氯化硝基四氮唑蓝对钢在盐酸溶液中的缓蚀作用
李向红 邓书端 付 惠
(1西南林业大学理学院,昆明650224; 2西南林业大学材料工程学院,昆明650224)
氯化硝基四氮唑蓝对钢在盐酸溶液中的缓蚀作用
李向红1,*邓书端2付 惠1
(1西南林业大学理学院,昆明650224;2西南林业大学材料工程学院,昆明650224)
采用失重法、动电位极化曲线、电化学阻抗谱(EIS)和扫描电子显微镜(SEM)研究了氯化硝基四氮唑蓝(NTBC)在1.0-5.0 mol·L-1HCl溶液中对冷轧钢(CRS)的缓蚀作用.结果表明:NTBC在1.0 mol·L-1HCl溶液中对冷轧钢具有良好的缓蚀作用,且在钢表面的吸附符合Langmuir吸附等温式.缓蚀率随缓蚀剂浓度的增加而增大,但随盐酸浓度和温度的增加而减小.求出了相应的吸附热力学(吸附自由能ΔG0,吸附焓ΔH0,吸附熵ΔS0)和腐蚀动力学参数(腐蚀速率常数k,动力学常数B),并根据这些参数讨论了缓蚀作用机理.动电位极化曲线表明:NTBC为混合抑制型缓蚀剂;EIS谱在高频区呈容抗弧,在低频区出现感抗弧,电荷转移电阻随缓蚀剂浓度的增加而增大.SEM再次表明NTBC对钢在盐酸介质中的腐蚀产生了明显的抑制作用.
缓蚀剂;氯化硝基四氮唑蓝;钢;盐酸;吸附
1 Introduction
Using inhibitors is one of the most practical methods for protection against corrosion,especially in acidic media.1It is well known that N-heterocyclic compounds are considered to be the most effective corrosion inhibitors on steel in acid media.2N-heterocyclic compound inhibitors act by adsorption on the metal surface,and the adsorption of N-heterocyclic inhibitor takes place through nitrogen heteroatom,as well as those with triple or conjugated double bonds or aromatic rings in their molecular structures.Generally,inhibition efficiency of N-heterocyclic organic inhibitor always increases with the number of aromatic systems and the availability of electronegative atoms in the molecule.3
Tetrazole derivatives whose molecules possess many aromatic systems and electronegative N atoms have also been received some attentions.In 1996,Kertit and Hammouti4studied the inhibition of 1-phenyl-5-mercapto-1,2,3,4-tetrazole(PMT) on the corrosion of iron in 1.0 mol·L-1HCl,and its maximum inhibition efficiency(E)was 98%at 2 mmol·L-1.Bensajjay et al.5extended their work to study the inhibition effect of PMT on steel in 0.5 mol·L-1H2SO4and 1/3 mol·L-1H3PO4.Results obtained showed that E reached an optimum value of 98%at 10-3mol·L-1PMT.Afterwards,Morales-Gil et al.6reported the inhibition effect of 5-mercapto-1-tetrazoleacetic sodium salt (MTAc)on steel in 1.0 mol·L-1H2SO4.Their results showed that E was about 69%at 200 mg·L-1MTAC,while then decreased with the MTAc concentrations.Noticeably,these tetrazole compounds studied contain only one tetrazole ring,the literature available to date about the corrosion inhibition of the tetrazole compound which simultaneously possesses two tetrazole rings is very scarce.
In our laboratory,much work has been conducted to study the corrosion inhibition of tetrazole derivatives on the corrosion of steel in strong acidic media.In our recent works,7-9the inhibition effect of red tetrazolium(RT),which contained only one tetrazole ring,on the corrosion of cold rolled steel(CRS) in HCl,H2SO4,and H3PO4solutions was investigated.The results showed that maximum E values of 500 mg·L-1RT at20°C were 94%in 1.0 mol·L-1HCl solution,779%in 1.0 mol·L-1H2SO4solution,8and 63%in 3.0 mol·L-1H3PO4solution.9With respect to the tetrazole derivative containing two tetrazole rings,we investigated the inhibition effect of blue tetrazolium (BT)on steel in 1.0 mol·L-1HCl solution,10and the maximum E was 94%(BT)with 0.1 mmol·L-1BT.In addition,BT exhibited better inhibition performance than the traditional tetrazole compound with only one tetrazole ring,which could be attributed to the simultaneous adsorption of the two tetrazole rings inducing greater adsorption of the inhibitor molecules onto the steel surface.10Nitrotetrazolium blue chloride(NTBC)was the tetrazole compound containing two tetrazole rings,which could also be seemed as a good potential inhibitor.However,to the best of our knowledge,NTBC has not been treated as a corrosion inhibitor for steel in HCl solution.
The present work is an extension of the earlier work and investigates the inhibition effect of NTBC on CRS in 1.0 mol· L-1HCl solution using weight loss,potentiodynamic polarization curves,and electrochemical impedance spectroscopy (EIS)methods.Meanwhile,the steel surface was examined by scanning electron microscope(SEM).Moreover,the adsorption isotherm of NTBC on CRS surface was obtained.Both thermodynamic parameters(adsorption enthalpy ΔH0,adsorption free energy ΔG0,adsorption entropy ΔS0)and kinetic parameters(rate constant k,reaction constant B)have been obtained by adsorption theory and kinetic equations.A probable inhibitive mechanism was proposed from the viewpoint of adsorption theory.
2 Experimental
2.1 Materials and inhibitor
Tests were performed on a cold rolled steel(CRS)of the following composition(w/%):0.07 C,0.3 Mn,0.022 P,0.010 S, 0.01 Si,0.030Al,and the remainder Fe.
Nitrotetrazolium blue chloride(NTBC)was obtained from Shanghai Chemical Reagent Company of China.Fig.1 shows the molecular structure of NTBC.Clearly,it contains two tetrazole rings(eight N heteroatoms),substitution groups of―NO2and―OCH3(six O atoms),and many aromatic systems.Also, it could be easily protonated in acid solution,and a great deal of lone electrons and π-electrons exist in this molecule.
2.2 Weight loss measurements
Weight loss tests were conducted under total immersion conditions of 250 mL in test solutions(1.0 mol·L-1HCl without and with different concentrations of NTBC)maintained at 25°C controlled by water thermostat.Three parallel CRS sheets of 2.5 cm×2.0 cm×0.06 cm were abraded by a series of emery paper(grade 320-500-800)and then washed with distilled water and acetone.After weighing accurately by digital balance with sensitivity of±0.1 mg,the specimens were suspended in a beaker containing test solutions using glass hooks and rods.All tests were made in non-deaerated solutions.The specimens were taken from test solutions after immersion for 6 h,washed with bristle brush under running water in order to remove the corrosion product,dried with a hot air stream,and reweighed accurately.The mean weight loss of three parallel CRS sheets was obtained and reported.Then the tests were repeated at different temperatures and HCl concentrations.The corrosion rate (v)was calculated from the following equation:


