Effect of Synthesis Temperature on Structure and Ceramization Process of Polyaluminasilazanes
LI Song ZHANG Yue
(1Key Laboratory of Aerospace Materials and Performance,School of Materials Science and Engineering, Beihang University,Beijing 100191,China)
(2State Key Laboratory of Advanced Fibre Composites,Beijing Composite Materials CO.,Ltd.Beijing 102101,China)
Effect of Synthesis Temperature on Structure and Ceramization Process of Polyaluminasilazanes
LI Song1,2ZHANG Yue*,1
(1Key Laboratory of Aerospace Materials and Performance,School of Materials Science and Engineering, Beihang University,Beijing100191,China)
(2State Key Laboratory of Advanced Fibre Composites,Beijing Composite Materials CO.,Ltd.Beijing102101,China)
Polyaluminasilazanes with nominal Si/Al molar ratio of 3 were synthesized by reaction of poly (methylvinyl)silazane with aluminum isopropoxide at 90,100 and 120℃,respectively.The structures of the precursors were characterized by FTIR and NMR.The results indicate that,with the synthesis temperature increasing,the intensities of Al-N FTIR vibration peak (1 450 cm-1)and AlON2(8 ppm)and AlO2N (-1 ppm) groups of27Al NMR increase.The higher the reaction temperature is,the more the Al-N bonds are.The dehydrocoupling process may be a three-stage reaction process and the structure of polyaluminasilazane synthesized at 120℃ is the most complicated one due to the highest synthesis temperature.The pyrolysis processes of the precursors were studied by TG/DTA,FTIR and gas chromatography (GC).The synthesis temperatures have no significant effect on ceramization process and ceramic yield.Based on DTA curve,a further crosslink takes place at 475℃ during pyrolysis process.Besides,the released gases during pyrolysis process are identified by GC analysis as oligosilazane,CH4,C2H4,H2and NH3.According to the XRD and SEM results,the product pyrolyzed at 1200℃under nitrogen atmosphere is a homogenous amorphous phase.
synthesis;polyaluminasilazane;structure;thermolysis
The pyrolysis of precursors is an interesting approach to the fabrication of near-net shape products with high purity and homogeneity[1-3].In addition,the process offers a promising route for preparation of ceramic and fiber-reinforced ceramic matrix composites with desirable shapes,as well as fibers and coatings[4-6]. Many precursor-derived ceramics are used in hightemperature oxidization environments,thus property of the oxidation resistance is of importance.Due to their excellent oxidation resistance[7-11],high hot-corrosion resistance[8,10],as well as good thermal stability against crystallization[12],polyaluminasilazanes-derived SiAlCN ceramics are extremely attractive.
The polyaluminasilazanes(PASZs)were generally synthesized via reaction of silazanes with organo aluminum compounds.Seyferth et al.[12-13]prepared PASZs from reaction of oligosilazanes with various amounts of trimethylalumium or triethylaluminum or dimethylaluminum amide.In other literatures[14-15],the aluminasilazane precursor was formed via reaction between trimethylaluminum and hexamethyldisilazane at room temperature.In addition,aluminasilazanes can also be fabricated by reaction of aluminum isopropoxide[8-11]orotherorganoaluminumcompounds[16-20]or aluminum hydride[7,21-23]with silazanes.Among of those organoaluminum compounds,aluminum isopropoxide is the cheapest and the most stable one in air.
The SiAlCN ceramics own much better oxidation resistance with aluminum content increasing[9]. However,An and co-workers[7-11]merely prepared PASZs with low aluminum content(nSi∶nAl≥25∶3)via polycondensation reaction between polysilazane and aluminum isopropoxide.To the best of our knowledge, the PASZs with high aluminum content have not been synthesized via reaction of aluminum isopropoxide with silazane. And the relations among synthesis temperature,structureofPASZ and ceramization process remain to be clarified.So the present work is mainly involved in the preparation of PASZ with high aluminum content(nSi∶nAl=3∶1)via reaction of stable and low-cost aluminum isopropoxide with polysilazane,and studies on relations among synthesis temperature, structure and ceramization process of PASZ.The PASZ should own better oxidation resistance than those with low aluminum content.
Here we report PASZs with high aluminum content (nSi∶nAl=3∶1)prepared via reaction between aluminum isopropoxide and a oligosilazane polymer by ammonolysis of dichloromethylvinylsilane at different temperatures.The structure and ceramization process of PASZsarecharacterizedbyNMR,IR,TG/DTAandGC.
1 Experimental
1.1 Synthesis and pyrolysis of PASZs
Since chlorosilanes,silazanes and aluminasilazane are sensitive to moisture and air,the synthesis of PASZs was performed in protective nitrogen atmosphere to avoid from impurities.
