Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
YIN Jiamin (印佳敏)* and WU Zhansong (吴占松)
Corrosion Behavior of TP316L of Superheater in Biomass Boiler with Simulated Atmosphere and Deposit
YIN Jiamin (印佳敏)* and WU Zhansong (吴占松)
Key Laboratory for Thermal Science and Power Engineering of Ministry of Education, Department of Thermal Engineering, Tsinghua University, Beijing 100084, China
Corrosion behavior of TP316L was investigated with simulated atmosphere and ash deposition for the superheater in biomass boiler. Corrosion dynamic curves were plotted by mass gain. The results showed that the corrosion was dependent on temperature and was greatly accelerated by ash deposition. The mass gain was distinctly reduced in the presence of SO2with and without ash deposition on the specimens. Corrosion rates with ash deposit at different temperatures were calculated. Two feasible methods were provided to avoid serious high-temperature corrosion in the biomass boiler.
high-temperature corrosion, TP316L, superheater, biomass boiler, HCl
1 INTRODUCTION
High contents of potassium and chlorine in biomass are harmful to corrosion protection of superheater tubes in biomass boiler [1-4]. Traditionally, high- temperature corrosion is not a serious problem in biomass boiler, mainly because the temperature of superheater tubes is not so high. However, the temperature of superheated steam will be raised in order to improve the efficiency of biomass boiler.
The corrosion during biomass combustion is mostly related to chlorine [5]. Chlorine influences the corrosion of superheater tubes in many ways. Metals transfer continuously away from the metal surface toward the place with higher oxygen partial pressure [6-8], during which chlorine acts as a catalyst. The corrosion rate is dependent on temperature, gas composition and ash deposition [9-13]. Miltner. [14] have pointed out that the risk of high-temperature corrosion is relatively low as long as the molar ratio of sulfur to chlorine is higher than 4. Hence co-firing of straw and coal has been proposed [15, 16].
High-temperature corrosion of superheater tubes in biomass boiler was investigated in the atmosphere [10], but the factors affecting the corrosion could not be confirmed. Some investigations focused on the corrosion by gaseous HCl in municipal solid waste (MSW) boiler [17, 18]. However, little is known about the mechanism of high-temperature corrosion with ash deposition. The objective of this work is to describe the corrosion behavior of austenitic stainless steel TP316L, and to give advice for avoiding serious high-temperature corrosion in biomass boiler.
2 EXPERIMENTAL
Figure 1 gives the schematic diagram of the corrosion experiments. Different gases from gas cylinders and through mass flow controllers were mixed sufficiently in gas mixing vessel. In addition to HCl and SO2, the mixed gas contained 6% O2and 12% CO2, with N2balance. The total flow rate was 60 ml·min-1. The metal specimens were put on the top of quartz sledge and placed in the middle of the furnace, where the temperature difference was less than 3°C. The exhaust gas was led into NaOH solution to adsorb redundant HCl and SO2.

Figure 1 Schematic diagram of the lab-scale corrosion experiment

Table 1 The element contents of TP316L(GB 13296-91)

Table 2 The original composition of ash (%)
The metal specimens were cut from the superheater tube, with the size 20 mm×10 mm×2 mm. The specimens were polished by sand paper with grit size up to 800, decreased with acetone, washed with de-ionized water and then laid in electric blast drying oven for 2 hours. The element contents of the metal are listed in Table 1. Ash deposit covered on the surface of the metal specimens was collected in biomass power plant. The composition of ash is shown in Table 2, analyzed by X-ray fluorescence spectrometer. Carbon was found in the ash because of incomplete combustion, with the content of about 10%. The original ash was put into muffle for 1 hour to make sure all the carbon in the ash was burned before the experiments with ash deposit, so that the mass loss caused by the burnout of carbon was avoided during the corrosion experiments.
The metal specimens were exposed to the simulated flue gas for 168 hours. The temperature of the gas in the quartz tube was kept at 500-600°C. The corrosion experiments were conducted according to the criterion ASTM G54-77 [19]. After exposure, three parallel specimens were withdrawn from the oven and kept dry in a box containing silica gel. Three specimens were cast in epoxy, polished by a polishing machine, cleaned with clear water, wiped up and then kept dry in order to observe the morphology of cross section. For the specimens covered with ash deposit, the ash was swept by a brush gently before the casting process.
