离子液体[Cnpy][NTf2](n=2,4,5)的动力粘度和电导率
刘青山 颜佩芳 杨 淼 谭志诚,2,* 李长平,3,* WELZ-BIERMANN Urs
(1中国科学院大连化学物理研究所,中国离子液体实验室,辽宁大连116023;2中国科学院大连化学物理研究所,热化学实验室,辽宁大连116023;3大连大学,环境与化学工程学院,辽宁大连116622)
1 Introduction
Ionic liquids(ILs)as organic salts,often exhibit interesting properties such,as low melting points,good solvation properties,and non-volatility,which are required both by industry and scientific communities for their broad applications as electrolytes in batteries and supercapacitors1,2or reaction media in nanoscience,3physical chemistry,4,5and many other areas. Most ILs are hydrophilic,so that the anions,such as hexafluorophosphate[PF6]-,bis(trifluoromethylsulfonyl)imide[NTf2]-, trifluorotris(perfluoroethyl)phosphate[PF3(CF2CF2)3]-,trifluo-romethyltrifluoroborate[BF3CF3]-,and perfluoroethyltrifluoroborate[BF3C2F5]-ILs,have been attracted much attention as the hydrophobic components.6-13Some of them have been used in the potential electrolytes.14-19However,the uses of the ILs as the potential electrolytes require the data support of the dynamic viscosity and conductivity.The ILs with[NTf2]-anion were studied widely because the dynamic viscosities of this type ILs were low enough to be applied in electrochemical devices.16-21
Recently we22have reported the densities and surface tensions of the ILs[Cnpy][NTf2](n=2,4,5)and Tokuta et al.23published the dynamic viscosity and conductivity of IL[C4py] [NTf2].In the present study we also measured the dynamic viscosity and conductivity of IL[C4py][NTf2]for comparison.In order to obtain further understanding and extend the range of applications of the ILs[Cnpy][NTf2](n=2,4,5)family,the temperature dependence of dynamic viscosity and conductivity was studied from 298.15 to 338.15 K and from 283.15 to 338.15 K,respectively.
2 Experimental
2.1 Chemicals
The syntheses and characterizations of ILs[Cnpy][NTf2](n= 2,4,5)have been described in our previous research.22For the samples,no impurity was found from the1H,13C-NMR measurements.All samples were dried under vacuum at 353 K for 24 h before measurement.This step is extremely important to reduce the impurities(including water),because the trace impurities influence the experimental results of the dynamic viscosity and the electrical conductivity.The anion Br-was determined using a silver nitrate test and no anion Br-was found,indicating that the concentration of Br-was out of the determination limit range,i.e.,less than 5×10-5.Since the trace water still existed in the ILs after vacuum drying,the presence of water became the most problematic impurity and needed to be confirmed before measurement.The dynamic viscosity measurement was carried out at the atmosphere,so,the absorption of the water content was also determined after the measurement. The mass fractions of water were the average of three measurements using a Cou-Lo Aquamax Karl Fischer Moisture Meter (v.10.06)and are listed in Table 1.The purities of the ILs [Cnpy][NTf2](n=2,4,5)were estimated to be better than 99% by mass.The effect of the absorbed water on the dynamic viscosity measurement can be ignored as seen from Table 1.Theconductivity values were determined under the dry argon. Some values of the dynamic viscosity and conductivity of the IL[C4py][NTf2]from 283.15 to 333.15 K are listed in Table 2 together with recent literature values.23

Table 1 Mass fraction(w)of water before and after measurements of dynamic viscosity,conductivity
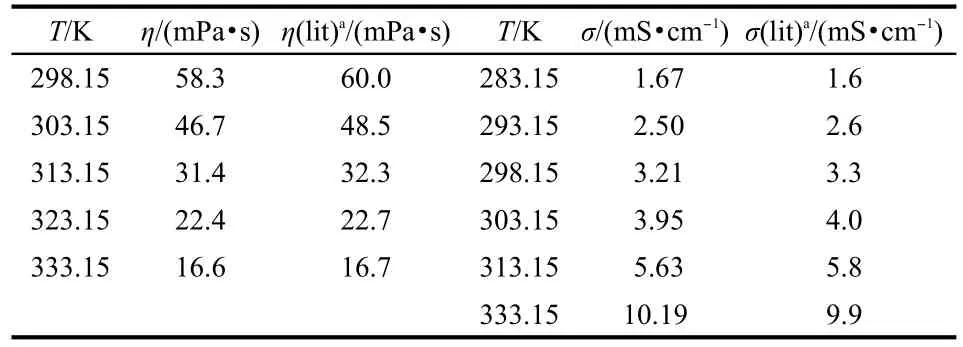
Table 2 Comparison of experimental values with literature values of dynamic viscosity and conductivity of IL[C4py][NTf2]
2.2 Dynamic viscosity
The dynamic viscosities of the ILs[Cnpy][NTf2](n=2,4,5) were measured using an Ostwald viscometer in the temperature range from 298.15 to 338.15 K.The temperature was controlled using a thermostatic bath with stability of±0.1 K.The time for attaining thermal equilibrium in the viscosity determination was 30 min.The values were recorded at a regular interval of 5 K.Each datum point of dynamic viscosity was the average value of three measurements and the uncertainties were estimated to be±1%.The data are listed in Table 3.
