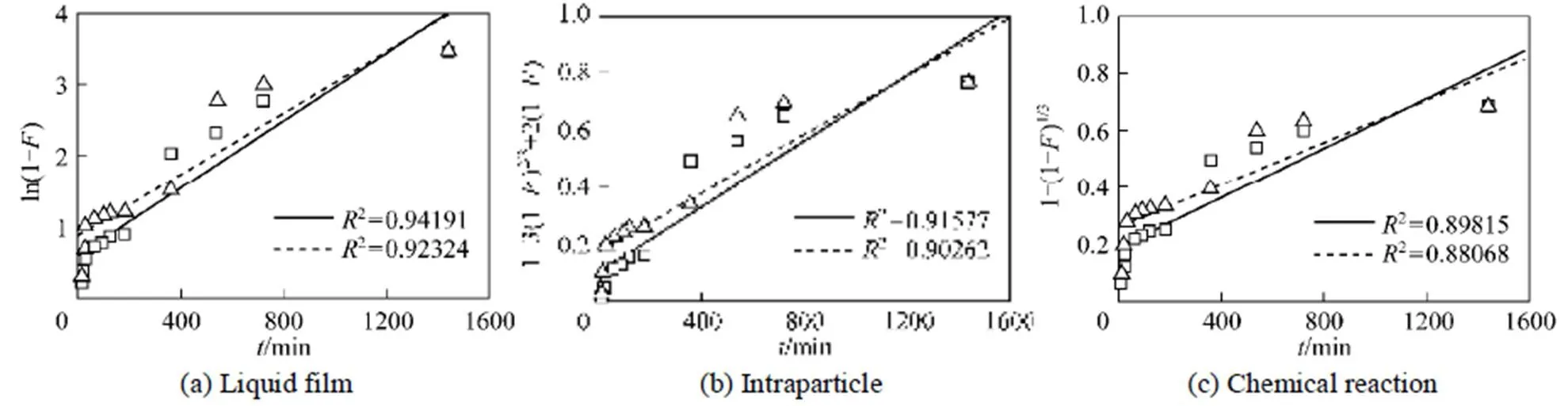
Figure 5 Fitted regression line of MB on adsorbents FA-ZA and FA-ZX by three models □ FA-ZA; △ FA-ZX
Figure 6 Plots of kinetics of MB adsorption on adsorbents FA-ZA and FA-ZX □ FA-ZA; △ FA-ZX
3.3 Adsorption isotherms
For the solid-liquid system, the studies of adsorption isotherms are very important to realize information about adsorption capacity of adsorbents. The widely used isotherm equations for evaluating the adsorption equilibrium are Langmuir and Freundlich isotherms. The Langmuir isotherm is obtained under an assumption that the adsorption occurs at a specific homogeneous surface of the adsorbent [39]. The linearly transformed Langmuir isotherm is used to fit the adsorption data in this study and is expressed as

whereeis the equilibrium concentration of MB in solution (mg·L-1),eis the amount of MB adsorbed on adsorbents (mg·g-1), andmandare the monolayer adsorption capacity (mg·g-1) and the binding constant, respectively. The Freundlich isotherm is an empirical equation which is used for the heterogeneous systems and is represented as [40, 41]
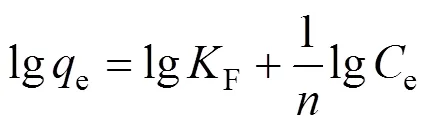
whereFandare indicative of the extent of the adsorption and the adsorption intensity, respectively.
Figure 7 shows the linearized Langmuir and Freundlich adsorption isotherms of MB on adsorbents FA-ZA and FA-ZX with different temperatures. The model parameters obtained from these isotherms are given in Table 2.