3 RESULTS AND DISCUSSION
3.1 Effect of temperature on corrosion by gaseous HCl
According to the preliminary results of corrosion test, it is very difficult to remove corrosion products, thus corrosion dynamic curves are plotted by mass gain. Fig. 2 shows the effect of temperature on corrosion by gaseous HCl. The mass gain is strongly increased with temperature. Each corrosion dynamic curve follows the parabolic kinetics.
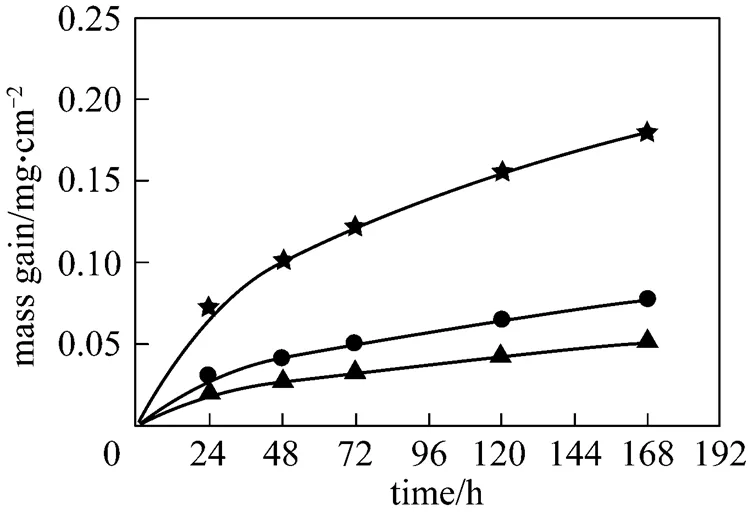
Figure 2 Corrosion results in 6%O2-12%CO2-0.01% HCl-N2
temperature/°C:▲ 550;● 575;★ 600
The surface morphology of the metal specimens after corrosion was investigated by scanning electron microscopy (SEM), as shown in Fig. 3. Some tiny holes were observed for the specimens at 550°C, with about 1.5%Cl detected by energy dispersive spectrometer(EDS). Spots of rust appeared when the temperature increased up to 575°C. The rust was porous and loose, in the shape of needle or flake. In addition, many little particles were found from the SEM photographs. The element analysis to the spots of rust showed only Fe, Cr and O, without Ni.
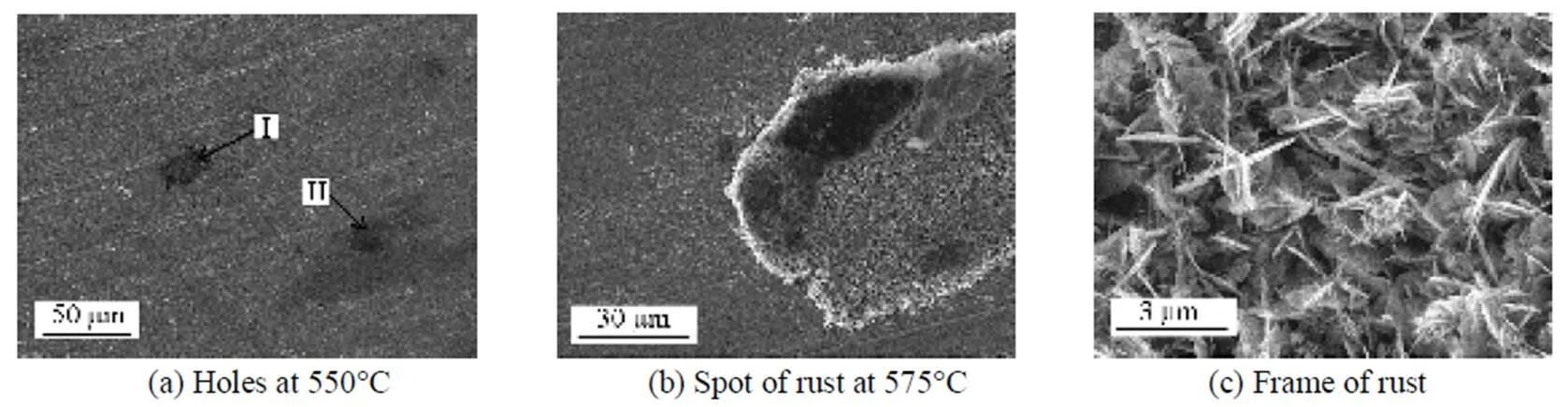
Figure 3 SEM micrographs of the corroded specimens in 6%O2-12%CO2-0.01% HCl-N2
Figure 4 SEM micrographs of the cross section of corroded specimens in 6%O2-12%CO2-0.01% HCl-N2
The morphology of cross section for the specimens after corrosion is shown in Fig. 4. A very thin oxide scale stuck to the metal surface while another scale departed away from the surface. The outer scale was easy to break off. According to Fig. 2, the mass gain increases with temperature, indicating that the corrosion is more serious. As a result, the oxide scale at 575°C was thicker than at 550°C. However, when the temperature increased to 600°C, the oxide scale was thinner and closer to the metal, probably because the observed oxide scale was formed after the old one broke off.