2.3 Conductivity
The conductivity measurements of ILs[Cnpy][NTf2](n=2,4, 5)were carried out on a MP522 conductivity instrument with the cell constants of 1 cm-1(the cell was calibrated with the aqueous KCl solution)under argon gas of high purity in the temperature range from 283.15 to 338.15 K.The uncertainties were estimated to be±1%.The samples were placed in a cell with a jacket.The temperature was controlled using a thermostat with temperature stability of±0.05 K.The time for attaining thermal equilibrium was 30 min.The values were recorded at interval of 5 K.The data are listed in Table 3.
3 Results and discussion
From Table 2,it can be seen that the dynamic viscosity and conductivity of[C4py][NTf2]in the present work have relatively small discrepancies with the reported values23at different temperatures.Pan et al.24have also published the dynamic viscosity and conductivity of[C4py][NTf2],the values are 59.90 mPa·s and 3.245 mS·cm-1at 298.15 K,respectively.Our experimental values are 58.3 mPa·s for dynamic viscosity and 3.21 mS·cm-1for conductivity.The agreement of our measurements with literature values is good.The comparison of our ex-perimental dynamic viscosity and conductivity of[C4py][NTf2] with literature23is also shown in Fig.1.The temperature dependence of dynamic viscosity and conductivity was plotted for ILs[Cnpy][NTf2](n=2,4,5)(see Fig.2 and Fig.3).
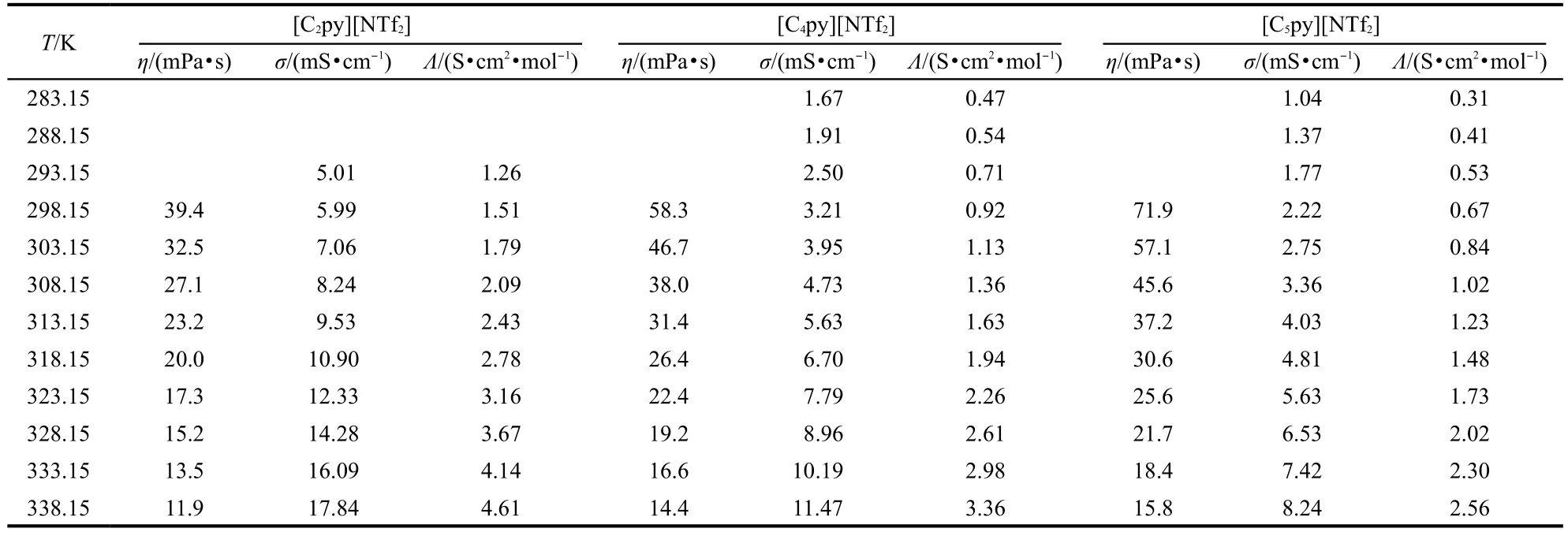
Table 3 Experimental values of viscosity,η,conductivity,σ,and molar conductivity,Λ of ILs[Cnpy][NTf2](n=2,4,5)