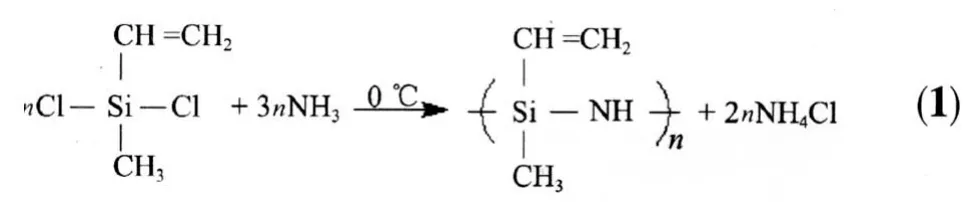
Ammonia(NH3)was dried by passing it through a column filled with pellets of anhydrous potassium hydroxide (KOH).The oligosilazane polymer was obtained by ammonolysis reaction of commercial dichloromethylvinylsilane(CH3Si(CHCH2)Cl2)(Alfa Aesar) dissolved in toluene with the pure NH3at 0℃.Eq.(1) shows the ammonolysis reaction[24].
The byproduct of ammonolysis, ammonium chloride,was removed through vacuum filter.According to Eq.(2)[25],the PASZ with nSi/nAlstoichiometric ratio of 3 could been obtained via dehydrocoupling reaction between commercial aluminum isopropoxide (AIP,Al (OCH(CH3)2)3)(Sinopharm Chemical Reagent Co.,Ltd.) and the polysilazane obtained from Eq.(1).After the mixture of the AIP and the polysilazane was stirred at 90,100 and 120℃ for 4 h,the pale yellow liquid PASZ1,PASZ-2 and PASZ-3 wereobtained by removing the solvent under reduced pressure, respectively.
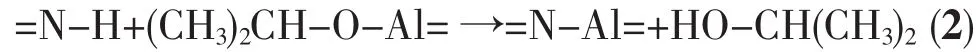
Then,the liquid precursor was cured at 120℃under nitrogen atmosphere to obtain powder product. Accordingly,the powder product was pressed into disks under 140 MPa at ambient temperature.Subsequently, the disks were placed in a corundum crucible in a corundum tubefurnace filled with nitrogen and thermolyzed at different temperatures for 2 h under flowing nitrogen (80 mL·min-1).The heating and cooling rate was 3℃·min-1.
1.2 Instrumentation
Fourier transform Infra-red (FTIR)spectra were recorded on a Thermo Nicolet NEXUS-470 FTIR spectrometer using KBr pellets at room temperature.Nuclear magnetic resonance (NMR)analysis of the precursors was carried out on a BRUKER AVANCEⅢ-400 spectrometer using a 4 mm magic angle spinning (MAS)probe.29Si,27Al,13C,1H NMR experiments were performed at 79.30,104.01,100.37 and 399.16 MHz,respectively.27Al and13C chemical shifts were respectively referenced to hydrated Al ion Al(H2O)63+(1 mol·L-1aluminum nitrate)and adamantine as external standards.
Thermolysis behavior was investigated by coupled thermogravimetry/differentialthermalanalysis(TG/ DTA)using a Beijing Hengjiu HCT-2 under nitrogen flow (30 mL·min-1)with heating rate of 10℃·min-1up to 1 000℃.The evolved gases during pyrolysis process were identified by gas chromatography (GC)using an Agilent 6890N(N2carrier gas of 30 mL·min-1,splitless injection at 25℃,stainless steel TDX-01 column of 2 mm×2 m in column oven at 80℃,thermal conductivity detector at 180℃).Ammonia among the pyrolysis gas was identified by visible spectrophotometer using 722S visible spectrophotometer.The residues of thermolysis at different temperatures were analyzed by FTIR and xray diffraction (XRD)using a D/max-2200PC (monochromated Cu Kα radiation,λ=0.154 184 nm,at 40 kV and 40 mA,10°~80°,SC-70 scintillation counter).The microstructure and elemental content of the pyrolyzed residues were investigated by using Cam Scan 3400 backscattered electron imaging of scanning electron microscopy (BEI-SEM)(20kV acceleration voltage)with an OXFORD INCAPentaFETX3 energy dispersive spectrometer(EDS).
2 Results and discussion
2.1 Characterization of precursors
The ammonolysis product and PASZs(nominal Si/ Al molar ratio of 3)were characterized by FTIR.Fig.1 demonstrates the FTIR spectra for (a)ammonolysis product and polyaluminasilazanes of(b)PASZ-1,(c) PASZ-2 and(d)PASZ-3.Compared to the spectrum of (a),the relative intensities of the N-H and Si-NH-Si absorption bands at about 3 400 and 959 cm-1decrease in Fig.1(b),1(c)and 1(d).The absorption peak at 1 450 cm-1is characteristic band of Al-N bond[26].These indicate that the reaction (Eq.(2))occurs during the synthesis process and aluminum atom is introduced into the backbone of the polysilazane.