Fig.1 Chemical molecular structure of nitrotetrazolium blue chloride(NTBC)
where W is the average mass loss of three parallel CRS sheets, S is the total area of one CRS specimen,and t is immersion time(6 h).With the calculated corrosion rate,the inhibition efficiency(Ew)was calculated as follows:

where v0and v are the corrosion rates without and with inhibitor,respectively.
2.3 Electrochemical measurements
Electrochemical experiments were carried out in the conventional three-electrode cell with a platinum counter electrode (CE)and a saturated calomel electrode(SCE)coupled to a fine Luggin capillary as the reference electrode.To minimize ohmic contribution,the Luggin capillary was close to working electrode(WE)which was in the form of a square CRS embedded in polyvinyl chloride(PVC)holder using epoxy resin so that the flat surface was only surface in the electrode.The working surface area was 1.0 cm×1.0 cm,prepared as described above (Section 2.2).Before measurement the electrode was immersed in test solution at open circuit potential(OCP)for 2 h at 25°C to be sufficient to attain a stable state.All electrochemical measurements were carried out using PARSTAT 2273 advanced electrochemical system(Princeton Applied Research).Each experiment was repeated at least three times to check the reproducibility.
The potential of potentiodynamic polarization curves was increased at 0.5 mV·s-1and started from a potential of-250 mV to+250 mV versus OCP.Inhibition efficiency obtained from polarization curves(Ep)is defined as:

where Icorrand Icorr(inh)represent corrosion current densities without and with inhibitor,respectively.
Electrochemical impedance spectroscopy(EIS)was carried out at OCP in the frequency range of 0.01 Hz-100 kHz using a 10 mV root mean square(r.m.s)voltage excitation.Inhibition efficiency obtained from EIS(ER)is calculated by the following equation:

where Rt(0)and Rt(inh)are charge transfer resistances for CRS in the absence and presence of inhibitor,respectively.
2.4 Scanning electron microscopy(SEM)
Samples of dimension 2.5 cm×2.0 cm×0.06 cm were prepared as described above(Sect.2.2).After immersion in 1.0 mmol·L-1HCl without and with 0.10 mmol·L-1NTBC at 25°C for 6 h,the specimens were thoroughly washed with distilled water,dried with a cold air blaster.SEM experiments were performed with model S-3000N scanning electron microscope (Hitachi High-Tech Science Systems Corporation,Japan).
3 Results and discussion
3.1 Effect of NTBC on inhibition efficiency

Fig.2 Relationship between inhibition efficiency(Ew)and the concentration of NTBC(cNTBC)in 1.0 mol·L-1HCl
The values of inhibition efficiencies obtained from the mass loss for different inhibitor concentrations in 1.0 mol·L-1HCl are given in Fig.2.It is found that when the concentration of NTBC is less than 0.05 mmol·L-1,Ewincreases sharply with the increase in concentration,while a further increase causes no appreciable change in performance.At 0.1 mmol·L-1,the maximum Ewvalues are 98.3%,92.7%,86.5%,76.4%,and 70.4%at 25,30,35,40,and 45°C,respectively.At 25°C,the inhibition efficiency is estimated to be higher than 81.3%even at 0.01 mmol·L-1NTBC,and it is>90%at 0.03 mmol·L-1, which indicates that NTBC is a good inhibitor for CRS in 1.0 mol·L-1HCl.Under similar conditions,Ewof 1-phenyl-5-mercapto-1,2,3,4-tetrazole(PMT)is 34.8%at 0.5 mmol·L-1;4and of red tetrazolium(RT),66.7%at 50 mg·L-1(about 0.15 mmol·L-1).7Therefore,comparing with PMT and RT,NTBC shows better inhibition performance.This result could be explained as follows:the main adsorption centre of tetrazole compound is the tetrazole ring.4-10PMT or RT has just one tetrazole ring,while NTBC contains two tetrazole rings,which favors more adsorption of NTBC and thus improves the inhibition performance.Furthermore,the molecule weight of NTBC is 817.60 g·mol-1,which is higher than that of PMT(178.01 g· mol-1)or RT(334.81 g·mol-1),and likely to efficiently cover more surface area(due to adsorption)of steel.
Fig.2 also shows that inhibition efficiency decreases with increasing the experimental temperature,which can be attributed to that the higher temperatures might cause desorption of NTBC from the steel surface.
3.2 Adsorption isotherm
Assuming the increase of the inhibition is caused by the adsorption of inhibitor on the steel surface and obeys Langmuir adsorption isothermal equation:10