Correlation coefficient (2) shows that the Langmuir model is better than the Freundlich model in simulation of the adsorption isotherm. The agreement of the Langmuir model with the experimental results suggests that a monolayer coverage of MB on the outer surface of both adsorbents. For adsorbent FA-ZA, the greatest adsorption capacitymvalue was obtained for MB at 20°C, that is 23.20 mg·g-1, which increase to 25.98 mg·g-1at 30°C and 43.76 mg·g-1at 40°C. Similarly, for FA-ZX, the greatest adsorption capacitymvalue was obtained for MB at 20°C, that is 31.90 mg·g-1, which increase to 35.42 mg·g-1at 30°C and 51.20 mg·g-1at 40°C. Themvalues of FA-ZA and FA-ZX increased with the increase in temperature, indicating the endothermic nature of the adsorption process. The apparent equilibrium constant (a) determined by the product ofandmcan be used as relative indicators of the affinity of adsorbents toward MB. It is indicated from theseavalues that the affinity of FA-ZX for MB is quite higher in comparison of FA-ZA. Table 2 also illustrates that FA-ZX shows higher adsorption capacity than FA-ZA. The crystal structure of zeolites may be responsible for MB removal. Zeolites A and X, in general, have the effective pore size of 0.42 nm and 0.74 nm, respectively. MB molecules