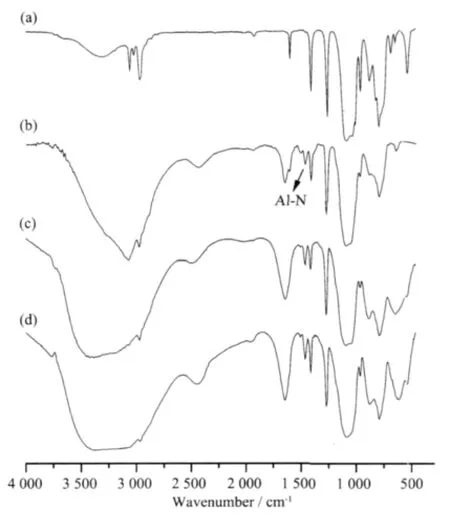
Fig.1 FTIR spectra of(a)ammonolysis product and polyaluminasilazanes of(b)PASZ-1, (c)PASZ-2,(d)PASZ-3
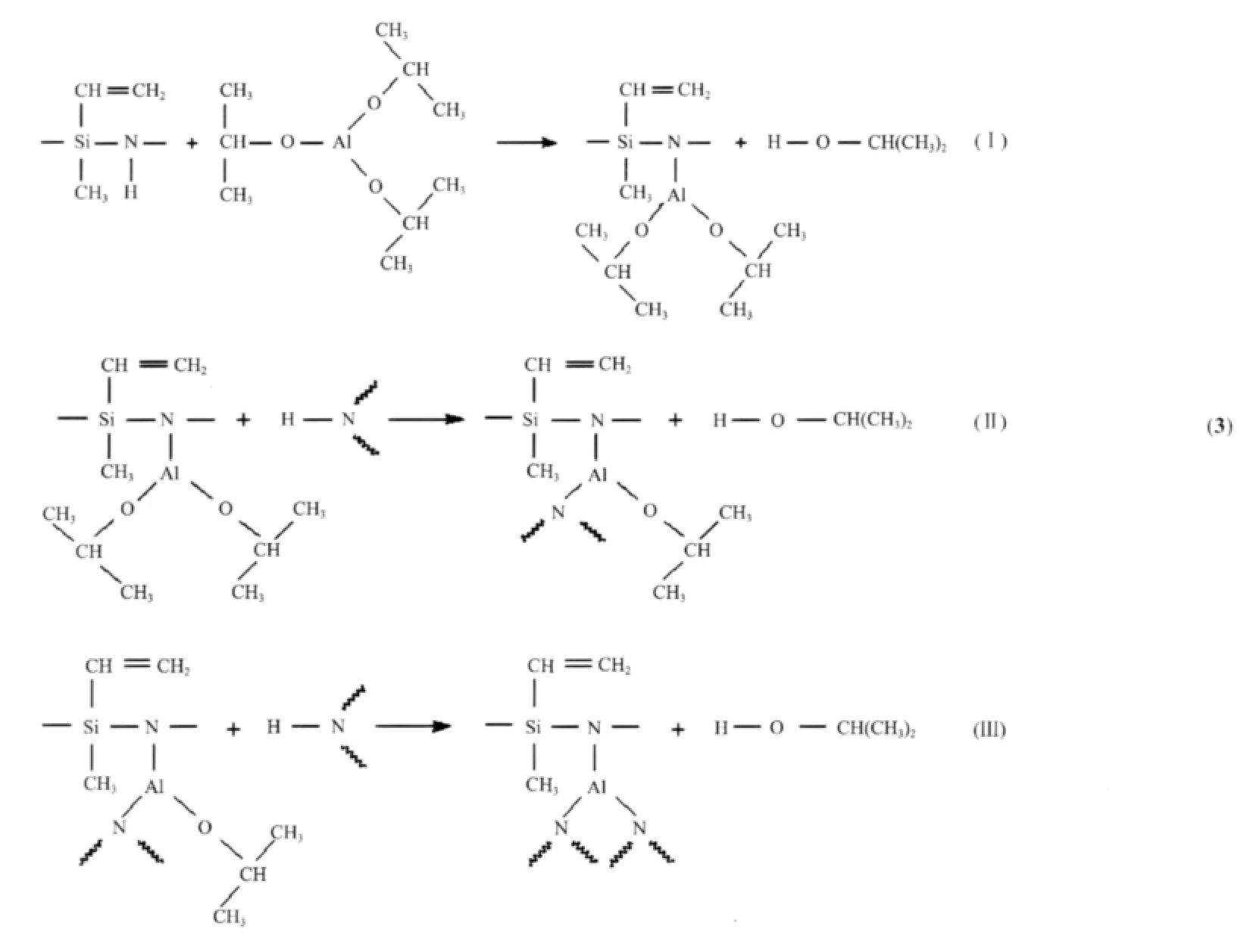
With the increase of synthesis temperature,the relative intensities of the CH=CH2bond peak at 1 600 cm-1,Si-CH3groups at 1 260 cm-1,H-C(methyl) bending vibration at 1 400 cm-1and methyl vibration at 2 960 cm-1gradually increase.The reason for this needs a further study.However,the intensity of Al-N groups at 1 450 cm-1increases with increasing synthesis temperature,because the reaction (Eq.(2))could be progressed more completely at higher synthesis temperature.We propose that the dehydrocoupling reaction is a three-stage reaction(Eq.(3))includingⅠ-stage(formation of one Al-N bond),Ⅱ-stage(formation of two Al-N bond)andⅢ-stage(formation of three Al-N bond).While the synthesis process at high temperature can be more likely to occur in theⅡ-stage andⅢ-stage reactions than that at lower temperature.So,the PASZ-3 synthesized at 120℃can possess more complicated network structure than the PASZ-1 and the PASZ-2 prepared at relatively low temperature.