From Figs.2 and 3,it can be seen that the dynamic viscosity decreases with increasing the temperature.But the conductivity behaves inversely.The conductivity decreases with the extension of the alkyl side chain of the cation for the series of ILs [Cnpy][NTf2](n=2,4,5).However,the dynamic viscosity changes in contrast with the conductivity.This is because the volume of cation increases with the extension of the alkyl side chain and the transfer resistance of ions increases with the increasing volume.It can be concluded that the conductivity decreases with the increase of dynamic viscosity for the three ILs.
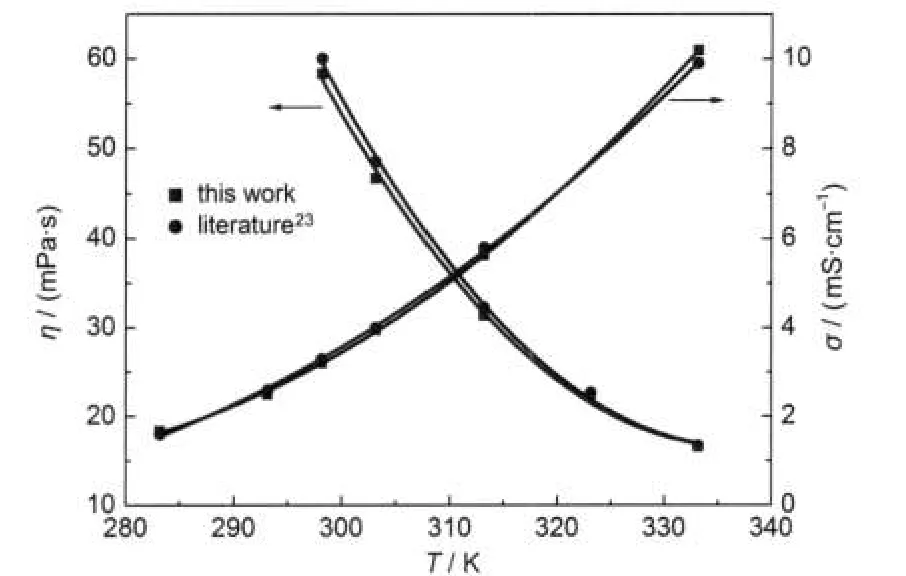
Fig.1 Plots of the experimental dynamic viscosity,η,and conductivity,σ,against T for IL[C4py][NTf2]

Fig.2 Dynamic viscosity of ILs[Cnpy][NTf2](n=2,4,5)as a function of temperature
The dynamic viscosities and conductivities of[Cnpy][NTf2] (n=2,4)were also compared with the reported values25,26at 298 and 299 K.These ILs have the same anion(NTf2-)and one same alkyl side chain in the cations.The dynamic viscosity and conductivity and the corresponding experimental values23,25,27are listed in Table 4.It can be seen that the order of the dynamic viscosity for ILs with ethyl side chain is[(n-C4H9)(CH3)3N]>[DMES]>[C2py]>[Emim],the conductivity behaves inversely; the order of the dynamic viscosity for ILs with butyl side chain is[P14]>[bmpro]>[BDMS]>[C4py]>[Bmim],but the conductivity has a different order:[BDMS]>[Bmim]>[C4py]>[bmpro]>[P14].From the comparison,it can be seen that the dynamic viscosities and conductivities of[Cnpy][NTf2](n=2,4)have moderate values,favoring for their application.