can pass easily into the pores from the channels and may be adsorbed on the interior surface of zeolite X in comparison with zeolite A. Table 3 gives the greatest adsorption capacitymvalue of MB adsorption on both adsorbents investigated by us and other researchers.
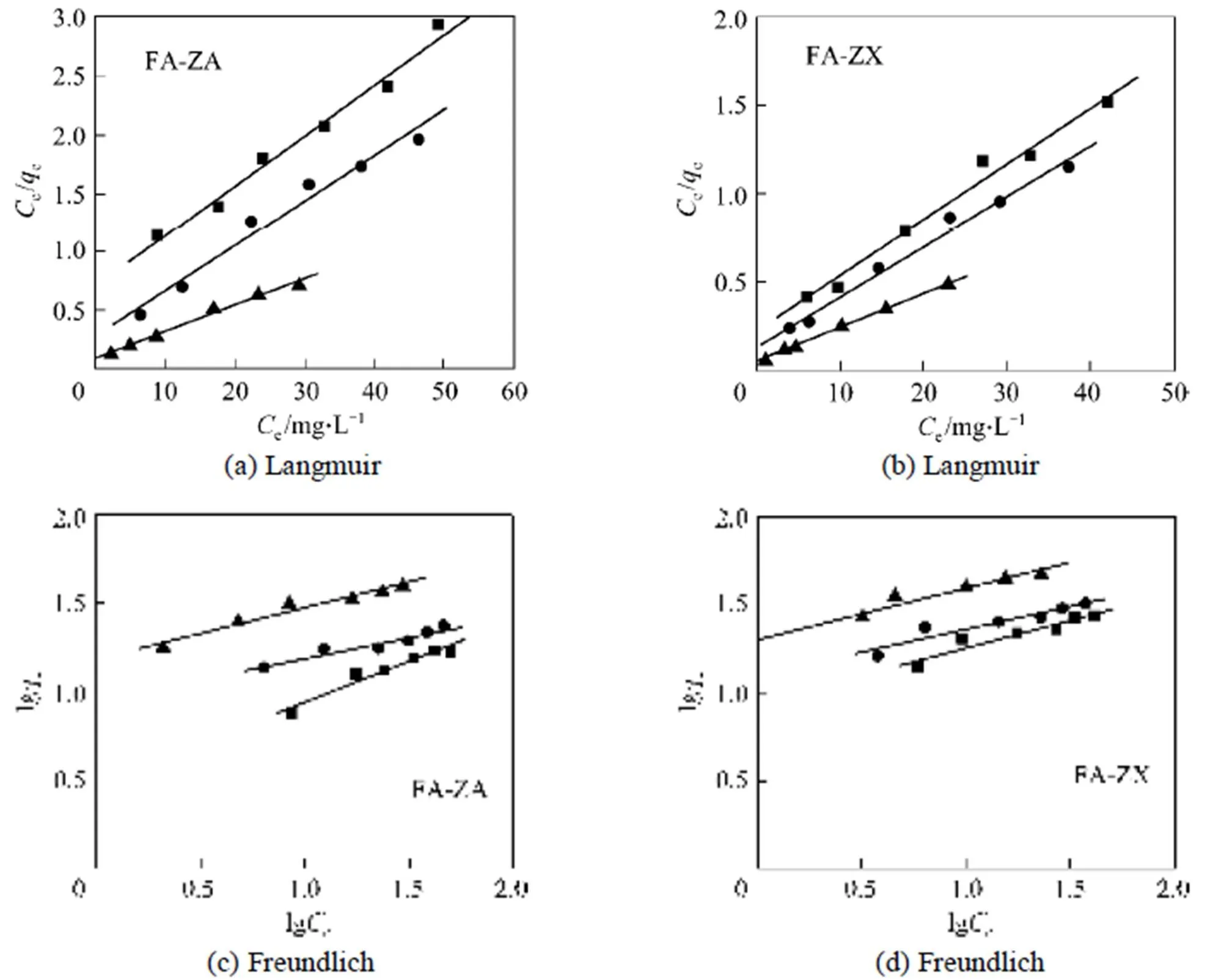
Figure 7 Experimental MB adsorption isotherms of adsorbents FA-ZA and FA-ZX with different temperatures■ 20°C;● 30°C;▲ 40°C
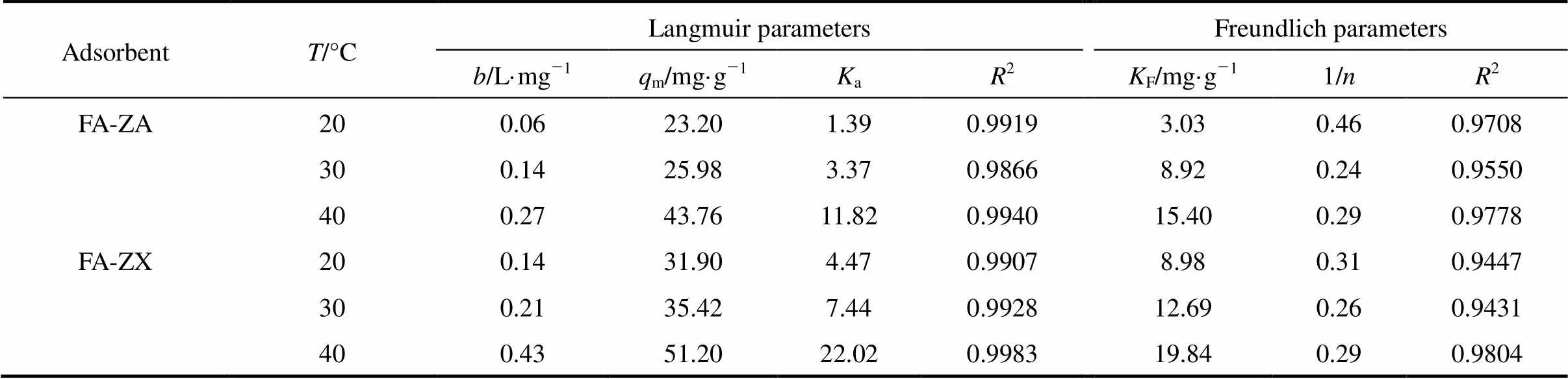
Table 2 Langmuir and Freundlich parameters for MB adsorption on adsorbents FA-ZA and FA-ZX
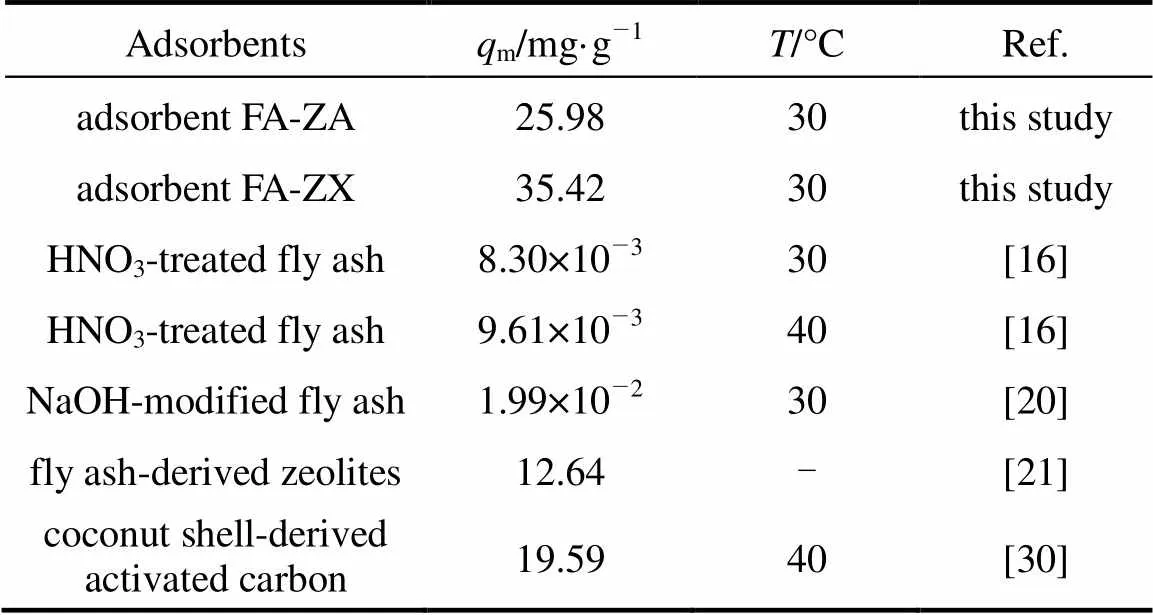
Table 3 The greatest adsorption capacity of MB on various adsorbents
It can be seen that results obtained in this study are higher than the results obtained by other investigations. This clearly indicates that FA-ZA and FA-ZX can be fruitfully used as an adsorbent for dye removal.
3.4 Thermodynamic parameters
Thermodynamic parameters, that is enthalpy (Δ), entropy (Δ), and free energy (Δ) changes, are calculated using the following equations:
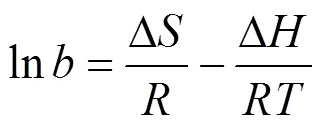
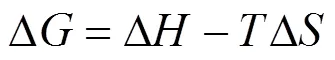
whereis the Langmuir constant at. All the values of Δ, Δ, and Δare listed in Table 4.

Table 4 Thermodynamic parameters for MB adsorption
The negative values of Δindicate the feasibility and the spontaneous nature of MB adsorption on both adsorbents. It was also observed that with the increase in temperature, the values of Δbecome more negative, which justify that the increase in temperature contributes to the adsorption process. The positive values of Δand Δagain indicate the endothermic nature of adsorption process and increased disorderliness in the solid/solution interface. A similar trend has been reported for the adsorption of crystal violet and rosaniline hydrochloride onto fly ash [13].
3.5 Comparison of original and regenerative adsorbents
The purpose of the heat treatment is to burn off the residual MB existing in zeolitic adsorbents. Generally, burning temperature should be determined in the range of the corresponding temperature obtained from endothermic and exothermic peaks of the zeolitic adsorbents. This not only can remove zeolitic water and organic matters but also maintain the structure of zeolitic adsorbents. To check the adsorption capacity, regenerative adsorbents are reloaded with MB solution of known concentrations. Fig. 8 gives the removal performance of MB on original and regenerative adsorbents with different initial pH value, temperature, and adsorbent dosage, respectively.