Above-mentioned FTIR results indicate that aluminum is introduced into the backbone of the polysilazane.Due to the formation of complicated network structure via the Ⅱ-stage and Ⅲ-stage dehydrocoupling reaction, the higher reaction temperature,the stronger intensity of the characteristic peak of Al-N bond.Besides,the FTIR spectra of(b),(c) and (d)are similar to the spectrum of aluminum isopropoxide (AIP)at 3 000~3 600 cm-1.It is possible that the dehydrocoupling reaction occurs partially.Thus,there are still partial groups of AIP remained within the PASZ synthesized at different temperatures (including N2Al(OCH(CH3)2)and NAl(OCH(CH3)2)2).
Fig.2 shows the29Si NMR spectra of(a)PASZ-1, (b)PASZ-2 and(c)PASZ-3.29Si NMR signals at-21.7 ppm,-34.2 ppm and-65.7 ppm are ascribed to the SiC2O2,SiC2ON and SiC2N2,respectively.With the reaction temperature increasing,theⅡ-stage andⅢ-stage dehydrocoupling reaction (Eq.(3))occurs more easily.Therefore,the signals of SiC2N2(Al)(-56.2 ppm) and SiC2N2(Al2) (-45.5 ppm)is the strongest in spectrum (c)compared to the other two spectra.There are no significant variations for other signals(-21.7 ppm,-34.2 ppm,-65.7 ppm)in the three spectra.
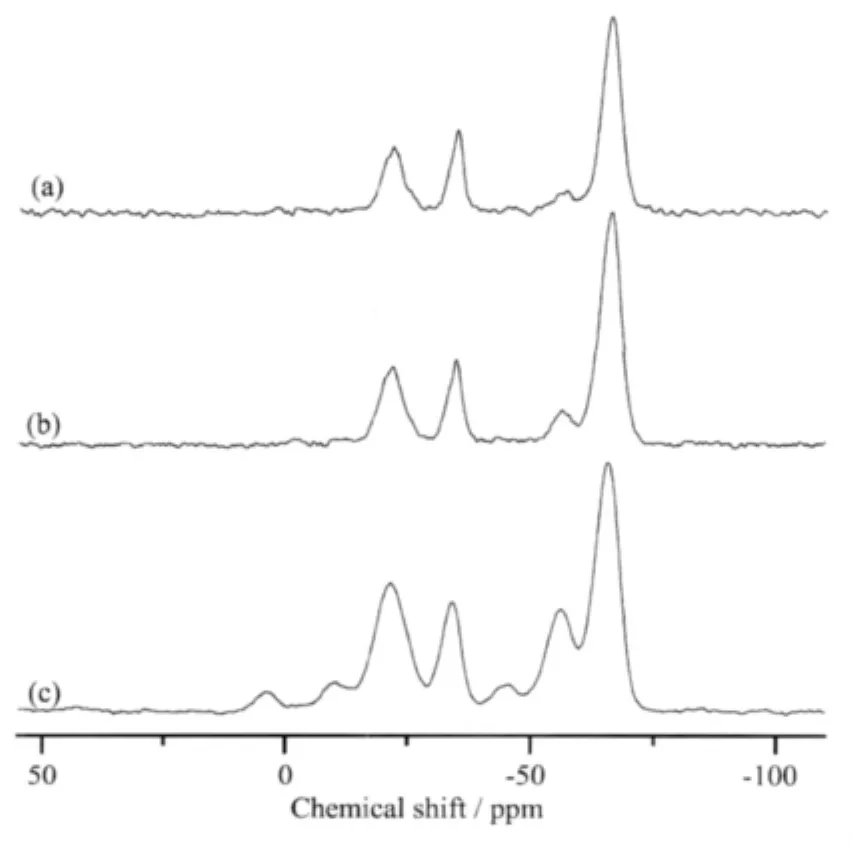
Fig.2 29Si NMR spectra of(a)PASZ-1,(b)PASZ-2, (c)PASZ-3
The27Al NMR spectra of(a)PASZ-1,(b)PASZ-2 and (c)PASZ-3 are shown in Fig.3.There is only one broad peak ascribed to the overlapping of AlON2(8 ppm)and AlO2N(-1 ppm)units in the range of 15 ppm~20 ppm.The area of the signal in Fig.3(C)is obviously larger than that in the other two spectra, suggesting that PASZ-3 owns the largest number of Al-N unit among the three PASZs.The intensity of AlON2unit increases slowly with the synthesis temperature increasing.It suggests that the effect of synthesis temperature is not significant on formation of AlON2unit.In contrast,the area of the peak corresponding to AlO2N environmentincreasesobviously with the synthesis temperature increasing.This result suggests that the formation of AlO2N is easier than that of AlON2unit with the synthesis temperature increasing.It is evident that PASZ-3 owns more AlN and AlN2groups as a result of easier formation of Al-N bond in theⅠ-stage andⅡ-stage dehydrocoupling reaction at high reaction temperature.