杰克在收拾自己的用品,苏婷婷进来,问:杰克,要出差吗?杰克沉着脸:最近事情多,我搬到公司里去。傍晚,杰克走进一家饭店,问:这里需要服务员吗?柜台人员转身喊:老板!老板从里屋出来:啥事?柜台人员指着杰克:这位外籍先生想当服务员。 老板看看杰克,问:会端盘子吗?杰克回答:当然,我在唐人街端过盘子。
For dynamic viscosity,a fitting equation can be obtained according to theArrhenius equation:8,16

where η is the dynamic viscosity,η∞is the empirical constant, and Eηdenotes the activation energy for viscous flow.The values of η∞,Eη,andcorrelationcoefficients,r,arelistedinTable5.
The values of dynamic viscosity of ILs[Cnpy][NTf2](n=2, 4,5)were also fitted by using the Fulcher equation that is commonly used for ILs:14,27,28

where η0,B,T0are the empirical constants.The best fitted values of η0,B,T0,andr arelistedinTable5.
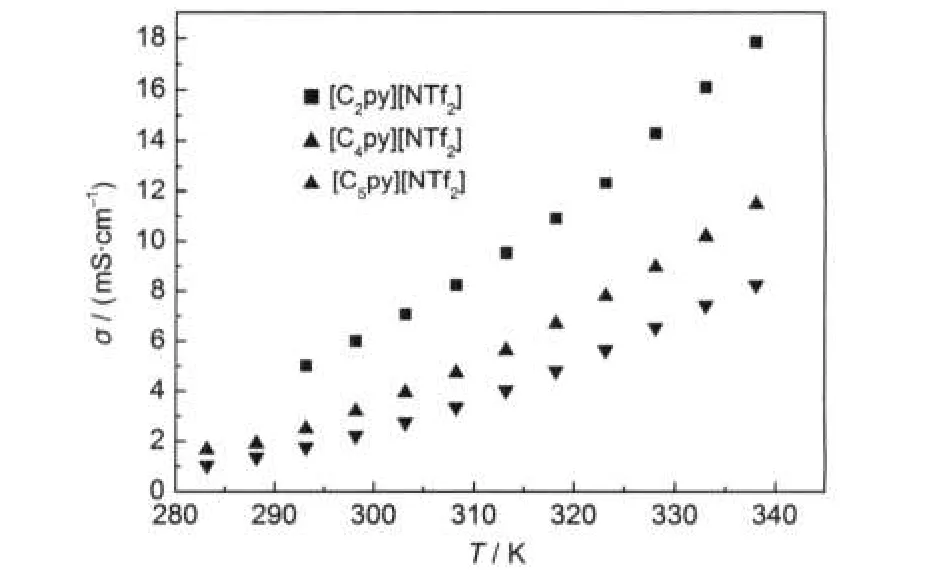
Fig.3 Conductivity of ILs[Cnpy][NTf2](n=2,4,5)as a function of temperature

Table 4 Comparison of dynamic viscosity and conductivity of ILs with same anion[NTf2]-and different cations at 298 K
For conductivity,an expression can be obtained according to theArrhenius equation:29

where σ is the conductivity,σ∞denotes the empirical constant, and Eσstands for the activation energy for electrical conduction(which indicates the energy needed for an ion to jump a free hole).The values of σ∞,Eσ,and r are listed in Table 6.