The composition of corrosion products analyzed by X-ray diffractometer (XRD) is shown in Fig. 5. Fe2O3was detected above 575°C although the peak value was very low. The limited content of corrosion product Fe2O3indicated good properties of corrosion- resistance for TP316L. Cr and O were both detected by EDS while no oxide of chromium was detected by XRD. Oxide of chromium is the effective component for corrosion-resistance. When the content of chromium in the steel is over 19%, the surface passivation film is noncrystal [20].
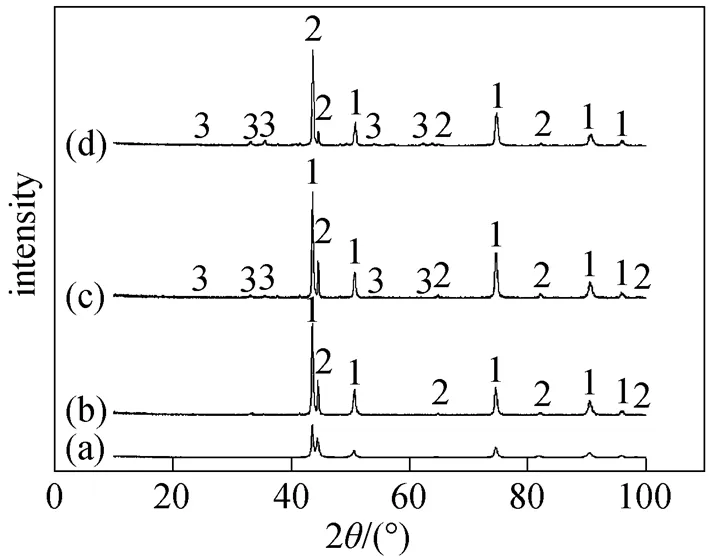
Figure 5 Corrosion products in 6%O2-12%CO2-0.01% HCl-N2
(a) without corrosion; (b) 550°C; (c) 575°C; (d) 600°C
1—Cr0.19Fe0.7Ni0.11; 2—Fe-Cr; 3—Fe2O3
3.2 Effect of SO2 on corrosion by gaseous HCl
Figure 6 shows the corrosion dynamic curves in 6%O2-12%CO2-0.01%HCl-0.01%SO2-N2. Compared with Fig. 2, the mass gain is markedly reduced with SO2in the simulated flue gas.

Figure 6 Corrosion results in 6%O2-12%CO2-0.01% HCl-0.01%SO2-N2
temperature/°C:▲ 500;● 550;★ 575;■ 600
The main corrosion process caused by chlorine is active oxidation, which leads to serious corrosion without chlorine consumption. As a result, the corrosion occurs continuously after chlorine penetrates through the protective oxide scale. Sulfur corrosion also occurs in the presence of SO2. The corrosion rate of sulfur is lower than that of chlorine and the corrosion product FeSO4forms a compact scale on the surface of the metal, which serves as a diffusion barrier against the transfer of chlorine.