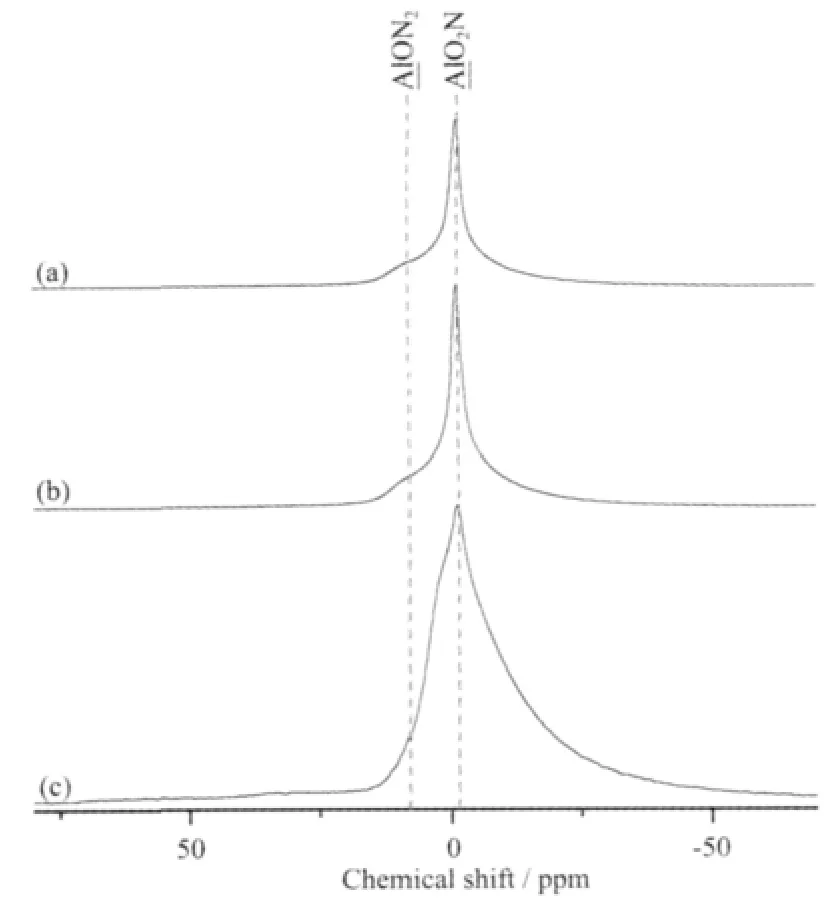
Fig.3 27Al NMR spectra of(a)PASZ-1,(b)PASZ-2, (c)PASZ-3
Fig.4 shows the13C NMR spectra of(a)PASZ-1,(b) PASZ-2 and(c)PASZ-3.13C NMR signals at 136.3 ppm, 28.8 ppm,23.1 ppm and 1.2 ppm are ascribed to the Si-CH=CH2,OCH(CH3)2,OCH(CH3)2and SiCH3groups, respectively.Because of easy occurrence of the I-stage andⅡ-stage dehydrocoupling reaction at 120℃,the intensities of OCH(CH3)2(28.8 ppm)and OCH(CH3)2(23.1 ppm)signals decrease in Fig.4(c).Whereas,there are unreacted Al-OCH(CH3)2groups remaining in the structure of PASZ-3 precursor.

Fig.4 13C NMR spectra of(a)PASZ-1,(b)PASZ-2, (c)PASZ-3
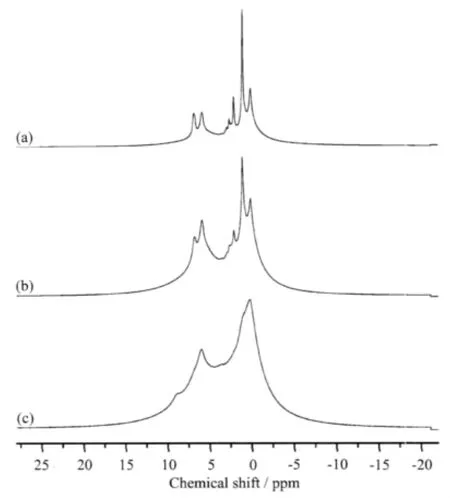
Fig.5 1H NMR spectra of(a)PASZ-1,(b)PASZ-2, (c)PASZ-3
The1H NMR spectra of(a)PASZ-1,(b)PASZ-2, (c)PASZ-3 are shown in Fig.5.The resonances at 6.7 ppm and 5.9 ppm are attributed to the-CH=CH2and -CH =CH2groups, respectively. As synthesis temperature increasing,the two signals intensify and combine lastly into one broad signal in Fig.5(c).1H NMR signals at 2.1 ppm,1.1 ppm and 0.1 ppm are ascribed to the OCH(CH3)2,OCH(CH3)2and SiCH3groups,respectively.The intensities of the signals corresponding to OCH(CH3)2(1.1 ppm)and OCH(CH3)2(2.1 ppm)unit decrease gradually.While the intensity of the signal corresponding to SiCH3(0.1 ppm)unit increases.The three signals combine into one signal in Fig.5(c).These also can be attributed to the more complicated network structure of PASZ-3 in contrast with PASZ-1 and PASZ-2.
Above-mentioned variationsofintensitiesfor NMR signals indicate that the reaction (Eq.(2)) process is affected by synthesis temperature.The dehydrocoupling reaction is a three-stage reaction, with increase in the synthesis temperature,theⅠ-stage andⅡ-stage(Eq.(3))reactions occur more easily. The reaction (Eq.(2))at 120℃ is more completed as compared to those at other temperature,however,even at 120℃ the occurrence of theⅢ-stage reaction is rare.Thus,there are still many unreacted Al-OCH (CH3)2groups in PASZ-3.