The values of conductivity of ILs[Cnpy][NTf2](n=2,4,5) were also fitted by using the Fulcher equation:14,27,28

where σ0,B,T0are the empirical constants.The best fitted values of σ0,B,T0,and r are listed in Table 6.
From Tables 5,6,it can be seen that the activation energies of dynamic viscosity and conductivity increase with the extension of the cationʹs alkyl side chain length;the discrepancies between the activation energies of dynamic viscosity and conductivity are relatively small.It is worth while mentioning that the same results were also got by Hagiwara et al.30And they gave the suggestion that the high conductivity can be explained by the low viscosity without introducing some special conduction mechanism,such as ion hopping.The values of correlation coefficient resulting from Fulcher equation are 0.9996 or better,which shows that the Fulcher equation represents very well for the experimental dynamic viscosity and conductivity data.
The molar conductivity was calculated according to the following equation:
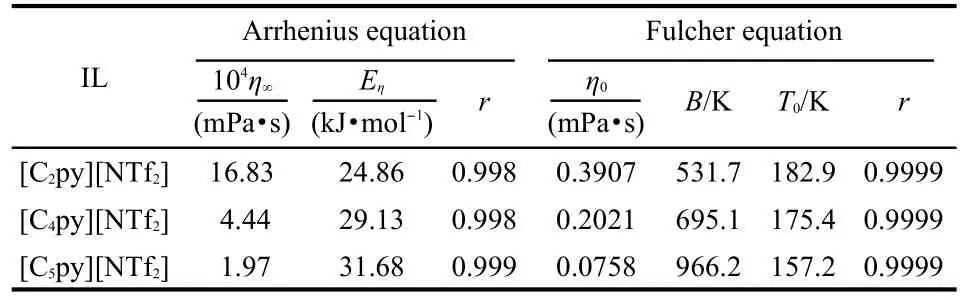
Table 5 Fitted values of dynamic viscosity of η∞,Eη,η0,B,T0,and r according to Fulcher equation andArrhenius equation
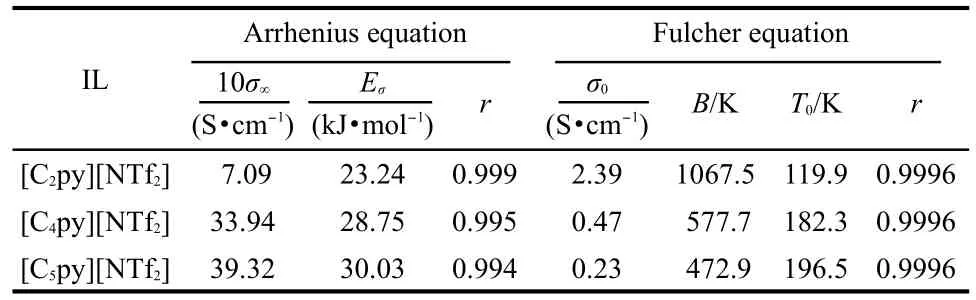
Table 6 Fitted values of conductivity of σ∞,Eσ,σ0,B,T0,and r according to Fulcher equation andArrhenius equation

where Λ is the molar conductivity,M is the molar mass,and ρ is the density.The values of the molar conductivity are also listed in Table 3.The temperature dependence of molar conductivity was also fitted according to the Fulcher equation:

where Λ0,B,and T0are constants.The best-fitted parameters are listed in Table 7.
The relationship between the molar conductivity and dynamic viscosity has been described by Walden rule:31-34

where k is a temperature dependent constant.The Walden products are 60(S·cm2·mol-1)(mPa·s)for[C2py][NTf2],54(S· cm2·mol-1)(mPa·s)for[C4py][NTf2],and 48(S·cm2·mol-1) (mPa·s)for[C5py][NTf2]at 298.15 K,respectively.
lgΛ dependence on lgη-1was ploted for ILs[Cnpy][NTf2](n= 2,4,5)from 298.15 K to 338.15 K(see Fig.4).From Fig.4,the curves are approximate to straight lines,which shows that the ILs obey the Walden rule.The position of the ideal line was established using aqueous KCl solutions at high dilution.The lines for ILs[Cnpy][NTf2](n=2,4,5)are below and close to the ideal KCl line.Most of the ILs has the same tendency.28,31-37The slopes of the lines for ILs[C2py][NTf2],[C4py][NTf2],and [C5py][NTf2]are 0.941,0.935,and 0.893,respectively.The results indicate that the conductivity fluidity relationship remains constant.