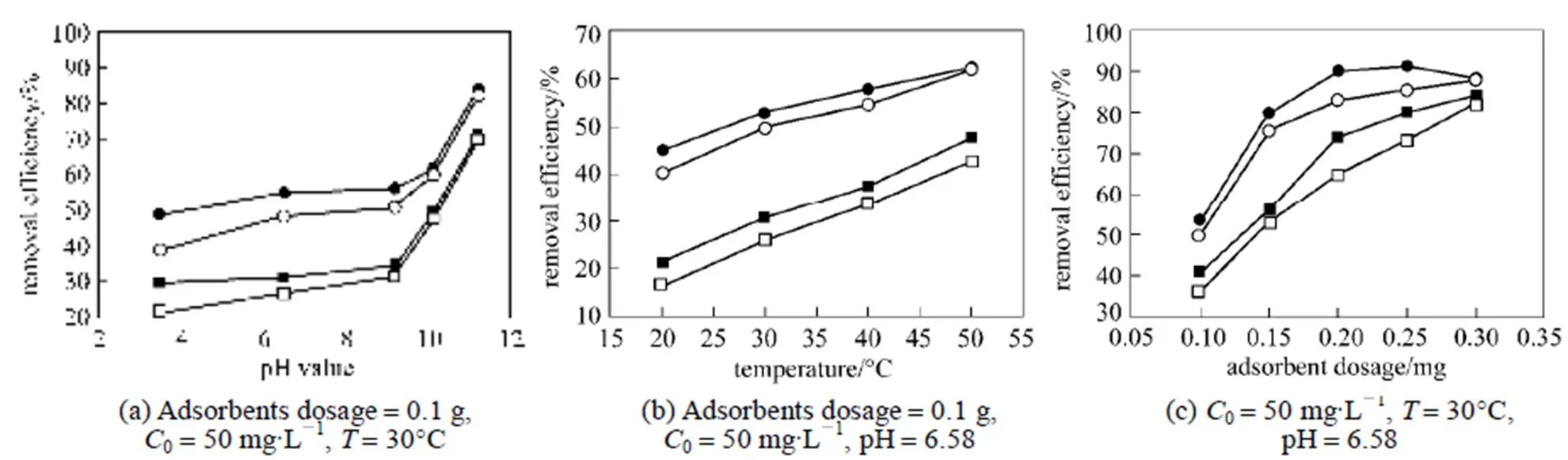
Figure 8 Comparison of the removal performance of MB on original and regenerative adsorbents■ original FZ-ZA;● original FZ-ZX;□ regenerative FA-ZA;○ regenerative FA-ZX
4 CONCLUSIONS
This study indicated that both fly ash-based adsorbents (FA-ZA and FA-ZX) have the potential to act as adsorbents for the removal of MB cationic dye from aqueous solutions. The step of the overall removal rate was governed by liquid film diffusion with the experimental data mathematically described using liquid film diffusion, intraparticle diffusion, and chemical reaction equations, and the adsorption process followed Ho’ pseudo-second-order model. The adsorption data correlated well with the Langmuir equation. Thermodynamic parameters were evaluated for MB and revealed that the adsorption of the dye is endothermic in nature. The values ofaindicated that adsorbent FA-ZX exhibits higher adsorption capacity for MB when compared with adsorbent FA-ZA. It also showed that the removal performance of MB on original and regenerative adsorbents was dependent on these parameters, such as initial pH value of the solution, temperature, and adsorbent dosage. Generally, the removal efficiency of MB increased with a rise of initial pH value of the solution, temperature, and adsorbent dosage.
The fly ash synthesized zeolitic adsorbents may be an alternative to more costly adsorbents, such as activated carbon and commercial grade zeolites for the treatment of wastewaters containing the dyes.
1 Namasivayam, C., Muniasamy, N., Gayatri, K., Rani, M., Ranganathan, K., “Removal of dyes from aqueous solutions by cellulosic waste orange peel”,.., 57 (1), 37-43 (1996).