2.2 Ceramization of PASZs
Fig.6 shows TG/DTA curves for PASZs up to 1 000℃.The ceramic yield increases with reaction temperature arising,and the mass fraction remaining for PASZ-3(68%)is higher than that of PASZ-1(65%)and PASZ-2(66%).This result can be explained by the more complicated network structure of PASZ-3 due to the higher synthesis temperature.
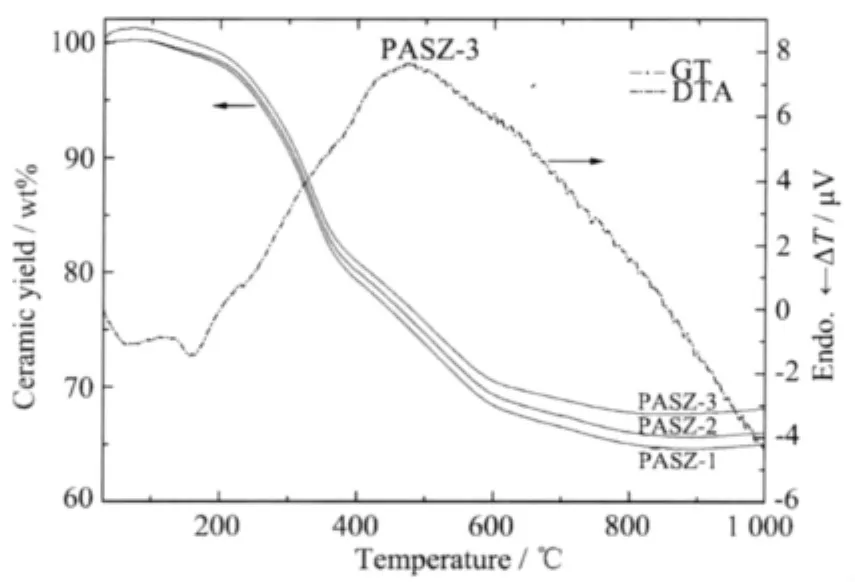
Fig.6 TG/DTA curves of PASZs
The small endothermic peak at 160℃ might be owing to solvent and oligopolymer volatilizing.The mass loss is merely 1%up to 200℃in the TG curve.There is a significant broad exothermic peak at 475 ℃ because of further crosslinking and releasing of CH4,H2and NH3identified by GC analysis.A large mass loss is present at around 200℃(99%)up to 600℃(71%),and there is only 3%mass loss from 600 to 800℃(68%)at which conversion ofprecursor into ceramic is essentially completed.Allbond formations and decompositions in the structure are completed at 800℃as shown in Fig.6.
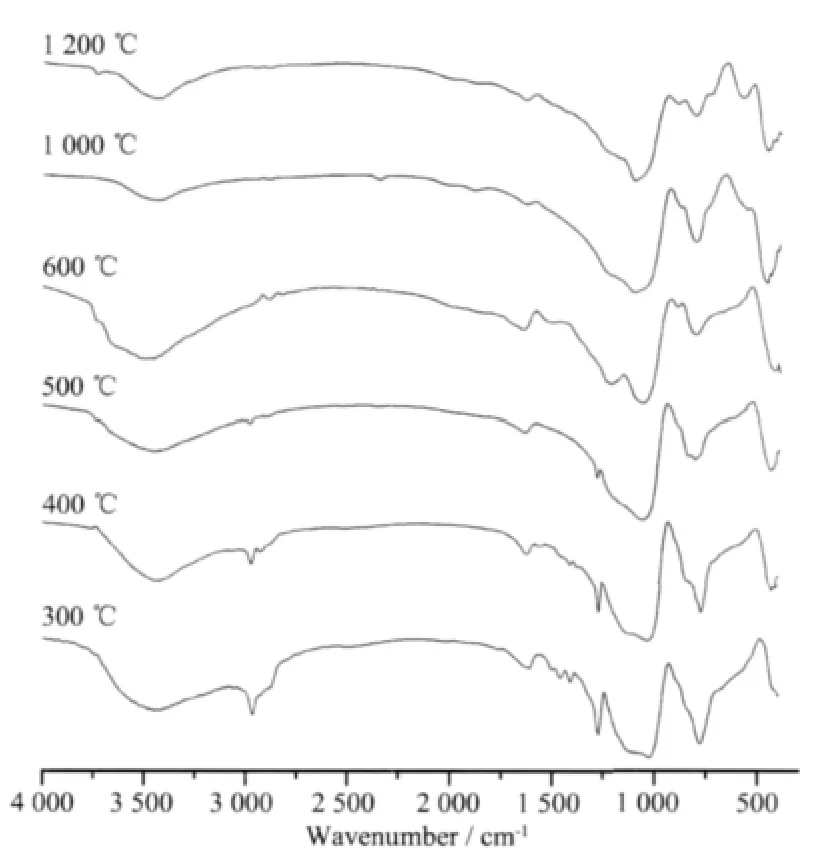
Fig.7 FTIR spectra of PASZ-3 at various stage of the thermolysis process
The thermolysis process of PASZ-3 with the highest ceramic yield will be mainly discussed.Fig.7 demonstrates the FTIR spectra of PASZ-3 pyrolyzed at different temperatures.From 300 to 400℃,the C-H vibration (3 050 cm-1)of vinyl almost vanishes and-CH=CH2(1 600 cm-1)absorption peak obviously diminishes compared to FTIR spectrum of PASZ-3. According to GC,the gases captured from 300 to 400℃include CH4and C2H4.Thus,the results suggest that the following reactions(Eq.(4),(5)and(6))may occur from 300 to 400℃[27].