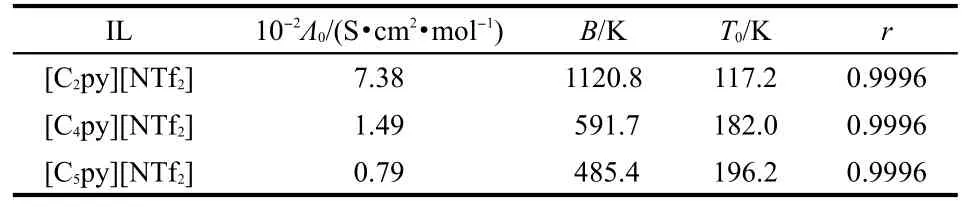
Table 7 Fitted values of molar conductivity of Λ0,B,T0,and r according to Fulcher equation

Fig.4 Walden plots for series ILs[Cnpy][NTf2](n=2,4,5)from 298.15 to 338.15 KThe solid straight line is the ideal line for 0.01 mol·L-1aqueous KCl solution.
4 Conclusions
The effect of temperature on dynamic viscosity and conductivity of the ILs[Cnpy][NTf2](n=2,4,5)was experimentally determined in the temperature range from 298.15 to 338.15 K and from 283.15 to 338.15 K,respectively.The dynamic viscosity decreases with the increase in temperature.However, the conductivity increases with the decrease of dynamic viscosity for the three ILs.The activation energies of viscous flow and electrical conduction were obtained from Arrhenius equation.These values increase with the extension of the alkyl side chain in the cation.The relationship between the molar conductivity and dynamic viscosity was described in terms of Walden rule.The Walden products are 60(S·cm2·mol-1)(mPa·s)for [C2py][NTf2],54(S·cm2·mol-1)(mPa·s)for[C4py][NTf2],and 48(S·cm2·mol-1)(mPa·s)for[C5py][NTf2]at 298.15 K,respectively.
(1)Tsunashima,K.;Sugiya,M.Electrochem.Commun.2007,9, 2353.
(2) Seki,S.;Kobayashi,Y.;Miyashiro,H.;Ohno,Y.;Usami,A.; Mita,Y.;Watanabe,M.;Terada,N.Chem.Commun.2006,544.
(3) Itoh,H.;Naka,K.;Chujo,Y.J.Am.Chem.Soc.2004,126,3026.
(4)Du,Z.;Yu,Y.L.;Wang,J.H.Chem.Eur.J.2007,13,2130.
(5) Endres,F.;Abedin,S.Z.E.Phys.Chem.Chem.Phys.2006,8, 2101.
(6) Fuller,J.;Carlin,R.T.;Long,H.C.D.;Haworth,D.J.Chem. Soc.Chem.Commun.1994,299.
(7) Gordon,C.M.;Holbrey,J.D.;Kennedy,R.;Seddon,K.R. J.Mater.Chem.1998,8,2627.
(8) Bonhôte,P.;Dias,A.P.;Papageorgiou,N.;Kalyanasundaram, K.;Grätzel,M.Inorg.Chem.1996,35,1168.
(9) Cammarata,L.;Kazarian,S.G.;Salter,P.A.;Welton,T.Phys. Chem.Chem.Phys.2001,3,5192.
(10) Liu,Q.B.;Janssen,M.H.A.;Rantwijk,F.V.;Sheldon,R.A. Green Chem.2005,7,39.
(11) Zhou,Z.B.;Matsumoto,H.;Tatsumi,K.Chemistry Letters 2004,33,680.
(12) Zhou,Z.B.;Matsumoto,H.;Tatsumi,K.Chemistry Letters 2004,33,886.