2 McKay, G., Otterburn, M.S., Sweeney, A.G., “Surface mass transfer processes during color removal from effluent using silica”,.., 15 (3), 327-331 (1981).
3 Asfour, H.M., “Color removal from textile effluents using hardwood as an adsorbent”,...., 35A, 28-32 (1985).
4 Japan Fly Ash Association Coal Ash Handbook, Kankyo Gijyutsu Kyokai and Nippon Fly ash Kyokai, Tokyo (2000).
5 Inada, M., Eguchi, Y., Enomoto, N., Hojo, J., “Synthesis of zeolite from coal fly ashes with different silica-alumina composition”,., 84 (2/3), 299-304 (2005).
6 Davini, P., “Investigation of flue gas desulphurization by fly ash and calcium hydroxide mixtures”,..., 15 (3), 193-201 (1995).
7 Kastner, J.R., Melear, N.D., Das, K.C., “Catalytic oxidation of gaseous reduced sulphur compounds using coal fly ash”,..., 95 (1/2), 81-90 (2002).
8 Lu, G.O., Do, D.D., “Adsorption properties of fly ash particles for NOremoval from flue gases”,..., 27 (1), 95-107 (1991).
9 Ayala, J., Blanco, F., Garcia, P., Rodriguez, P., Sancho, J., “Asturian fly ash as a heavy metals removal material”,., 77 (11), 1147-1154 (1998).
10 Dasmahapatra, G.P., Pal, T.K., Bhadra, A.K., Bhattacharya, B., “Studies on separation characteristics of hexavalent chromium from aqueous solution by fly ash”,..., 31 (5), 2001-2009 (1996).
11 Panday, K.K., Prasad, G., Shingh, V.N., “Copper (II) removal from aqueous solutions by fly ash”,.., 19 (7), 869-873 (1985).
12 Gupta, V.K., Mittal, A., Krishnan, L., Gajbe, V., “Adsorption kinetics and column operations for the removal and recovery of malachite green from wastewater using bottom ash”,..., 40 (1), 87-96 (2004).
13 Mohan, D., Singh, K.P., Singh, G., Kumar, K., “Removal of dyes from wastewater using fly ash: a low-cost adsorbent”,...., 41 (15), 3688-3695 (2002).
14 Acemioğlu, B., “Adsorption of Congo red from aqueous solution onto calcium-rich fly ash”,...., 274 (2), 371-379 (2004).
15 Janoš, P., Buchtová, H., Rỳznarová, M., “Sorption of dyes from aqueous solutions onto fly ash”,.., 37 (20), 4938-4944 (2003).
16 Wang, S., Boyjoo, Y., Choueib, A., Zhu, Z.H., “Removal of dyes from aqueous solution using fly ash and red mud”,.., 39 (1), 129-138 (2005).
17 Aksu, Z., Yener, J., “A comparative adsorption/biosorption study of mono-chlorinated phenols onto various sorbents”,.., 21 (8), 695-702 (2001).
18 Davidovits, J., “Geopolymers—inorganic polymeric new materials”,..., 37, 1633-1656 (1991).
19 Xu, H., VanDeventer, J.S., “The geopolymerisation of alumino-silicate minerals”,...., 59 (3), 247-266 (2000).
20 Wang, S., Soudi, M., Li, L., Zhu, Z.H., “Coal ash conversion into effective adsorbents for removal of heavy metals and dyes from wastewater”,..., B133 (1-3), 243-251 (2006).
21 Woolard, C.D., Strong, J., Erasmus, C.R., “Evaluation of the use of modified coal ash as a potential sorbent for organic waste streams”,.., 17 (9), 1159-1164 (2002).
22 Li, L., Wang, S., Zhu, Z., “Geopolymeric adsorbents from fly ash for dye removal from aqueous solution”,...., 300 (1), 52-59 (2006).
23 Hollman, G.G., Steenbruggen, G., Janssen-Jurkovicova, M., “A two-step process for the synthesis of zeolites from coal fly ash”,., 78 (10), 1225-1230 (1999).