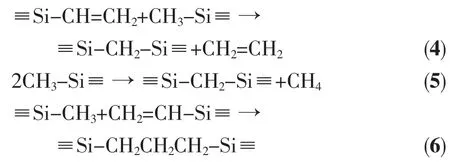
At 600℃,the FTIR spectrum is similar to that obtained at 500℃.The vibration peaks for C-H (2950 cm-1)and Si-CH3(1 250 cm-1)disappear and gases including H2,CH4and NH3are identified by GC.From 400 to 600℃,the reaction (Eq.(5))still occurs and other possible reactions associated with the evolution of hydrogen and ammonia can be expressed by Eq.(7)to (9)[26-27].

The thermolysis process essentially completes up to 800℃as shown in Fig.6,and there are no variations between FTIR spectrum at 1 000℃and at 1 200℃(in Fig.7).As shown in Fig.8,the product of pyrolysis is still amorphous phase up to 1 200℃.

Fig.8 XRD patterns of the products pyrolyzed at 1 000 and 1 200℃

Fig.9 BEI-SEM image showing the cross-section of PASZ-3 disk pyrolyzed at 1 200℃
Fig.9 shows the BEI-SEM image of the crosssection of PASZ-3 disk pyrolyzed at 1 200℃ for 2 h under nitrogen atmosphere.These residual pores could be induced by the escape of gaseous species formed during thermolysis process without auxiliary pressure.It is evident that the PASZ-3 derived ceramic is a homogenously amorphous glass for no differences in contrastfeatures.The semi-quantitative elemental analysis of the residue of PASZ-3 pyrolyzed at 1 200℃was estimated by EDS spectra,while the result is shown in Table 1.The oxygen content of the residue is determined by those unreacted Al-O groups of AIP.The O/Al molar ratio of the residue pyrolyzed at 1 200℃is 1.70.Based on the oxygen content,about 43%Al-O units in AIP occur dehydrocoupling reaction with N-H in poly(methylvinyl)silazane.The Si/Al molar ratio of the residue pyrolyzed at 1 200℃is slightly lower than that of PASZ-3.It can be proposed that silicon is lost by the gaseous oliogsilanes during pyrolysis process.

Table 1 Composition of the residue of PASZ-3 pyrolyzed at 1 200℃
3 Conclusions
The FTIR vibration at 1 450 cm-1is ascribed to characteristic Al-N absorption band,and27Al NMR signals at 8 ppm and-1 ppm are attributed to the AlON2and AlO2N,respectively.According to the results of NMR and FTIR,PASZ (nominal Si/Al molar ratio of 3) can be synthesized via polycondensation between AIP and poly(methylvinyl)silazane at 90,100 and 120℃. The synthesis process is a three-stage dehydrocoupling reaction process. With increase in synthesis temperature,theⅠ-stage andⅡ-stage reactions occur more easily.Thus,the intensities of Al-N FTIR vibration peak and AlON2environment NMR signal of PASZ-3 are the strongest.At the same time,there are unreacted Al-O groups in PASZ precursor.The ceramic yields of PASZ-1,PASZ-2 and PASZ-3 as measured by TG are 65%,66%and 68%,respectively.The synthesis temperatures have no significant effect on ceramization process and ceramic yield.Due to the most complicated network structure of PASZ-3,the yield of it is the highest.Based on the GC result and DTA curve,further crosslinking reactions take place at 475℃.Because of releasing oligosilazane and other gases during pyrolysis process,the Si/Al molar ratio decreases and the residue of PASZ-3 pyrolyzed at 1 200℃ is amorphous phase. The further research about polyaluminasilazane with different Si/Al molar ratios will be focused on how to decrease oxygen content of PAS and increase ceramic yield.
Acknowledgements:The authors gratefully thank the support of Beijing Composite Materials CO.,Ltd.,Specialized Research Fund for the Doctoral Program of Higher Education(No. 20091102110002)from Chinese Ministry of Education and National natural Science Foundation of China(No.51072010).