3.3 Effect of ash deposition on corrosion
Figure 7 shows the corrosion dynamic curves with about 20 mg biomass ash covered on the surface of the metal specimens. In the temperature range of 550-600°C, the mass gain after 168 hours is nearly 50 times larger than that without ash deposition. Corrosion is greatly accelerated by ash deposition. The corrosion dynamic curve at 600°C follows the linear kinetics, indicating that the metal is unprotectable.

Figure 7 Corrosion results with ash deposition in 6%O2-12%CO2-0.01%HCl-N2
temperature/°C: ▲ 500;● 550;★ 575;■ 600
The surface morphology of the specimens after corrosion is shown in Fig. 8. Protuberant spots of rust appeared at 500°C, while the whole metal surface was covered with loose frame in the shape of flake over 550°C, which was confirmed to be Fe2O3by XRD analysis.
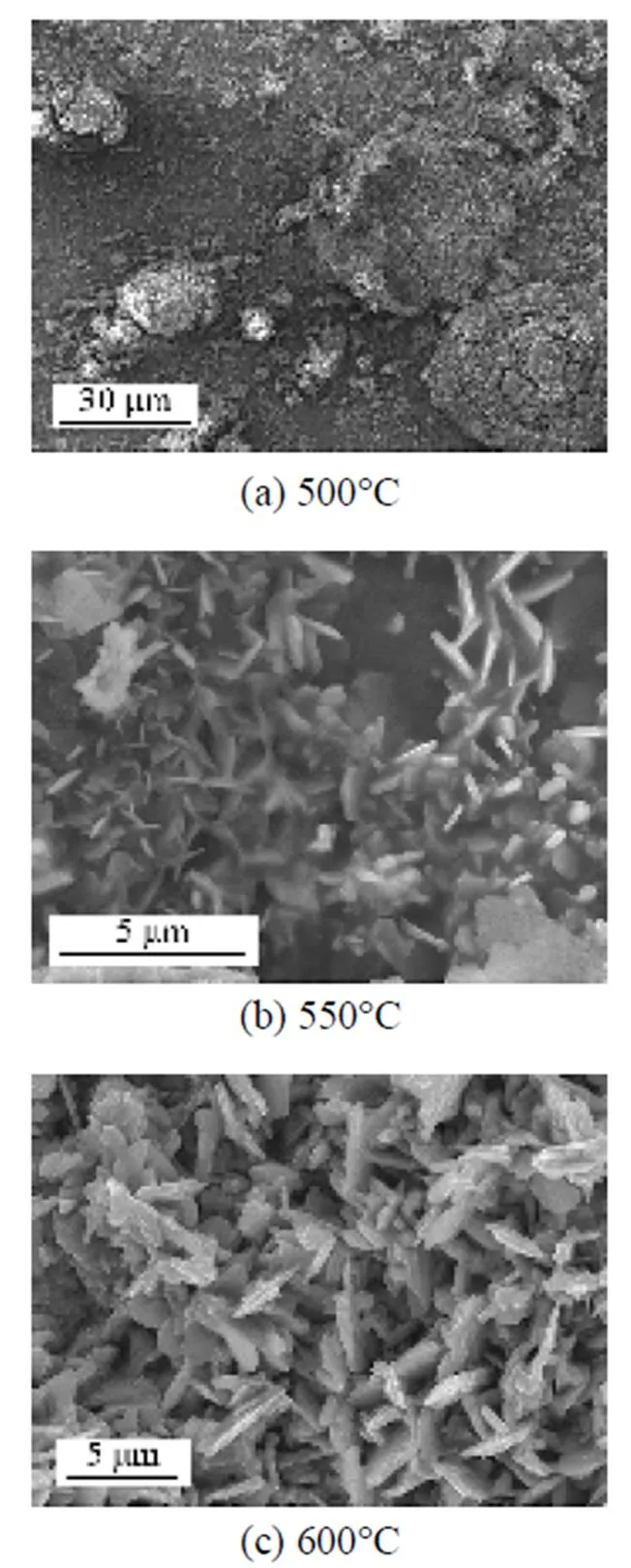
Figure 8 SEM micrographs of the corroded specimens in 6%O2-12%CO2-0.01%HCl-N2with ash deposition
Some green condensed chlorides were found in colder regions of the quartz tubes near the metal specimens. The outward diffusion of volatile chlorides through the oxide scale was confirmed. In addition, K2CrO4was detected by XRD at the surface of some specimens although the peak value was very low. Thus the corrosion acceleration by ash deposition can be attributable to the effect of KCl in the ash. Solid KCl reacts with metal oxide and releases chlorine [reaction (1)], which has better penetrability than oxygen in the oxide. Chlorine will reach the oxide/metal interface, react with metal and release volatile metal chlorides MCl2[reaction (2)], which will be oxidized [reaction (3)] during the diffusion outward to the atmosphere. The formation of oxide in the oxide