24 Treacy, M.M.J., Higgins, J.B., Collection of Simulated XRD Powder Patterns for Zeolites, 4th edition, Elsevier, Amsterdam (2001).
25 Molina, A., Poole, C., “A comparative study using two methods to produce zeolite from fly ash”,.., 17 (2), 167-173 (2004).
26 Weitkamp, J., Puppe, L., Catalysis and Zeolite, Fundamentals and Applications, Springer, Germany, Chapters 1-2 (1999).
27 Tanaka, H., Sakai, Y., Hino, R., “Formation of Na-A and Na-X zeolites from waste solutions in conversion of coal fly ash to zeolites”,..., 37 (11), 1873-1884 (2002).
28 Petrovic, I., Navrotsky, A., Davis, M.E., Zones, S.I., “Thermochamical study of the stability of frameworks in high silica zeolites”,.., 5 (12), 1805-1813 (1993).
29 Valentin, P.V., Lubomira, T., Krassimir, N.B., “Synthesis of zeolite nanocrystals at room temperature”,., 21 (23), 10724-10729 (2005).
30 Breck, D.W., Zeolite Molecular Sieves, Chemistry, and Use, John Wiley & Sons, New York (1974).
31 Christophliemk, P., Gerike, P., Potokar, M., Handbook of Environmental Chemistry, Springer-Verlag, Berlin (1992).
32 Gloxhuber, C., Potokar, M., Pittermann, W., Wallat, S., Bartnik, F., Reuter, H., Braig, S., “Zeolite A—A phosphate substitute for detergents-toxicological investigation”,..., 21 (2), 209-220 (1983).
33 Vaughan, D., Crystal Engineering: The Design and Application of Functional Solids, Kluwer Academic Publishers, The Netherlands, 451-472 (1999).
34 McKay, G., “The adsorption basic dye onto silica from aqueous-solution solid diffusion-model”,..., 39, 129-138 (1984).
35 Boyd, G.E., Adamson, A.W., Myers, L.S., “The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics”, J...., 69 (11), 2836-2848 (1947).
36 Reichenberg, D., “Properties of ion-exchange resins in relation to their structure. III. Kinetics of exchange”,...., 75 (3), 589-597 (1953).
37 Lagergren, S., “About the theory of so-called adsorption of soluble substances, Kung”,..., 24, 1-39 (1898).
38 Ho, Y.S., McKay, G., “Pseudo-second order model for sorption processes”,., 34 (5), 451-465 (1999).
39 Langmuir, I., “Adsorption of gases on plain surface of glass, mica and platinum”,...., 40 (9), 1361-1403 (1918).
40 Namasivayam, C., Jeyakumar, R., Yamuna, R.T., “Dye removal from waste-water by adsorption on waste Fe(III)/Cr(III) hydroxide”,.., 14 (7), 643-648 (1994).
41 Namasivayam, C., Yamuna, R.T., Jayanthi, J., “Removal of methylene blue from wastewater by adsorption on cellulosic waste, orange peel”,..., 37 (7), 333-339 (2003).
42 Lazaridis, N.K., Peleka, E.N., Karapantsios, T.D., Matis, K.A., “Copper removal from effluents by various separation techniques”,., 74 (1/2), 149-156 (2004).
43 Barthomeuf, D., “Basic zeolites: Characterization and uses in adsorption and catalysis”,..., 38, 521-612 (1996).
44 Al-Qodah, Z., “Adsorption of dyes using shale oil ash”,.., 34 (17), 4295-4303 (2000).
2008-07-01,
2008-12-27.
the Specialized Research Fund for the Doctoral Program of Higher Education of China (20060288008) and the Cultivation Fund of the Key Scientific and Technical Innovation Project, Ministry of Education of China (708049).
** To whom correspondence should be addressed. E-mail: wanglj@mail.njust.edu.cn
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