where c is the concentration of inhibitor,K is the adsorptive equilibrium constant,and θ is the surface coverage,calculated by the following equation:11

where vminis the smallest corrosion rate with inhibitor.
The linear regressions between c/θ and c at different temper-atures were calculated by the computer,and the corresponding parameters are listed in Table 1.Fig.3 is the straight line of c/θ versus c at 25°C.At each temperature,the linear correlation coefficient(r)is almost equal to 1 and the slope is close to 1, indicating the adsorption of NTBC on steel surface obeys Langmuir adsorption isotherm.Noticeably,the slope at 45°C is deviated from unity,which indicates that there is a repulsion force in the adsorption layer.12
The adsorptive equilibrium constant(K)value decreases with increase in temperature,which indicates that it is easily and strongly adsorbed onto the steel surface for the inhibitor at relatively lower temperature.But when the temperature was relatively higher,the adsorbed inhibitors tended to desorb from the steel surface.
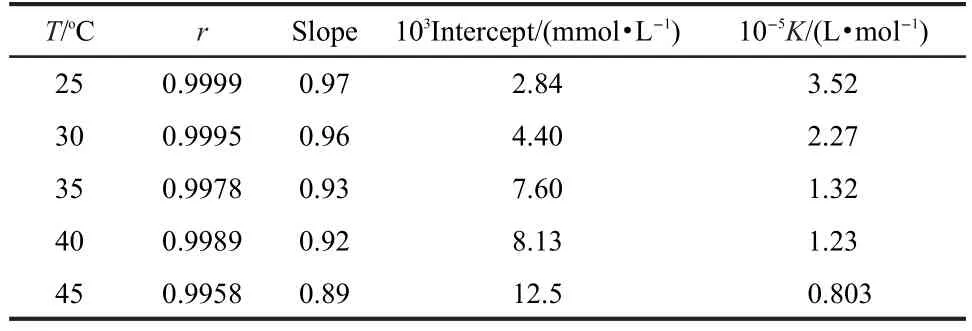
Table 1 Parameters of the linear regression between c/θ and c
3.3 Thermodynamic parameters
Thermodynamic parameters play an important role in understanding the inhibitive mechanism.The standard adsorption enthalpy(ΔH0)could be calculated according to the Vanʹt Hoff equation:8

where R is the gas constant(8.314 J·mol-1·K-1),T is the absolute temperature(K).According to Eq.(7),-ΔH0/R is the slope of the straight line of lnK-1/T as shown in Fig.4.
The adsorptive equilibrium constant(K)is related to the standard free energy of adsorption(ΔG0)as shown in the following equation:13

where the value 55.5 is the concentration of water in solution expressed in mol·L-1.13Then the standard adsorption entropy (ΔS0)can be obtained by the following basic thermodynamic equation:

Fig.3 Relationship between cNTBC/θ and cNTBCin 1.0 mol·L-1HCl solution at 25°C

All the calculated thermodynamic parameters are listed in Table 2.The negative value of ΔH0shows that the adsorption of inhibitor is an exothermic process,which indicates that adsorption amount decreases with increase in temperature.Such behavior can be interpreted on the basis that the increase in temperature resulted in desorption of some adsorbed inhibitor molecules from the metal surface.Generally,values of ΔG0up to-20 kJ·mol-1are consistent with the electrostatic interaction between the charged molecules and the charged metal(physical adsorption),while those more negative than-40 kJ·mol-1involve sharing or transfer of electrons from the inhibitor molecules to the metal surface to form a coordinate type of bond (chemisorption).13In the present study,the value of ΔG0is slightly negative than-40 kJ·mol-1;probably means that the adsorption mechanism of the NTBC on steel in 1.0 mol·L-1HCl solution involves chemisorption.Noticeably,it is generally accepted that physical adsorption is preceding stage of chemisorption of inhibitors on metal surface.14In other words,chemisorption is always accompanied by physisorption.15Therefore,in the adsorption process of NTBC on steel surface,the adsorption will be further discussed in detail as shown in the following parts.Inspection of Table 2 reveals that the sign of ΔS0is negative.The negative values of ΔS0mean that the process of adsorption is accompanied by a decrease in entropy.It might be explained as follows:before the adsorption of inhibitor onto the steel surface,the chaotic degree of steel surface is high,but when inhibitor molecules are orderly adsorbed onto the steel surface,as a result,a decrease in entropy.
3.4 Effect of HCl concentration on corrosion inhibition