scale destroys the integrity of scale, which causes easier diffusion of chlorine to the metal. A portion of chlorine diffuses inwardly and returns to the oxide/metal interface leading continuous corrosion. Only a small amount of chlorides evaporate into the gas flow and condense in colder regions of the furnace [21].


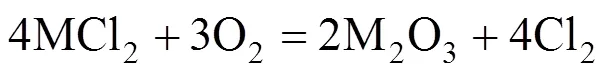
3.4 Effect of SO2 on corrosion by ash deposition
Figure 9 shows the corrosion dynamic curves in 6%O2-12%CO2-0.01%HCl-0.01% SO2-N2with about 20 mg biomass ash covered on the surface of the metal specimens. Compared with Fig. 7, the mass gain is evidently reduced in the presence of SO2. This result is in contradiction with that by Nielsen. [6] but in agreement with that by Grabke[13]. The corrosion dynamic curve at 600°C follows the parabolic kinetics. It indicates that the metal has acceptable properties of corrosion-resistance.
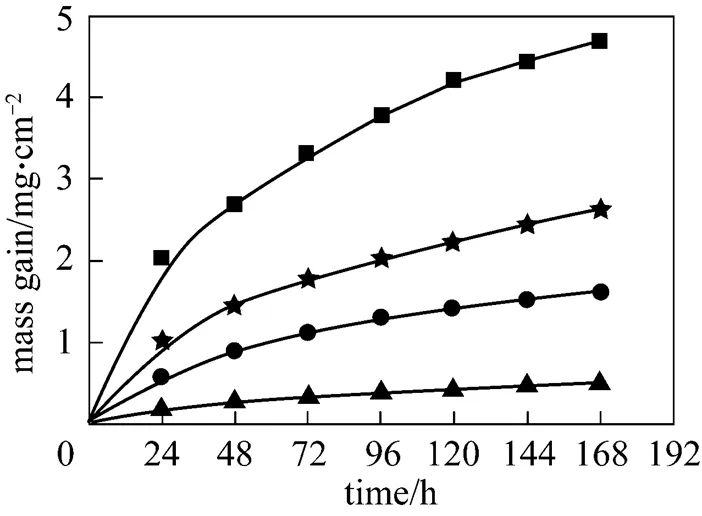
Figure 9 Corrosion results with ash deposition in 6%O2-12%CO2-0.01%HCl-0.01%SO2-N2
temperature/°C: ▲ 500;● 550;★ 575;■ 600
The effect of SO2can be explained with the conversion of KCl into K2SO4, which is less corrosive according to reaction (4).
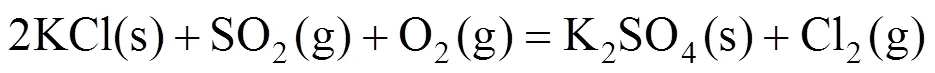
3.5 Discussion
According to the element analysis, the metal specimens react with oxygen and form oxide scale, so that the mass gain is from oxygen. The corrosion rate can be calculated by converting the mass gain into the amount of metal corrosion. Table 3 gives the corrosion rates with ash deposition at different temperatures. For sub-high temperature and pressure biomass boiler, the temperature of superheater is about 535°C. The corrosion rate is below 0.25 mm·a-1. Thus TP316L can be used as superheater tubes.