[1]Greil P,Seibold M.J.Mater.Sci.,1992,27(9):1053-1060
[2]Greil P.J.Eur.Ceram.Soc.,2008,18(13):1905-1914
[3]Jamet J,Spann J R,Rice R W,et al.Ceram.Eng.Sci.Proc., 1984,5(728):677-694
[4]Ziegler G,Kleebe H J,Motz G,et al.Mater.Chem.Phys., 1999,61(1):55-63
[5]Shah S R,Raj R.Acta Mater.,2002,50(16):4093-4103
[6]Sarkar S,Chunder A,Fei W,et al.J.Am.Ceram.Soc.,2008, 91(8):2751-2755
[7]Mller A,Gerstel P,Butchereit E,et al.J.Eur.Ceram.Soc., 2004,24(12):3409-3417
[8]An L,Wang Y,Bharadwaj L,et al.Adv.Eng.Mater.,2004, 5(6):337-340
[9]Wang Y,An L.J.Am.Ceram.Soc.,2005,88(11):3075-3080
[10]Wang Y,Fei W,An L.J.Am.Ceram.Soc.,2006,89(3): 1079-1082
[11]Wang Y,Fan Yi,Zhang L,et al.Scripta Mater.,2006,55(4), 295-297
[12]Boury B,Seyferth D.Appl.Organomet.Chem.,1999,13(6): 431-440
[13]Seyferth D,Brodt G,Boury B.J.Mater.Sci.Lett.,1996,15 (4):348-349
[14]Salles V,Foucaud S,Laborde E,et al.J.Eur.Ceram.Soc., 2007,27(1):357-366
[15]Salles V,Foucaud S,Goursat P,et al.J.Eur.Ceram.Soc., 2008,28(6):1259-1266
[16]Koyama S,Takeda H,Saito Y,et al.J.Mater.Chem.,1996, 6(6):1055-1058
[17]Koyama S,Nakashima H,Sugahara Y,et al.Chem.Lett., 1998,27(2):191-192
[18]Nakashima H,Koyama S,Kuroda K,et al.J.Am.Ceram. Soc.,2002,85(1):59-64
[19]Mori Y,Ueda T,Kitaoka S,et al.J.Ceram.Soc.Jpn., 2006,114(6):497-501
[20]Mori Y,Sugahara Y.Appl.Organomet.Chem.,2006,20(8): 527-534
[21]Fooken U,Khan M A,Wehmschulte R J.Inorg.Chem., 2001,40(6):1316-1322
[22]Berger F,Weinmann M,Aldinger F,et al.Chem.Mater., 2004,16(5):919-929
[23]Toyoda R,Kitaoka S,Sugahara Y.J.Eur.Ceram.Soc., 2008,28(1):271-277
[24]kricheldorf H R,Burger C,Hertler W R,et al.Silicon in Polymer Synthesis.Berlin Heidelberg:Springer-Verlag,1996: 271.
[25]Dhamne A,Xu W,Fookes B,et al.J.Am.Ceram.Soc., 2005,88(9):2415-2419
[26]Chu Z Y,Feng C X,Song Y C,et al.Chinese J.Chem., 2003,21(7):975-978
[27]Li Y,Kroke E,riedel R,et al.Appl.Organomet.Chem., 2001,15(10):820-32
合成温度对聚铝硅氮烷的结构和陶瓷化过程的影响
李 松1,2张 跃*,1
(1空天材料与服役教育部重点实验室,北京航空航天大学材料科学与工程学院,北京 100191)
(2特种纤维复合材料国家重点实验室,北京玻钢院复合材料有限公司,北京 102101)
采用聚甲基乙烯基硅氮烷与异丙醇铝在90、100和120℃下反应分别合成出Si/Al物质的量的比为3的聚铝硅氮烷。利用红外(FTIR)和核磁(NMR)对前驱体结构进行表征。结果表明:Al-N键的红外振动强度(1 450 cm-1)和核磁铝谱中的AlON2(8 ppm)及AlO2N(-1 ppm)基团的强度随合成温度的增加而增加。反应温度越高,形成的Al-N键就越多。这个脱氢耦合合成过程可能是一个三级反应过程,而在最高温120℃下所合成的聚铝硅氮烷的结构最复杂。前驱体的裂解过程通过耦合热重/差热分析(TG/DTA)、FTIR和气相色谱(GC)进行研究。合成温度对陶瓷化过程和陶瓷产率并没有明显的影响。根据DTA曲线可知, 475℃发生进一步的交联。另外,GC数据表明裂解时所释放的气体为低分子量硅氮烷、CH4、C2H4、H2和NH3。根据XRD和SEM可知,1200℃裂解后产物为均匀的非晶相。
合成;聚铝硅氮烷;结构;热解
O614.3+1;TQ174.1
A
1001-4861(2011)05-0943-08
2010-11-15。收修改稿日期:2010-12-17。
国家自然科学基金(No.51072010)和教育部高校博士点专项科研基金(No.20091102110002)资助项目。*
。E-mail:zhangy@buaa.edu.cn;Tel/Fax:+86 10 82316976;会员登记号:E410400016M。
- 无机化学学报的其它文章
- Influence of Carbon Nanotube Content on Microstructures and Mechanical Properties of Carbon/Carbon Composite
- Hydrothermal Syntheses and Crystal Structures of Two Metal Complexes with Mixed 1,2,4-Benzenetricarboxylic Acid and 1,3,5-Tris(1-imidazolyl)benzene Ligands
- Hydrothermal Syntheses,Crystal Structures and Luminescent Properties of Binuclear Zn(Ⅱ)and Co(Ⅱ)Complexes Assembled by 4-Carboxymethoxy Phenylacetic Acid
- Thermal Stability and Antibacterial Activity of Phosphonium Salts Pillared Layered α-Zirconium Phosphates
- Synthesis of Porous Hydromagnesite Microspheres with Rosette-Like Morphology
- Syntheses,Crystal Structures and Electrochemical Properties of Cobalt and Nickel Complexes Containing Imidazole Ligand