Table 2 Thermodynamic parameters of the adsorption of NTBC on CRS surface in 1.0 mol·L-1HCl solution
Fig.5 shows the effect of HCl concentration(1.0-5.0 mol· L-1)at 25°C on the inhibition efficiency of 0.10 mmol·L-1NTBC.In all cases,increasing acid concentration results in decreasing Ew,and the minimum Ewis 67%in 5.0 mol·L-1HCl solution.
To study quantificationally the effect of HCl concentration on the corrosion inhibition of NTBC,assuming the corrosion rate(v)against the molar concentration of acid concentration obeys the expression proposed by Mathur and Vasudevan:16

where k is the rate constant,B is the reaction constant.Fig.6 shows the fitted lines of lnv versus acid concentration(cHCl).Inspection of Fig.6 reveals that there is a good linear relationship between lnv and cHCl.The straight lines show that the kinetic parameters could be calculated by Eq.(10),and listed in Table 3.
Table 3 reveals that the linear regression coefficients(r)are very close to 1,which indicates that the corrosion rate obeys the kinetic expression proposed by Mathur and Vasudevan.According to Eq.(10),k can be regarded as a commencing rate at zero acid concentration,so k means the ability of corrosion in acid for steel.16Table 3 shows that k decreases to more extent after adding NTBC in HCl solution,which indicates that the corrosion of steel is drastically inhibited by the NTBC inhibitor.Eq.(10)shows that B is the slope of the line lnv-cHCl,so B indicates the changed extent of corrosion rate with the acid concentration.The value of B in inhibited HCl is higher than that in uninhibited HCl,which indicates that the changed extent of v with cHClin inhibited HCl is bigger than that in uninhibited HCl.
3.5 Polarization properties
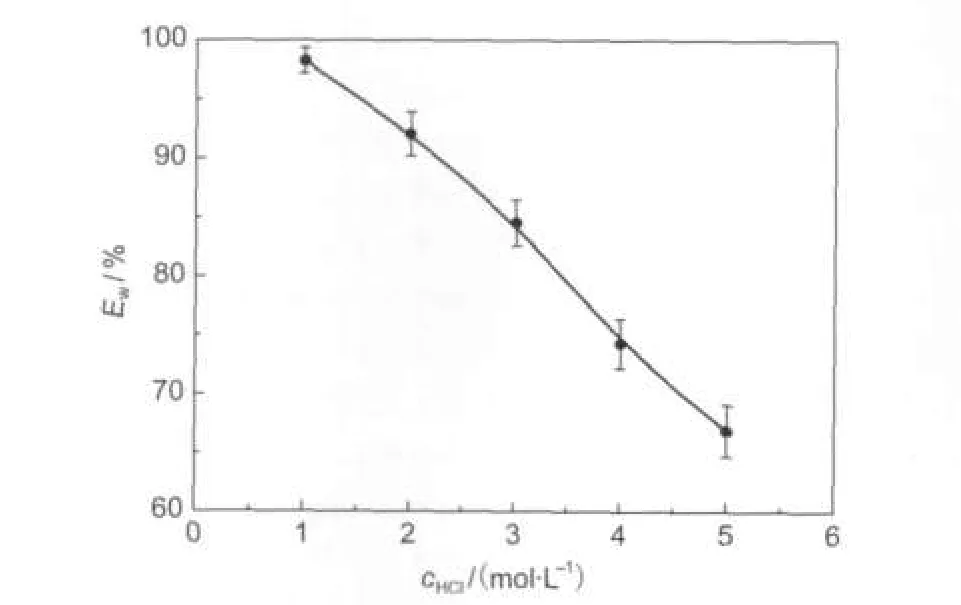
Fig.5 Relationship between inhibition efficiency(Ew)and the concentration of HCl at 25°C

Table 3 Parameters of the linear regression between lnv and cNTBC for the corrosion of steel in HCl solution containing NTBC at 25°C