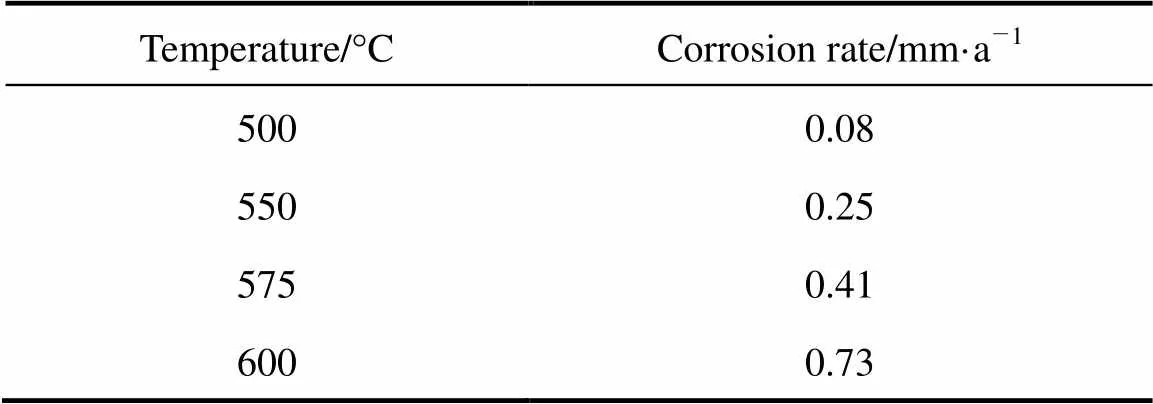
Table 3 Corrosion rate with ash deposition in 6%O2-12%CO2-0.01% HCl-0.01%SO2-N2
The corrosion is dependent on temperature and is greatly accelerated by ash deposition. In order to improve the efficiency of biomass boiler, the temperature of superheated steam should be raised. This will cause serious corrosion of superheater if the surface of the tube is covered with ash deposit. If ash blowing is regularly adopted for the superheater, the corrosion rate will be decreased and allowable.
The positive effect of SO2on corrosion control has been proved whether there is ash deposition or not. This provides another method to reduce the risk of high-temperature corrosion: co-firing. Co-firing of straw and coal will decrease the content of KCl in the ash, because sulfur from coal reacts with potassium from biomass forming potassium sulfate. The SO2emission will not cause environmental problem due to its low concentration.
4 CONCLUSIONS
Corrosion experiments were conducted for TP316L in oxidizing atmosphere containing 0.01% HCl in the temperature range of 500-600°C. The mass gain is strongly increased with temperature.
Corrosion is greatly accelerated by ash deposition because of KCl in the ash. KCl reacts with oxide scale directly and releases chlorine.
SO2has positive effect on controlling corrosion whether the surface of the metal is covered with ash deposition or not. Compact FeSO4scale forms on the surface of the metal and KCl is converted to K2SO4, which is less corrosive.
TP316L can be used as superheater tubes in sub- high temperature and pressure biomass boiler. Two feasible methods to avoid serious high-temperature corrosion in biomass boiler are ash blowing and co-firing.
1 Demirbaş, A., “Potential applications of renewable energy sources, biomass combustion problems in boiler power systems and combustion related environmental issues”,..., 31 (2), 171-192(2005).
2 Obernberger, I., Brunner, T., Bärnthaler, G., “Chemical properties of solid biofuels-significance and impact”,, 30 (11), 973-982 (2006).
3 Demirbaş, A., “Combustion characteristics of different biomass fuels”,..., 30 (2), 219-230 (2004).
4 Jenkins, B.M., Baxter, L.L., Miles, J.T.R., Miles, T.R., “Combustion properties of biomass”,.., 54 (1-3), 17-46 (1998).