(13) Zhou,Z.B.;Matsumoto,H.;Tatsumi,K.Chemistry Letters 2004,33,1636.
(14)Tokuda,H.;Hayamizu,K.;Ishii,K.;Susan,M.A.B.H.; Watanabe,M.J.Phys.Chem.B 2005,109,6103.
(15) Jacquemin,J.;Husson,P.;Padua,A.A.H.;Majer,V.Green Chem.2006,8,172.
(16) Fang,S.H.;Yang,L.;Wei,C.;Peng,C.X.;Tachibana,K.; Kamijima,K.Electrochem.Commun.2007,9,2696.
(17) Kazock,J.;Taggougui,M.;Anouti,M.;Willman,P.;Carré,B.; Lemordant,D.J.Appl.Electrochem.2009,39,2461.
(18)Sakaebe,H.;Matsumoto,H.Electrochem.Commun.2003,5, 594.
(19)Yang,L.;Zhang,Z.X.;Gao,X.H.;Zhang,H.Q.;Mashita,K. J.Power Sources 2006,162,614.
(20) Matsumoto,H.;Sakaebe,H.;Tatsumi,K.;Kikuta,M.;Ishiko, E.;Kono,M.J.Power Sources 2006,160,1308.
(21) Orita,A.;Kamijima,K.;Yoshida,M.J.Power Sources 2010, 195,7471.
(22)Liu,Q.S.;Yang,M.;Yan,P.F.;Liu,X.M.;Tan,Z.C.;Welz-Biermann,U.J.Chem.Eng.Data 2010,55,4928.
(23) Tokuda,H.;Tsuzuki,S.;Susan,M.A.B.H.;Hayamizu,K.; Watanabe,M.J.Phys.Chem.B 2006,110,19593.
(24) Pan,Y.;Boyd,L.E.;Kruplak,J.F.;Cleland,W.E.,Jr.;Wilkes, J.S.;Hussey,C.L.J.Electrochem.Soc.2011,158,F1.
(25)MacFarlane,D.R.;Meakin,P.;Sun,J.;Amini,N.;Forsyth,M. J.Phys.Chem.B 1999,103,4164.
(26)Orita,A.;Kamijima,K.;Yoshida,M.;Yang,L.J.Power Sources 2010,195,6970.
(27)Harris,K.R.;Woolf,L.A.;Kanakubo,M.J.Chem.Eng.Data 2005,50,1777.
(28)Wu,T.Y.;Su,S.G.;Gung,S.T.;Lin,M.W.;Lin,Y.C.;Lai,C. A.;Sun,I.W.Electrochim.Acta 2010,55,4475.
(29) Carpio,R.A.;King,L.A.;Kibler,F.C.,Jr.;Fannin,A.A.,Jr. J.Electrochem.Soc.1979,126,1650.
(30) Hagiwara,R.;Matsumoto,K.;Nakamori,Y.;Tsuda,T.;Ito,Y.; Matsumoto,H.;Momota,K.J.Electrochem.Soc.2003,150, D195.
(31)Yoshizawa,M.;Xu,W.;Angell,C.A.J.Am.Chem.Soc.2003, 125,15411.
(32)Angell,C.A.;Byrne,N.;Belieres,J.P.Accounts Chem.Res. 2007,40,1228.
(33) Xu,W.;Cooper,E.I.;Angell,C.A.J.Phys.Chem.B 2003,107, 6170.
(34) MacFarlane,D.R.;Forsyth,M.;Izgorodina,E.I.;Abbott,A.P.; Annat,G.;Fraser,K.Phys.Chem.Chem.Phys.2009,11,4962.
(35)Xu,W.;Wang,L.M.;Nieman,R.A.;Angell,C.A.J.Phys. Chem.B 2003,107,11749.
(36) Fraser,K.J.;Izgorodina,E.I.;Forsyth,M.;Scott,J.L.; MacFarlane,D.R.Chem.Commun.2007,3817.
(37)Matsumoto,K.;Hagiwara,R.Inorg.Chem.2009,48,7350.
——李振声