Fig.6 Fitted lines of lnv versus cHClat 25°C
Fig.7 shows the potentiodynamic polarization curves for CRS in 1.0 mol·L-1HCl without and with various concentrations of NTBC at 25°C.The presence of NTBC causes a prominent decrease in the corrosion rate,i.e.shifting both anodic and cathodic curves to lower values of current densities.Namely,both cathodic and anodic reactions of CRS electrode corrosion are drastically retarded by NTBC in 1.0 mol·L-1HCl.Values of corrosion current densities(Icorr),corrosion potential (Ecorr),cathodic Tafel slope(bc),anodic Tafel slope(ba),and inhibition efficiency(Ep)are listed in Table 4.Clearly,Icorrdecreases remarkably while Epincreases with the inhibitor concentration,and the maximum Epis up to 92.3%.There is no definite trend in the shift of Ecorrin the presence of NTBC, therefore,NBTC can be arranged as a mixed-type inhibitor, and the inhibition action is caused by geometric blocking effect.17Namely,the inhibition action comes from the reduction of the reaction area on the surface of the corroding metal.17Tafel slopes of bcand bado not change upon addition of NTBC,which indicates that adding NTBC does not change the reaction mechanism.
3.6 Electrochemical impedance spectroscopy
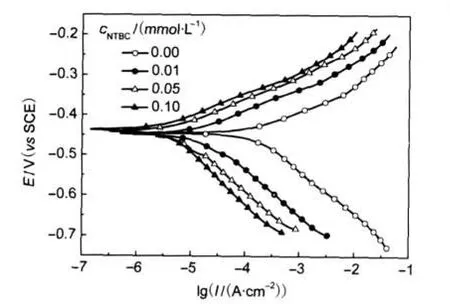
Fig.7 Potentiodynamic polarization curves for CRS in 1.0 mol·L-1HCl solution without and with different concentrations of NTBC at 25°C
Fig.8 shows the Nyquist diagrams for CRS in 1.0 mol·L-1HCl at 25°C with various concentrations of NTBC.These diagrams have similar shape.The shape is maintained throughout all tested concentrations,indicating that almost no change in the corrosion mechanism occurs due to the inhibitor addition.18The impedance spectra consist of large capacitive loop at high frequencies followed by a small inductive one at low frequency values.The high frequency capacitive loop is usually related to the charge transfer of the corrosion process and double layer behavior.On the other hand,the low frequency inductive loop may be attributed to the relaxation process obtained by adsorption species likeandor inhibitor species22on the electrode surface.It might be also attributed to the redissolution of the passivity surface at low frequencies.23
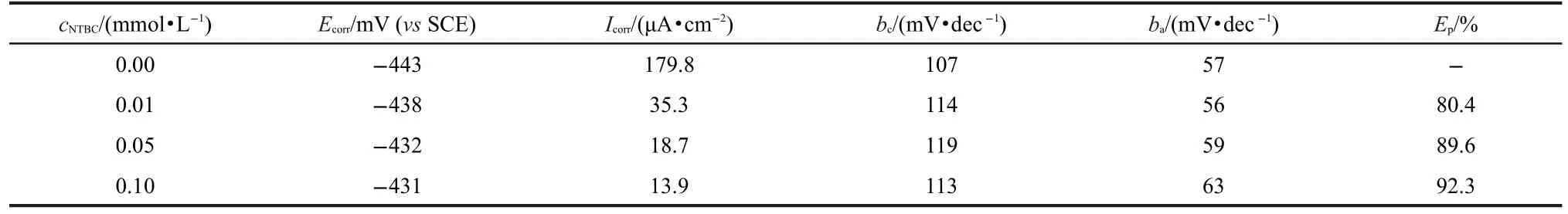
Table 4 Potentiodynamic polarization parameters for the corrosion of CRS in 1.0 mol·L-1HCl containing different concentrations of NTBC at 25°C
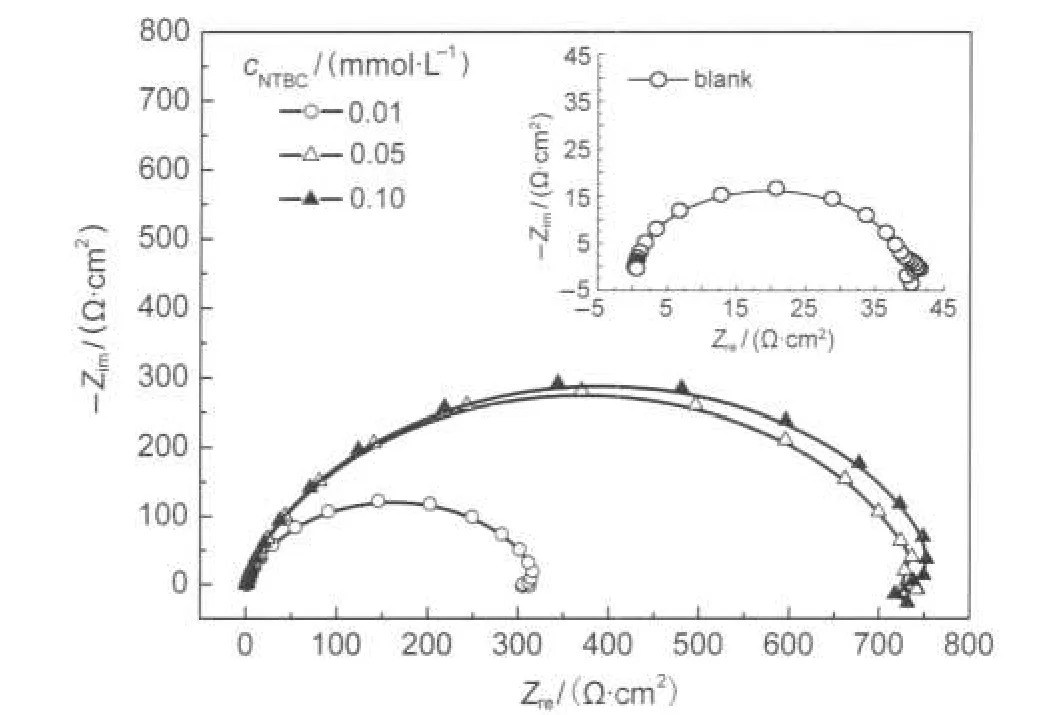
Fig.8 Nyquist diagrams for CRS in 1.0 mol·L-1HCl solution without and with NTBC at 25°C
These high frequency loops are not perfect semicircles which can be attributed to the frequency dispersion as a result of the roughness and inhomogeneousness of electrode surface.24Furthermore,the diameter of the capacitive loop in the presence of inhibitor is bigger than that in the absence of inhibitor(blank solution)and increases with the inhibitor concentrations.This indicates that the impedance of inhibited substrate increases with NTBC concentrations.
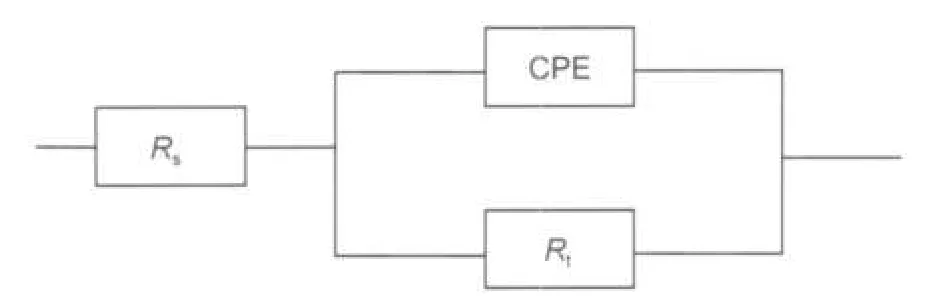
Fig.9 Equivalent circuit model of EISRs:solution resistance;Rt:charge transfer resistance;CPE:constant phase element
The EIS results of high frequency capacitive loops are simulated by the equivalent circuit shown in Fig.9 to pure electric models that could verify or rule out mechanistic models and enable the calculation of numerical values corresponding to the physical and/or chemical properties of the electrochemical system under investigation.25The circuit employed allows the identification of both solution resistance(Rs)and charge transfer resistance(Rt).It is worth mentioning that the double layer capacitance(Cdl)value is affected by imperfections of the surface, and this effect is simulated via a constant phase element (CPE).26The constant phase element is composed of a component Qdland a coefficient a.The parameter a quantifies different physical phenomena like surface inhomogeneousness resulting from surface roughness,inhibitor adsorption,porous layer formation,etc.So the capacitance is deduced from the following relation:27