5 Ma, X.Q., “The new development on the study of problems with alkali metals during straw combustion”,, 28 (12), 28-34, 63 (2006). (in Chinese)
6 Nielsen, H.P., Frandsen, F.J., Dam-Johansen, K., Baxter, L.L., “The implications of chlorine-associated corrosion on the operation of biomass-fired boilers”,..., 26 (3), 283-298 (2000).
7 Riedl, R., Dahl, J., Obernberger, I., Narodoslawsky, M., “Corrosion in fire tube boilers of biomass combustion plants”, In: Proceedings of the China International Corrosion Control Conference, Beijing, China (1999).
8 Abels, J.M., Strehblow, H.H., “A surface analytical approach to the high temperature chlorination behaviour of inconel 600 at 700°C”,.., 39 (1), 115-132 (1997).
9 Xu, Y., The Kinetics of Chemical Reaction, Chemical Industry Press, Beijing (2005). (in Chinese)
10 Nielsen, H.P., Frandsen, F., Dam-Johansen, K., Larson, O.H., “Deposition and high temperature corrosion in a 10 MW straw fired boiler”,.., 54 (1-3), 95-108 (1998).
11 Li, Y.S., Niu, Y., Wu, W.T., “Chlorination of metallic materials at high temperature”,...., 12 (1), 41-44 (2000). (in Chinese)
12 Li, Y.S., “High temperature oxidation and chlorination of metallic materials”, Ph. D. Thesis, Dalian Univ. of Technol., China (2001). (in Chinese)
13 Grabke, H.J., Reese, E., Spiegel, M., “The effects of chlorides, hydrogen chloride, and sulfur dioxide in the oxidation of steels below deposits”,.., 37 (7), 1023-1043 (1995).
14 Miltner, A., Beckmann, G., Friedl, A., “Preventing the chlorine-induced high temperature corrosion in power boilers without loss of electrical efficiency in steam cycles”,..., 26 (16), 2005-2011 (2006).
15 Aho, M., Ferrer, E., “Importance of coal ash composition in protecting the boiler against chlorine deposition during combustion of chlorine-rich biomass”,, 84 (2/3), 201-212 (2005).
16 Demirbaş, A., “Sustainable cofiring of biomass with coal”,.., 44 (9), 1465-1479 (2003).
17 Li, Y.S., Niu, Y., Liu, G., Wu, W.T., “High temperature corrosion of metallic materials in waste incineration environment”,..., 12 (4), 224-227 (2000). (in Chinese)
18 Pan, C.Y., “Study on the corrosion mechanism of superheater in MSW boiler”, Master Thesis, Zhejiang Univ., China (2004). (in Chinese)
19 Zhu, R.Z., He, Y.D., Qi, H.B., High Temperature Corrosion and Anti-corrosion Materials, Shanghai Science and Technology Press, Shanghai (1995). (in Chinese)
20 Zhu, R.Z., Corrosion of Meatals, Metallurgical Industry Press, Beijing (1989). (in Chinese)
21 Zahs, A., Spiegel, M., Grabke, H.J., “Chloridation and oxidation of iron, chromium, nickel and their alloys in chloridizing and oxidizing atmospheres at 400-700°C”,.., 42 (6), 1093-1122 (2000).
2009-01-04,
2009-04-16.
* To whom correspondence should be addressed. E-mail: yjm04@mails.tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2009年5期
Chinese Journal of Chemical Engineering2009年5期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular Simulation of CO2/H2 Mixture Separation in Metal-organic Frameworks: Effect of Catenation and Electrostatic Interactions*
- Deactivation Kinetics of Nitrile Hydratase in Free Resting Cells*
- Influence of A-type Zeolite on Methane Hydrate Formation*
- Effects of Sintering Atmosphere on the Microstructure and Surface Properties of Symmetric TiO2Membranes*
- Improvement of Isomerization Process of Crude Isoamylene with Tertiary-amyl-alcohol Addition
- Prediction of Thermophoretic Deposition Efficiency of Particles in a Laminar Gas Flow along a Concentric Annulus: A Comparison of Developing and Fully Developed Flows