where fmaxrepresents the frequency at which imaginary value reaches a maximum on the Nyquist plot.The electrochemical parameters of Rt,Cdland ERare calculated by ZSimpWin software and presented in Table 5.
Inspection of Table 5 reveals that Rtvalues increases prominently while Cdlreduces with the concentrations of NTBC.The decrease in Cdl,compared with that in blank solution(without inhibitor),results from a decrease in local dielectric constant and/or an increase in the thickness of the electrical double layer,suggesting the inhibitor molecule function by adsorption at the metal/solution interface.28ERincreases with the concentrations of NTBC,and the maximum ERreaches up to 95.1%, which further confirms NTBC exhibiting very good inhibitive performance for CRS in 1.0 mol·L-1HCl solution.
Inhibition efficiency(E)obtained from weight loss,potentiodynamic polarization curves,and EIS are a little difference owing to different conditions of three methods.The difference could be the difference in immersion time of polarization.EIS was 2 h,but the weight loss was 6 h.There is the discrepancy in the three experimental methods(weight loss gives average E,but polarization and EIS give the instantaneous value).But the general rule of inhibition obtained by three methods is similar.Namely,all three methods indicate that NTBC acts as a good inhibitor for CRS in 1.0 mol·L-1HCl solution,and E increases with the inhibitor concentration.
3.7 Scanning electron microscopy
Fig.10 presents the SEM photos of CRS surface in 1.0 mol·L-1HCl.It can be seen from Fig.10a that the CRS steel samples seems smooth before immersion and shows some abrading scratches on the surface.However,it appears some black holes,which may be attributed to the defect of steel.After immersion in uninhibited 1.0 mol·L-1HCl,Fig.10b reveals that the CRS surface appears an aggressive attack of the corroding medium on the steel surface.Furthermore,the corrosion products appear very uneven and lepidoteral-like morphology,and the surface layer is rather rough.In contrast,in the presence of 0.10 mmol·L-1NTBC,there is much less damage on the steel surface,which further confirms the inhibition action.Also, there is an adsorbed film adsorbed on CRS surface(Fig.10c), which does not exist in Fig.10(b).In accordance,it might be concluded that the adsorption film could efficiently inhibit the corrosion of steel.
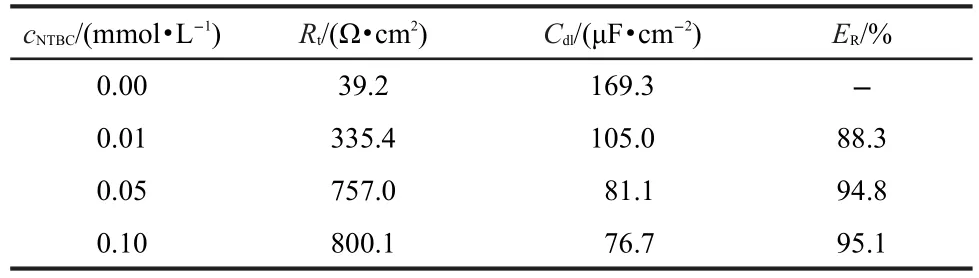
Table 5 EIS parameters for the corrosion of CRS in 1.0 mol·L-1 HCl solution containing NTBC at 25°C
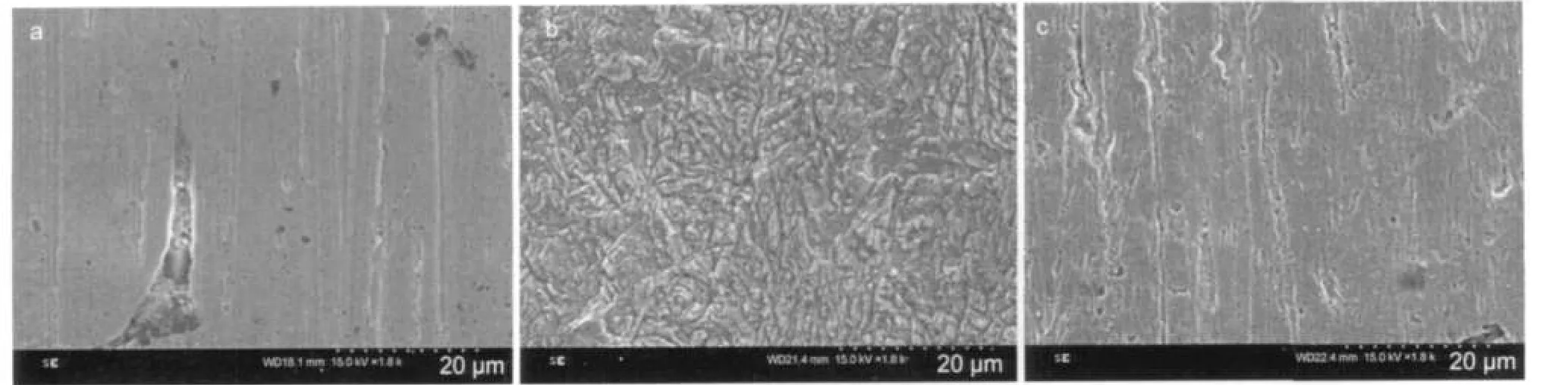
Fig.10 SEM micrographs of CRS surface(a)before immersion;(b)after 6 h of immersion in 1.0 mol·L-1HCl at 25°C;(c)after 6 h of immersion in 0.10 mmol·L-1NTBC+1.0 mol·L-1HCl at 25°C
3.8 Explanation for inhibition
The adsorption process is affected by the chemical structure of inhibitor,the nature and charged surface of the metal,and the distribution of charge over the whole inhibitor molecule.In general,owing to the complex nature of adsorption and inhibition of a given inhibitor,it is impossible for single adsorption mode between inhibitor and metal surface.In the present system,NTBC has many active sites to facilitate the adsorption process.Thus,four kinds of adsorption may take place in the inhibiting phenomena involving NTBC molecules on steel surface as follows:
(i)NTBC can be classified as an electrolyte,namely,the organic part(NTB2+)is the cation and the inorganic part(Cl-)is the anion:

Owing to neutral N atoms in NTB2+,NTB2+could further be protonated in the acid solution as following:

Thus,in aqueous acidic solutions,the NTBC exists as protonated cations of NTB2+and[NTBHx](x+2)+.The charge of the metal surface can be determined from the value of Ecorr-Eq=0(zero charge potential).29The value of Ecorrobtained in 1.0 mol·L-1HCl is-443 mV(vs SCE).The Eq=0of iron in HCl is-530 mV (vs SCE).30The steel surface charges positive charge in HCl because of Ecorr
-Eq=0>0.Since chloride ions(Cl-)have a smaller degree of hydration,being specifically adsorbed,they create excess negative charge towards the solution and favor more adsorption of the cations,NTB2+and[NTBHx](x+2)+may adsorb on the negatively charged metal surface,namely,i.e.,there may be a synergism between Cl-and protonated inhibitor.
(ii)It should be noted that the molecular structures of NTB2+and[NTBHx](x+2)+still contain the neutral N atoms.In accordance,NTB2+and[NTBHx](x+2)+may be adsorbed on the metal surface via the chemisorption mechanism,involving the displacement of water molecules from the metal surface and the sharing electrons between the N atoms and vacant d orbitals of Fe.
(iii)Also,the molecular structures of NTB2+and[NTBHx](x+2)+remain unchanged with respect to NTBC,that is,the N atoms on the ring remain strongly blocked.So NTB2+and[NTBHx](x+2)+molecules can be also adsorbed on the metal surface on the basis of donor-acceptor interactions between π electrons of the heterocycles and vacant d orbitals of Fe.The simultaneous adsorption of the two tetrazole rings induces greater adsorption of the inhibitor molecules on the surface of CRS.The larger molecule area plays an important role in retarding the corrosion.
(iv)Due to the good chelating action of tetrazole ring,some metal complexes of Fe2+-tetrazole compounds have been widely prepared.31,32Thus,NTB2+or[NTBHx](x+2)+may combine with freshly generated Fe2+ions on steel surface forming metal inhibitor complexes:

These complexes might adsorb onto steel surface by the van der Waals force to form a blocking barrier to keep CRS from corrosion.
4 Conclusions
(1)NTBC acts as a novel good inhibitor for the corrosion of CRS in 1.0 mol·L-1HCl solution.Inhibition efficiency values increase with the inhibitor concentrations,while decreases with the temperatures.
(2)The adsorption of NTBC obeys Langmuir adsorption isotherm.The adsorption process is spontaneous and exothermic accompanied by a decrease in entropy.
(3)The corrosion rates in the absence and presence of inhibitor obey the kinetic expression proposed by Mathur and Vasudevan.The rate constant(k)decreases obviously after adding NTBC in 1.0-5.0 mol·L-1HCl solution,while the reaction constant(B)increases.
(4)NTBC is a mixed-type inhibitor in 1.0 mol·L-1HCl solution,and the inhibition action is caused by geometric blocking effect.EIS spectra exhibit large capacitive loop at high frequencies followed by a small inductive one at low frequency values.The addition of NTBC to 1.0 mol·L-1HCl solutions enhances Rtvalues while reduces Cdlvalues.
(5)The introduction of NTBC into 1.0 mol·L-1HCl solution results in the formation of an adsorptive film on the CRS surface,which efficiently protects steel from corrosion.
(1) Trabanelli,G.Corrosion 1991,47,410.
(2) Bentiss,F.;Traisnel,M.;Gengembre,L.;Lagrenée,M.Appl. Surf.Sci.2000,161,194.
(3) Granese,S.L.;Rosales,B.M.;Oviedo,C.;Zerbino,J.O. Corrosion Sci.1992,33,1439.
(4) Kertit,S.;Hammouti,B.Appl.Surf.Sci.1996,93,59.
(5) Bensajjay,E.;Alehyen,S.;Achouri,M.E.;Kertit,S. Anti-Corros.Meth.Mater.2003,50,402.
(6)Morales-Gil,P.;Negrón-Silva,G.;Romero-Romoa,M.; Ángeles-Chávez,C.;Palomar-Pardavé,M.Electrochim.Acta 2004,49,4733.
(7) Li,X.H.;Deng,S.D.;Fu,H.Chin.J.Appl.Chem.2009,26, 1075.[李向红,邓书端,付 惠.应用化学,2009,26,1075.]
(8) Li,X.H.;Deng,S.D.;Fu,H.Corrosion Sci.2009,51,1344.
(9)Li,X.H.;Deng,S.D.;Fu,H.Mater.Chem.Phys.2009,115, 815.
(10) Li,X.H.;Deng,S.D.;Fu,H.Corrosion Sci.2010,52,2786.
(11) Sekine,I.;Hirakawa,Y.Corrosion 1986,42,272.
(12) Mu,G.N.;Li,X.H.;Qu,Q.;Zhou,J.Corrosion Sci.2006,48, 445.
(13) Cano,E.;Polo,J.L.;Iglesia,A.L.;Bastidas,J.M.Adsorption 2004,10,219.
(14)Wang,F.P.;Kang,W.L.;Jin,H.M.Corrosion Electrochemistry Mechanism,Methods and Applications;Chemical Industrial Engineering Press:Beijing,2008;p 242. [王凤平,康万利,敬和民.腐蚀电化学原理、方法及应用.北京:化学工业出版社, 2008:242.]
(15) Zhang,S.T.;Tao,Z.H.;Liao,S.G.;Wu,F.J.Corrosion Sci. 2010,52,3126.
(16) Mathur,P.B.;Vasudevan,T.Corrosion 1982,38,171.
(17) Cao,C.Corrosion Sci.1996,38,2073.
(18) Labjar,N.;Lebrini,M.;Bentiss,F.;Chihib,N.E.;El Hajjaji,S.; Jama,C.Mater.Chem.Phys.2010,119,330.
(19)Lenderink,H.J.W.;Linden,M.V.D.;De Wit,J.H.W. Electrochim.Acta 1993,38,1989.
(20) Veloz,M.A.;Gonźalez,I.Electrochim.Acta 2002,48,135.
(21)Amin,M.A.;Abd El-Rehim,S.S.;El-Sherbini,E.E.F.; Bayyomi,R.S.Electrochim.Acta 2007,52,3588.
(22) Zhang,Q.B.;Hua,Y.X.Mater.Chem.Phys.2010,119,57.
(23) Singh,A.K.;Quraishi,M.A.Corrosion Sci.2010,52,152.
(24) Lebrini,M.;Lagrenée,M.;Vezin,H.;Traisnel,M.;Bentiss,F. Corrosion.Sci.2007,49,2254.
(25) Priya,A.R.S.;Muralidharam,V.S.;Subramania,A.Corrosion 2008,64,541.
(26)Bommersbach,P.;Alemany-Dumont,C.;Millet,J.P.;Normand, B.Electrochim.Acta 2006,51,4011.
(27) Qu,Q.;Hao,Z.Z.;Li,L.;Bai,W.;Liu,Y.J.;Ding,Z.T. Corrosion Sci.2009,51,569.
(28) Lagrenée,M.;Mernari,B.;Bouanis,M.;Traisnel,M.;Bentiss, F.Corrosion Sci.2002,44,573.
(29) Schweinsberg,D.P.;Ashworth,V.Corrosion Sci.1988,28,539.
(30) Banerjee,G.;Malhotra,S.N.Corrosion 1992,48,10.
(31) Stassen,A.F.;Dova,E.;Ensling,J.;Schenk,H.;Gütlich,P.; Haasnoot,J.G.;Reedijk,J.Inorg.Chim.Acta 2002,335,61.
(32) Hassan,N.;Weinberger,P.;Kubel,F.;Molnar,G.;Bousseksou, A.;Dlhan,L.;Boča,R.;Linert,W.Inorg.Chim.Acta 2009,362, 3629.
July 19,2011;Revised:September 13,2011;Published on Web:September 30,2011.
Inhibition Effect of Nitrotetrazolium Blue Chloride on the Corrosion of Steel in Hydrochloric Acid Solution
LI Xiang-Hong1,*DENG Shu-Duan2FU Hui1
(1Faculty of Science,Southwest Forestry University,Kunming 650224,P.R.China;2Faculty of Materials Engineering,Southwest Forestry University,Kunming 650224,P.R.China)
The inhibition effect of nitrotetrazolium blue chloride(NTBC)on the corrosion of cold rolled steel(CRS)in 1.0-5.0 mol·L-1HCl solution was investigated by weight loss method,potentiodynamic polarization curves,electrochemical impedance spectroscopy(EIS),and scanning electron microscopy (SEM).The results show that NTBC is a good inhibitor for CRS in 1.0 mol·L-1HCl solution and the adsorption of NTBC on the CRS surface obeys the Langmuir adsorption isotherm.Inhibition efficiency increases with inhibitor concentration while it decreases with hydrochloric acid concentration and temperature.The adsorption thermodynamic parameters(adsorption free energy ΔG0,adsorption enthalpy ΔH0,adsorption entropy ΔS0)and corrosion kinetic parameters(corrosion rate constant k,kinetic reaction constant B)were also calculated.Based on these parameters the inhibitive mechanism is discussed. Potentiodynamic polarization curves show that NTBC acts a mixed-type inhibitor.EIS spectra exhibit one capacitive loop at high frequency and this is followed by a small inductive loop at low frequency,and the charge transfer resistance increases with inhibitor concentrations.SEM clearly shows that the addition of NTBC can drastically retard the corrosion of steel in hydrochloric acid media.
Corrosion inhibitor;Nitrotetrazolium blue chloride;Steel;Hydrochloric acid;Adsorption
The electrochemicalmeasurements were carried outusing PARSTAT 2273 advanced electrochemical system(Princeton Applied Research)provided by Advanced Science Instrument Sharing Center of Southwest Forestry University.
10.3866/PKU.WHXB20112841
∗Corresponding author.Email:xianghong-li@163.com;Tel:+86-871-3863377
O646

